Back to Journals » International Medical Case Reports Journal » Volume 16
Retinal Changes After COVID-19 Infection and COVID-19 Vaccination
Authors Leite J , Abreu AC , Furtado MJ , Lume M
Received 12 February 2023
Accepted for publication 20 April 2023
Published 24 July 2023 Volume 2023:16 Pages 433—442
DOI https://doi.org/10.2147/IMCRJ.S408306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
João Leite,1 Ana Carolina Abreu,1 Maria João Furtado,1,2 Miguel Lume1
1Department of Ophthalmology, Centro Hospitalar Universitário de Santo António, Oporto, Portugal; 2ICBAS - Instituto de Ciências Biomédicas Abel Salazar, Oporto, Portugal
Correspondence: João Leite, Ophthalmology, Centro Hospitalar Universitário de Santo António, Largo Do Prof. Abel Salazar, Oporto, 4099-001, Portugal, Tel +351916523942, Email [email protected]; [email protected]
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease was first reported in 2019 and was initially associated with respiratory pathology. With the improvement of knowledge about this disease, it was noticed that, among other symptoms, some patients presented visual acuity changes associated with retinal vascular changes, mainly associated with thrombotic phenomena. Later, with the development of vaccines against SARS-CoV-2 disease, cases of visual acuity alterations secondary to thrombotic phenomena were also reported.
Case Presentation: In this article, a series of clinical cases with retinal vascular alterations after COVID-19 infection and vaccination are described.
Conclusion: COVID-19 infection and vaccination increase the risk of retinal vascular events. The purpose of this article is to present a set of clinical cases with various manifestations of vascular changes in the retina associated with COVID-19 infection and COVID-19 vaccination observed in the Department of Ophthalmology of Centro Hospitalar Universitário de Santo António, in Porto, Portugal.
Keywords: COVID-19 infection, COVID-19 vaccination, retinal vascular events
Background
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, as a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 Although many patients are asymptomatic or have mild symptoms, this syndrome is characterized by a spectrum of multiorgan clinical manifestations:2 it was primarily characterized as a respiratory illness, presenting with dyspnea, anosmia, fever and cough.3 Eye symptoms included conjunctivitis, intraocular inflammation and retinal changes.3,4 As the disease became better known, reports of arterial and venous thromboembolic events emerged, probably associated with virus triggered immune-mediated thromboembolic events.2 Lately, many reports described retinal occlusive events after COVID-19 infection as central retinal vein occlusion, branch retinal vein occlusion, hemiretinal vein occlusion, central retinal artery occlusion and paracentral acute middle maculopathy.2,3,5–8
With the development of vaccination to SARS-CoV-2, in some cases, visual changes associated with retinal occlusive phenomena were observed after vaccination with the various vaccines available.9–11 However, the exact mechanisms of these adverse effects are unclear.10
The purpose of this article is to present a set of clinical cases with various manifestations of vascular changes in the retina associated with COVID-19 infection and COVID-19 vaccination, at Centro Hospitalar Universitário do Porto.
Case Presentation
Case 1
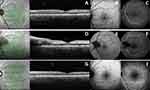
Caucasian 50-year-old man, with no known previous ophthalmologic history, no history of medication such as anticoagulants or antiplatelet agents and no previous history or previous exposure to COVID-19, with a history of chronic (unmedicated) hepatitis B. He came to the emergency department due to sudden-onset painless vision loss with 1 day of evolution in left eye. One day before the onset of symptoms, the patient was vaccinated for COVID-19 with the BioNTech/Pfizer – ComiRNATy vaccine. On ophthalmologic examination, he had a best corrected visual acuity (BCVA) 20/20 in the right eye (RE) and 20/400 in the left eye (LE). At biomicroscopy, the exam was normal except for the presence of relative afferent pupillary defect in LE. At mydriatic fundoscopy, no changes were found in the RE and but the LE presented venous engorgement and two retinal hemorrhages in the middle and extreme periphery (described in Table 1). Macular Spectral Domain – Optical Coherence Tomography (Macular SD-OCT) showed a parafoveal hyper-reflective band at the level of inner nuclear layer (shown in Figure 1A) and a Fundus Autofluorescence (FAF) revealing a fern-like pattern (Spectralis® OCT, version 1.10.2.0, Heidelberg Engineering, Heidelberg, Germany) (shown in Figure 1B), with no ischemia or vascular leakage on angiography on fluorescein angiography (FA) or indocyanine green angiography (ICGA) (shown in Figure 1C).
