Back to Journals » Clinical Ophthalmology » Volume 15
Retinal Detachment After Endophthalmitis: Risk Factors and Outcomes
Authors Wang T, Moinuddin O , Abuzaitoun R, Hwang M , Besirli C, Wubben TJ , Zacks DN
Received 21 January 2021
Accepted for publication 9 March 2021
Published 13 April 2021 Volume 2021:15 Pages 1529—1537
DOI https://doi.org/10.2147/OPTH.S302757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Tiantian Wang,1,2 Omar Moinuddin,1 Rebhi Abuzaitoun,1 Min Hwang,1 Cagri Besirli,1 Thomas J Wubben,1 David N Zacks1
1Department of Ophthalmology and Visual Sciences, University of Michigan W.K. Kellogg Eye Center, Ann Arbor, MI, USA; 2Department of Ophthalmology, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China
Correspondence: David N Zacks
University of Michigan, Kellogg Eye Center, 1000 Wall St., Ann Arbor, MI, 48105 Tel +1 734-936-0871
Email [email protected]
Purpose: To analyze the risk factors, clinical course, and visual and anatomic outcomes of retinal detachment (RD) after endophthalmitis.
Patients and Methods: This retrospective study included 108 patients diagnosed with endophthalmitis between August 2014 and May 2019 at a single tertiary referral center. Sixteen patients developed RD after endophthalmitis. Retrospective analysis was performed to compare the cohort of endophthalmitis alone versus the cohort that developed RD after endophthalmitis, with analysis of potential risk factors for RD after endophthalmitis and treatment outcomes.
Results: The incidence of RD after endophthalmitis was 14.8% (N=16/108). The median time to develop RD after endophthalmitis was 27 days (range: 1– 581 days, IQR: 25.3). Thirteen (81.3%) cases of RD occurred less than 2 months after the diagnosis of endophthalmitis. The incidence of aphakia (p=0.023) and posterior synechia (PS) (p=0.014) were significantly higher in the RD group. The mean initial and final visual acuity (VA) of the endophthalmitis alone group was 1.9± 0.8 logMAR and 1.2± 1.0 logMAR (p< 0.0001), respectively, and 1.9± 0.9 logMAR and 1.3± 1.2 logMAR (p=0.07) in the RD group, respectively. Enucleation or evisceration occurred in 31.3% of cases with RD after endophthalmitis. The rate of final retinal re-apposition for the RD cohort was 56.3%.
Conclusion: The anatomic and functional outcomes for RD after endophthalmitis remain poor, with significant risk for permanent vision loss. Aphakia and posterior synechiae were seen more often in cases with RD after endophthalmitis.
Keywords: pars plana vitrectomy, intraocular inflammation, aphakia, posterior synechiae, vision loss
Introduction
Infectious endophthalmitis is an emergent ophthalmic condition characterized by purulent inflammation of the intraocular structures caused by endogenous or exogenous pathogens, and may lead to permanent and irreversible loss of vision.1,2 Intravitreal antibiotics and pars plana vitrectomy (PPV) are the mainstay of treatment, with systemic antibiotic treatment often added primarily for endogenous infections. Along with antibiotics, a vitreous and/or aqueous biopsy (with or without vitrectomy) with microbiologic testing is routinely performed to determine infectious etiology.3,4 A severe complication of endophthalmitis is the subsequent development of retinal detachment (RD), which has been reported in up to 25% of cases of endophthalmitis.5–9
While several previous study groups have explored potential precipitating factors and clinical course, as it stands, there does not exist an evidence-based consensus on the risk factors that contribute to the development of RD after endophthalmitis. Likewise, there is limited literature correlating examination findings at the time of diagnosis of endophthalmitis and infectious etiologies with RD incidence and prognosis. A few studies have suggested that the virulence of organisms, posterior capsular rupture after cataract surgery, and vitreous prolapse are risk factors for the development of RD after endophthalmitis.5,6 Diabetes and retinal vasculitis have also been reported to increase the likelihood of RD after exogenous endophthalmitis.6,8 Though seldom mentioned, RD may occur as a result of membranous scar tissue formation even after the inflammation has subsided.10 Unlike the majority of patients with primary, non-complex rhegmatogenous retinal detachment, patients who develop RD after endophthalmitis generally have a poor visual prognosis, with up to two-thirds of all patients achieving a best corrected visual acuity (BCVA) of 20/400 or less even after surgical repair.11
The purpose of this study was to assess the incidence, potential risk factors, and underlying mechanisms related to the occurrence and outcomes of RD after endophthalmitis in a manner that is not restricted to the etiology of endophthalmitis, severity of initial vision, or limited selection of operative procedure as in previous literature.5,6,8,12 Specifically, we discuss the clinical course, virulence of pathogens, and visual and anatomic outcomes.