 |
Table 1 Instituted Therapy |
The diagnosis of Paracentral Acute Middle Maculopathy (PAMM) and the applied therapy is described in Table 2. This diagnosis was corroborated by clinical observation carried out on the 4th day of symptoms, with flame-shaped and dot hemorrhages adjacent to retinal veins and central retinal vein, peripapillary edema and nasal subretinal fluid (shown in Figure 1D), in addition to hyperreflectivity changes in the inner layers and the fern-like pattern already observed on the 1st day (shown in Figure 1E), without vascular leakage (shown in Figure 1F).
 |
Table 2 Examination Finding |
During follow-up, the patient did not report any new episodes of vision loss and in the 4th month after the diagnosis, the patient showed progressive improvement with a visual acuity of 20/20. Although at fundoscopy, the retina still presented visible hemorrhages and macular disorganization with regression of the fern-like pattern (shown in Figure 1G and H) and without ischemia (shown in Figure 1I).
Case 2
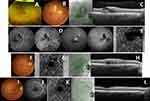
Caucasian 48-year-old woman, without known personal and ophthalmologic history, without medication or relevant family history, no history of medication such as anticoagulants or antiplatelet agents and no previous history or previous exposure to COVID-19 came to the emergency department due to sudden onset of visual loss with amputation of the upper visual field with approximately 6 hours of evolution in RE. She was vaccinated for COVID-19 with the BioNTech/Pfizer – ComiRNATy vaccine 6 days before. On objective examination, she had a BCVA 20/20 in RE and 20/20 in LE. At biomicroscopy, she had a decreased pupillary light reflex in RE, without no other relevant changes of the anterior segment (described in Table 1). On the mydriatic fundus, no changes were found in LE. In RE, pallor of the retina inferior to the optic disc was observed with preservation of the central macula (shown in Figure 2A) and macular SD-OCT showed an edema of the inner layers of the retina adjacent to the inferior temporal branch of the central retinal artery (shown in Figure 2B). Therefore, the most likely diagnostic hypothesis was a branch retinal artery occlusion and the patient was treated according therapy described in Table 2.
These changes remained constant in subsequent assessments during the first few weeks. At 1st month of follow-up, the patient kept a BCVA 20/20 in both eyes, without changes in the anterior segment. At fundoscopy it was observed in RE inferior papillary pallor, with some cotton-wool exudates along the inferior temporal artery, with good foveal reflex and no visible hemorrhages; at macular SD-OCT (shown in Figure 2C), the inner retina maintained the edema of the inner layers of the retina adjacent to the inferior temporal branch of the central retinal artery; FA (shown in Figure 2D) showed an anatomical variant (bifurcation of the superior temporal artery with the inferior macular branch), dilation of peripapillary capillaries with ischemic lesions in the inferior temporal artery, with signs of reperfusion and no signs of peripheral ischemia and 30–2 visual field (Humphrey Field Analyzer: HFA, Carl Zeiss Meditec, Dublin, CA) (shown in Figure 2E) with superior visual field amputation and preserved macula.