Patients and Methods
This is a retrospective, non-consecutive case series of patients seen at the University of Michigan/W.K. Kellogg Eye Center between August 2014 and May 2019. The Institutional Review Board of the University of Michigan (Ann Arbor, MI, USA) approved the research, and it conformed to the tenets of Health Insurance Portability and Accountability Act (HIPAA) and the Declaration of Helsinki. Patients who were included in this study were identified based on a review of International Classification of Diseases (ICD) diagnostic codes (see Supplemental Table). Patients with either endogenous or exogenous endophthalmitis alone or RD after endophthalmitis were included. As the study focused on the time point of RD after endophthalmitis, cases with RD before or concurrent with endophthalmitis were excluded. Only individuals diagnosed with retinal detachment after previously being diagnosed with endophthalmitis, and then undergoing vitreoretinal surgical repair with at least 3 months of documented follow-up were included in this study. The minimum follow-up of 3 months was set in order to best ascertain anatomic outcomes postoperatively.
In each case, a diagnosis of endophthalmitis was made based on clinical manifestations, such as eye pain, vitritis, loss of vision, hypopyon, red eye, periocular swelling, as well as review of each individual patient’s history of presenting illness. Data collected included demographic information, clinical and microbiological characteristics, and preoperative, perioperative, and postoperative information located in the electronic medical record (EMR). For culture-positive cases, microorganisms were categorized as less virulent (coagulase negative Staphylococcus (CoNS) and fungus) or more virulent (Staphylococcus aureus, gram-negative bacteria, Streptococcus species, and Bacillus species).6,11,13 Endophthalmitis cases were classified as endogenous or exogenous based on the presenting history, and the virulence was based on the results of microbiologic cultures. Endogenous endophthalmitis was defined as endophthalmitis in the setting of an existing systemic or localized organ infection that has reasonable potential to hematologically spread to the ocular tissues. Exogenous endophthalmitis was defined if occurred subsequent to surgery (postoperative), intravitreal injection, perforating or penetrating trauma, diagnosis of corneal ulcer within the past 6 months or associated with a filtering bleb. Postoperative endophthalmitis was divided into two sub-types. Late-onset postoperative exogenous endophthalmitis was defined as those cases that presented more than 42 days (6 weeks) after surgery. Acute postoperative exogenous endophthalmitis was defined as those cases that presented less than 42 days after surgery.9
The anatomic outcomes in this study included final status of retinal re-apposition and enucleation or evisceration. Visual acuity (VA) was based on the Snellen visual acuity, which was converted to logMAR for statistical analysis. Pinhole Snellen VA was used in place of best-corrected visual acuity due to the retrospective nature of this study. CF, hand motion (HM), light perception (LP) and no light perception (NLP) vision were converted to logMAR as follows: CF 1.9, HM: 2.3, LP: 2.7, NLP: 3.0.14,15
There were 127 patients identified. Ten patients with RD before the diagnosis of endophthalmitis, five patients with concurrent RD and endophthalmitis, two patients with RD in the fellow eye, and two patients with follow-up time less than 3 months after surgery were excluded. This resulted in 108 endophthalmitis patients being included in this study, 16 of which developed RD after endophthalmitis. Patients who underwent enucleation or evisceration (N=13) were not included in the analysis of the final VA, as there was no record of VA on the last office visit. As per the Institutional Review Board (IRB) at The University of Michigan, informed patient consent was not required to review patient medical data for research. Moreover, given that all patient information remained de-identified throughout data analysis and the drafting of this manuscript, informed patient consent was not deemed to be necessary by the IRB.
GraphPad Prism 8.0.2 (San Diego, CA) software was utilized for statistical analyses. The Shapiro–Wilk test of normality was performed to select parametric/non-parametric test. Fisher’s exact test and Student t-test (paired or unpaired) were separately used to measure the difference between groups for categorical and continuous variables, with P<0.05 necessary for significance.
Results
All Cases of Endophthalmitis (n=108)
The characteristics of all patients with endophthalmitis are summarized in Table 1. Overall, 108 patients with endophthalmitis were identified, with 51% being male (n=55). The mean age was 67 ± 17 years (range: 1–94 years). The mean follow-up time was 23.5 ±21.7 months (range: 2 days to 76 months). The etiology of endophthalmitis was exogenous in 93 (86.1%) cases and endogenous in 15 (13.9%) cases. Endophthalmitis was postoperative in 42 (38.9%) cases, associated with intravitreal injection in 21 (19.4%) cases, due to corneal ulcer in 17 (14.8%) cases, secondary to penetrating or perforating globe injury 6 (5.6%) cases, and bleb-associated in 6 (5.6%) cases. For patients in the postoperative cohort, the majority was acute (N=27, 64.3%). The mean VA at the time of diagnosis of endophthalmitis was 2.0±0.8 logMAR (range: 0.2–3 logMAR; Snellen equivalent, 20/1910 [range, 20/30-NLP]) with the majority of patients presenting with 20/400 or worse vision (N=85, 78.7%). As to lens status at the time of diagnosis of endophthalmitis, 63 patients were pseudophakic, 40 were phakic, and 5 were aphakic. Sixty-five (60.2%) patients had primary cataract surgery, after which 3 were aphakic. The remaining two patients were aphakic due to trauma or pars plana lensectomy. Microbial cultures were positive in 55.6% of the patients (60/108) and CoNS was the most commonly observed microorganism (N=22, 36.7%). All patients received intravitreal injection of antibiotics (IVAB), with the combination of vancomycin 1 mg/0.1 mL and ceftazidime 2.25 mg/0.1 mL being the most common treatment regimen (N=89, 82.4%). Twenty-six patients underwent pars plana vitrectomy (PPV) in addition to intravitreal antibiotic treatment. The mean final VA, excluding the 13 cases who had enucleation/evisceration, was 1.2±1.0 logMAR (range: −0.1 to 3 logMAR; Snellen equivalent, 20/332 [range, 20/15-NLP], N=95). Post-treatment vision was worse than 20/400 in 40 (42.1%) patients. The mean final VA of patients who underwent both PPV and IVAB was 1.20 ± 1.10 logMAR (range, 0 to 3 logMAR; Snellen equivalent, 20/317 [range, 20/20 to NLP], N=21), similar to 1.22±1.00 log MAR (range: −0.1 to 3 logMAR; Snellen equivalent, 20/332 [range, 20/15-NLP], N=74) for patients who received IVAB alone (p=0.94).
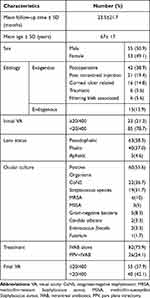 |
Table 1 Characteristics of All Patients with Endophthalmitis (n=108) |
RD After Endophthalmitis (n=16)
Sixteen patients developed RD subsequent to endophthalmitis diagnosis (Table 2). The mean age of this cohort was 60±18 years (range: 12–87), and 9 (56.3%) patients were male. The incidence of RD after endophthalmitis in this study was 14.8% (N=16/108). The frequency of RD was 11.5% (N=3/26) in patients who had PPV along with IVAB, as compared to 15.9% (N=13/82) for patients with IVAB alone. Five (7.9%) patients with pseudophakia developed RD. Among patients who had cataract surgery before endophthalmitis, six (9.2%) patients developed RD. The mean follow-up time for all 16 cases was 22.7±19.9 (range: 1–63) months.
 |
Table 2 Presenting Characteristics of Patients Who Developed RD Subsequent to Endophthalmitis |
Three quarters of the patients with RD had exogenous endophthalmitis (N=12/16). Among them, 5 (41.7%) had postoperative endophthalmitis, including two cases (No. 12, 16) after keratoprosthesis (KPRO), and three cases (No. 4, 8, 11) after Kelman phacoemulsification and intraocular lens implantation (KPE/IOL). Three of these cases (No. 4, 12, 16) were defined as late-onset postoperative endophthalmitis, as their endophthalmitis occurred 44, 75, and 77 days after ocular surgery, respectively. Of the remaining seven exogenous endophthalmitis cases with subsequent RD, 2 (12.5%) developed endophthalmitis after intravitreal injection, three developed endophthalmitis after open globe injury, and two others were secondary to corneal ulceration. In the four patients with endogenous endophthalmitis and subsequent RD, the source of endogenous infection was attributed to MRSA bacteriemia from a presumed primary pulmonary focus (No.2), Streptococcus bacteremia from meningitis (No. 3), urinary tract infection with negative culture (No.9), and Streptococcus bacteremia from an unknown source (No. 13).
Potential risk factors related to RD after endophthalmitis were aphakia (p=0.014) and the presence of posterior synechiae (PS) (p=0.023) (Table 3). The presence of pseudophakia (p=0.026) was higher in the endophthalmitis alone group. The median time to develop RD after endophthalmitis was 27 days (range: 1–581 days, IQR: 25.3). Thirteen (81.3%) cases of RD occurred less than 2 months after the diagnosis of endophthalmitis. Two patients (No. 13, 16) developed RD between 2 and 4 months after endophthalmitis. Only one patient (No. 7) developed RD over 1 year from the time of endophthalmitis, and this patient had a complicated ocular history of KPRO and anterior scleritis with scleral thinning.
 |
Table 3 Potential Factors Related to Occurrence of RD After Endophthalmitis |
Eleven patients (68.8%) in the RD cohort had positive ocular cultures, nine of which came from exogenous cases. Five cultures demonstrated less virulent pathogens with CoNS accounting for 4 cases (36.4%) and Fusarium accounting for the other (9.1%). The remaining six positive ocular cultures grew Streptococcus species (N=5, 45.5%) and MRSA (N=1, 9.1%).
Most of the patients (N=13, 81.3%) were treated primarily with IVAB alone. One (7.7%) patient received IVAB three times in 6 days, and four (30.8%) other patients received antibiotic injections twice. Three (17.6%) patients had PPV in addition to IVAB. Additionally, one patient (No. 8) received an intravitreal injection of dexamethasone 400 mcg/0.1mL.
Rhegmatogenous retinal detachment (RRD) was the most common type of RD (N=8, 50%) followed by tractional retinal detachment (TRD) (N=6, 37.5%). One (6.25%) patient had an exudative retinal detachment (ERD) and another patient (6.25%) had a combined TRD/RRD. The macula was detached at the time of RD diagnosis in six eyes (38%). Seven (43.8%) patients had no surgical intervention for RD: one had an ERD and 6 patients opted to not undergo surgery. For those who underwent surgical repair for RD (N=9, 56.3%), eight (88.9%) underwent PPV alone and one (11.1%) underwent PPV combined with a scleral buckle (SB). Two patients (No. 4, 8) required more than one surgery to achieve anatomic success, and one patient (No.1) who underwent repair did not achieve anatomic success and ultimately underwent enucleation. The rate of final retinal re-apposition was 56.3% (n=9/16) (Table 4).
 |
Table 4 Treatment Strategies and Outcomes for RD After Endophthalmitis |
The mean presenting VA at the time of RD diagnosis was 2.2±0.9 logMAR (range: 0.2–3; Snellen equivalent: 20/2891, [range: 20/150-NLP]; N=16) with 14 patients (87.5%) having a VA less than CF. The mean final VA was 1.3±1.2 logMAR (range: −0.1 to 3 logMAR; Snellen equivalent: 20/418, [range: 20/15-NLP]; N=11) in patients with RD after endophthalmitis (excluding the 5 patients who underwent enucleation or evisceration), with final VA of 20/400 or better in 6 (54.5%) patients. After treatment for RD, hypotony (IOP ≤5mmHg) developed in 3 (18.8%) patients, while 2 patients developed glaucoma or ocular hypertension. In addition, three (18.8%) patients developed proliferative vitreoretinopathy (PVR) after RD diagnosis.
Comparison of Outcomes in RD After Endophthalmitis (n=16) versus Endophthalmitis Alone (n=92)
Five patients (31.3%) with RD after endophthalmitis eventually underwent enucleation or evisceration, compared to eight patients (8.7%) with endophthalmitis alone (p=0.024). In the endophthalmitis alone cohort, VA was significantly improved after treatment compared to baseline, with mean initial and final VA of 1.9±0.8 logMAR and 1.2±1.0 logMAR (p<0.0001), respectively. The mean initial and final VA of the RD cohort was 1.9±0.9 logMAR and 1.3±1.2 logMAR (p=0.07), respectively, demonstrating an improvement. There was no significant difference in the mean final VA for endophthalmitis patients with or without RD who did not undergo enucleation or evisceration (p=0.73).
Discussion
Infectious endophthalmitis is a rare but serious ophthalmic emergency that may result in a poor visual outcome and permanent loss of vision. A major determinant for the prognosis of endophthalmitis is timely diagnosis that facilitates early intervention.16 However, despite early recognition of the infection and treatment with IVAB, the disease may progress and contribute to the formation of RD.
The most serious ocular sequala of endophthalmitis is the need to remove the eye or the intraocular contents. In this series, 8.7% of patients with endophthalmitis alone underwent enucleation or evisceration. However, in patients with RD, this rate was a significantly higher at 31.3%, confirming the inferior outcomes of patients who had RD secondary to endophthalmitis.
The incidence of RD after endophthalmitis was 14.8% in this series, which is within the range reported in previous series in the literature (8.3%–25%) over the past 35 years.5–9 The possibility that patients might have predisposing factors before the diagnosis of RD was considered here. Aphakia was associated with RD formation in this study, which is consistent with previous literature.17–19 Patients with aphakia might have a higher risk of retinal tears and RD, probably due to posterior capsular rupture and vitreous loss during lens removal. The rate of pseudophakia in the RD group was consistent with previous literature.20–22 Interestingly, the presence of pseudophakia was higher in the endophthalmitis alone group, with only 9.2% of the patients who had cataract surgery before endophthalmitis later developing an RD. We do not suggest that pseudophakia was protective against the formation of RD. Rather the difference between our results and those of previous reports23–25 might be due to different characteristics of the study population.
Severity of intraocular inflammation at the time of endophthalmitis diagnosis likely contributes to the risk of developing RD. Interestingly, the presence of hypopyon or the virulence of the organism did not correlate with RD formation in this study. However, we interestingly find the presence of PS was significantly higher in the RD group as compared to the endophthalmitis alone group. The finding that PS but not hypopyon is associated with RD after endophthalmitis, in conjunction with the finding that some patients developed retinal detachment several months after completing antibacterial treatment, suggests that chronic inflammation persisting beyond the infectious window of endophthalmitis may be involved in the pathophysiology of late retinal detachment.26
Another potential contributor to the development of RD is the invasive nature of the intraocular procedures such as IVAB and PPV used for treating endophthalmitis.5 These procedures can cause iatrogenic retinal breaks that may precipitate or predispose patients to the development of RD. It has been suggested that a “jet stream” effect induced by intravitreal injection could create a retinal tear, especially in patients who have undergone vitrectomy.5 The “jet stream” effect happens when high velocity of fluid stream from the needle damages fragile retina with no vitreous cushion. However, the frequency of RD was 11.5% in patients who received both PPV and IVAB compared to 15.9% in the IVAB alone group in this study, which suggested this “jet stream” effect had minimal effect in this cohort of patients.
In the current study, the anatomic and functional outcomes for RD after endophthalmitis was poor. Nearly one-third patients required enucleation or evisceration. The final VA was 20/400 or better in only 37.5% who did not undergo enucleation or evisceration. This was similar to the 39.7% observed by Dave et al.12 Initial and final mean VA in this series were 1.93±0.97 logMAR and 1.32±1.18 logMAR, respectively, compared to 1.50±0.66 logMAR and 1.35±0.70 logMAR in that study. The mean final VA in the two cohorts was similar, reflecting the generally poor functional outcomes of RD after endophthalmitis. The better initial VA in Dave et al may have resulted from the exclusion of inoperable eyes to evaluate silicone oil in RD repair, which usually had poor initial VA. Dave et al noted that the final reattachment was successful in 62.5% of cases, while either enucleation/evisceration or the final VA lower than CF occurred in 62.5% of cases. The fact that patients still had poor VA even with reattached retina, which was consistent with the Endophthalmitis Vitrectomy Study (EVS),8 demonstrated that the ocular damage to retinal cells caused by severe inflammation might be irreversible.
A key limitation of our study was its retrospective nature. As a result, there were inherent differences in management protocol and baseline characteristics among subjects. The relative rarity of endophthalmitis and especially RD after endophthalmitis caused statistical analysis between many subgroups to be unfeasible or underpowered due to inadequate sample size within the groups. Additionally, the fact that this study is not restricted to the etiology of endophthalmitis or is limited only to select ophthalmic procedures or surgeries introduces a number of unmeasured variables that can not be reasonably quantified. Furthermore, the exclusion of patients with follow-up time less than 3 months after surgery for RD has the potential to lead to surveillance bias, as those with RD repair may have a longer observation period than some of those with endophthalmitis alone.
Conclusion
In summary, RD after endophthalmitis is often associated with poor anatomic and visual outcomes. Our data demonstrate that the presence of aphakia and posterior synechiae are associated with the development of RD after endophthalmitis. Enucleation or evisceration occurred in approximately one-third of cases of RD after endophthalmitis, highlighting the high rate of poor anatomic and functional outcome despite intervention.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Novosad BD, Callegan MC. Severe bacterial endophthalmitis: towards improving clinical outcomes. Expert Rev Ophthalmol. 2010;5(5):689–698. doi:10.1586/eop.10.52
2. Durand ML. Bacterial and Fungal Endophthalmitis. Clin Microbiol Rev. 2017;30(3):597–613. doi:10.1128/CMR.00113-16
3. López-Cabezas C, Muner DS, Massa MR, Mensa Pueyo JM. Antibiotics in endophthalmitis: microbiological and pharmacokinetic considerations. Curr Clin Pharmacol. 2010;5(1):47–54. doi:10.2174/157488410790410597
4. Clarke B, Williamson TH, Gini G, Gupta B. Management of bacterial postoperative endophthalmitis and the role of vitrectomy. Surv Ophthalmol. 2018;63(5):677–693. doi:10.1016/j.survophthal.2018.02.003
5. Nelsen PT, Marcus DA, Bovino JA. Retinal detachment following endophthalmitis. Ophthalmology. 1985;92(8):1112–1117. doi:10.1016/s0161-6420(85)33916-7
6. Chiquet C, Aptel F, Combey-de Lambert A, et al. Occurrence and risk factors for retinal detachment after pars plana vitrectomy in acute postcataract bacterial endophthalmitis. Br J Ophthalmol. 2016;100(10):1388–1392. doi:10.1136/bjophthalmol-2015-307359
7. Lee CS, Khan M, Patrie J, Bajwa A, Shildkrot YE. Pars plana vitrectomy for endophthalmitis: microbiologic spectrum and clinical outcomes. Ocul Immunol Inflamm. 2020;1–6. doi:10.1080/09273948.2019.1698750
8. Doft BM, Kelsey SF, Wisniewski SR. Retinal detachment in the endophthalmitis vitrectomy study. Arch Ophthalmol. 2000;118(12):1661–1665. doi:10.1001/archopht.118.12.1661
9. Feng HL, Robbins CB, Fekrat S. A nine-year analysis of practice patterns, microbiologic yield, and clinical outcomes in cases of presumed infectious endophthalmitis. Ophthalmol Retina. 2020;4(6):555–559. doi:10.1016/j.oret.2020.03.009
10. Kimura D, Sato T, Suzuki H, et al. A case of rhegmatogenous retinal detachment at late stage following endogenous bacterial endophthalmitis. Case Rep Ophthalmol. 2017;8(2):334–340. doi:10.1159/000477160
11. Foster RE, Rubsamen PE, Joondeph BC, Flynn HW, Smiddy WS. Concurrent endophthalmitis and retinal detachment. Ophthalmology. 1994;101(3):490–498. doi:10.1016/s0161-6420(94)31308-x
12. Dave VP, Pathengay A, Relhan N, et al. Endophthalmitis and concurrent or delayed-onset rhegmatogenous retinal detachment managed with pars plana vitrectomy, intravitreal antibiotics, and silicone oil. Ophthalmic Surg Lasers Imaging Retina. 2017;48(7):546–551. doi:10.3928/23258160-20170630-05
13. Cornut PL, Thuret G, Creuzot-Garcher C. Relationship between baseline clinical data and microbiologic spectrum in 100 patients with acute postcataract endophthalmitis. Retina. 2012;32(3):549–557. doi:10.1097/IAE.0b013e3182205996
14. Baughman DM, Su GL, Tsui I, Lee CS, Lee AY. Validation of the Total Visual Acuity Extraction Algorithm (TOVA) for automated extraction of visual acuity data from free text, unstructured clinical records. Transl Vis Sci Technol. 2017;6(2):2. doi:10.1167/tvst.6.2.2
15. Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247(1):137–142. doi:10.1007/s00417-008-0926-0
16. Ching Wen Ho D, Agarwal A, Lee CS, et al. A review of the role of intravitreal corticosteroids as an adjuvant to antibiotics in infectious endophthalmitis. Ocul Immunol Inflamm. 2018;26(3):461–468. doi:10.1080/09273948.2016.1245758
17. Byer NE. Natural history of posterior vitreous detachment with early management as the premier line of defense against retinal detachment. Ophthalmology. 1994;101(9):1503–1513. doi:10.1016/s0161-6420(94)31141-9
18. Bhardwaj G, Walker RJE, Ezra E, Mirza Z, Muqit MMK. A 21-year study of vitreoretinal surgery for aphakic retinal detachment: long-term surgical outcomes and complications. Ophthalmol Retina. 2019;3(9):784–790. doi:10.1016/j.oret.2019.04.002
19. Petousis V, Sallam AA, Haynes RJ, et al. Risk factors for retinal detachment following cataract surgery: the impact of posterior capsular rupture. Br J Ophthalmol. 2016;100(11):1461–1465. doi:10.1136/bjophthalmol-2015-307729
20. Ramos M, Kruger EF, Lashkari K. Biostatistical analysis of pseudophakic and aphakic retinal detachments. Semin Ophthalmol. 2002;17(3–4):206–213. doi:10.1076/soph.17.3.206.14784
21. Russell M, Gaskin B, Russell D, Polkinghorne PJ. Pseudophakic retinal detachment after phacoemulsification cataract surgery: ten-year retrospective review. J Cataract Refract Surg. 2006;32(3):442–445. doi:10.1016/j.jcrs.2005.12.095
22. Karczewicz D, Lubiński W, Podboraczyńska-Jodko K, Spoz E. Odwarstwienie siatkówki w oczach po operacji zaćmy metoda fakoemulsyfikacji [Pseudophakic retinal detachment after cataract surgery by phacoemulsification]. Ann Acad Med Stetin. 2006;52(2):79–82.
23. Haug SJ, Bhisitkul RB. Risk factors for retinal detachment following cataract surgery. Curr Opin Ophthalmol. 2012;23(1):7–11. doi:10.1097/ICU.0b013e32834cd653
24. Coppé AM, Lapucci G. Posterior vitreous detachment and retinal detachment following cataract extraction. Curr Opin Ophthalmol. 2008;19(3):239–242. doi:10.1097/ICU.0b013e3282fc9c4a
25. Qureshi MH, Steel D. Retinal detachment following cataract phacoemulsification-a review of the literature. Eye. 2020;34(4):616–631. doi:10.1038/s41433-019-0575-z
26. Kunavisarut P, Srisomboon T, Patikulsila D, et al. Risk factors for development of rhegmatogenous retinal detachment in patients with uveitis. Ocul Immunol Inflamm. 2019;27(4):681–685. doi:10.1080/09273948.2018.1424343
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