Case 3
Caucasian 49-year-old female with no past ophthalmic diseases, no history of medication such as anticoagulants or antiplatelet agents and no previous history or previous exposure to COVID-19 and a history of severe ileocolic Crohn’s disease with 2 sigmoid strictures, being treated with azathioprine (25mg/d), adalimumab (every 2 weeks), calcifediol 0.266 mg (1 tablet per month) and cyanocobalamin 1mg (1 tablet per day). She went to the emergency department with complaints of blurred vision in the right eye with 2 weeks of evolution, which started 1 day after vaccination for COVID-19 with the BioNTech/Pfizer – ComiRNATy vaccine. On objective examination, she had BCVA 20/100 in RE and 20/20 in LE, without relevant changes of the anterior segment at biomicroscopy. On the mydriatic fundus, no changes were found in the LE; in the RE it was observed a well-defined whitish area adjacent to the superior temporal arcade, with intraretinal hemorrhages (shown in Figure 3A and B) (described in Table 1). Macular SD-OCT showed macular edema (shown in Figure 3C), FA and ICGA and Optical Coherence Tomography Angiography (OCT-A) showed an area of non-perfusion in the upper nasal macula (shown in Figure 3D and E).
After ruling out infectious pathology, given the concomitant immunomodulatory therapy, the hypothesis of occlusive retinal vasculopathy, with ischemic macular lesion and secondary macular edema, was assumed and the patient started the therapy described in Table 2.
In the 1st month of follow-up, the patient´s BCVA improved to 20/20 in the RE, and despite the maintenance of a whitish lesion in the upper nasal macula (shown in Figure 3F), a decrease in papillary hemorrhages was observed. Macular SD-OCT showed improvement of the macular edema and a smaller macular hyper-reflective lesion; OCT-A showed maintenance of non-perfusion area in the upper nasal macula (shown in Figure 3G and H). After 6 months of follow-up, the patient maintained BCVA of 20/20. At fundoscopy, it was observed regression of the lesion in the upper nasal macula (shown in Figure 3I and J), with microvascular alterations and rare hemorrhages, a maintenance of non-perfusion area in the upper nasal macula (shown in Figure 3K) and macular SD-OCT showed no macular edema (shown in Figure 3L).
Case 4
Caucasian 45-year-old woman with a personal history of endometriosis, only medicated with intrauterine device (with levonorgestrel), with no history of medication such as anticoagulants or antiplatelet agents and no previous history or previous exposure to COVID-19. She went to the emergency department due to blurred vision with 2 weeks of evolution. It should be noted that the user tested positive for COVID-19 three weeks before this assessment. On objective examination, she had a BCVA 20/20 in both eyes, without changes at biomicroscopy; the fundoscopy was unremarkable on the RE but in the LE there were blurry hemorrhages near the superior temporal arcade, without macular involvement (shown in Figure 4A) (described in Table 1). In macular SD-OCT, there was thickening of the inner layers of the retina at the level of hemorrhage (shown in Figure 4B and C) without macular edema. In this sense, the most likely diagnostic hypothesis was a branch retinal vein occlusion and no therapy was instituted (Table 2). Clinical observation was maintained and during follow-up, she remained clinically stable without new complaints. One year after the diagnosis, she had BCVA 20/20 in both eyes and without fundoscopic changes (shown in Figure 4D and E).
Case 5
Caucasian 66-year-old woman with a history of thyroid cancer (submitted to surgical treatment 20 years before), hypertension and no history of medication such as anticoagulants or antiplatelet agents and no previous history or previous exposure to COVID-19. The patient came to the emergency department due to blurred vision with 1 week of evolution in LE. She tested positive against COVID-19 2 weeks before the onset of symptoms. The BCVA was 20/40 in RE and 20/30 in LE. No changes were observed at biomicroscopy; at fundoscopy a patterned dystrophy of the retinal pigment epithelium was observed in both eyes; in LE there was also cotton-wool hemorrhages and exudates in the upper retina (shown in Figure 5A) (described in Table 1), without macular edema on macular SD-OCT (shown in Figure 5B). FA showed hypofluorescent spots due to blocking effect with slight contrast diffusion (shown in Figure 5C). Therefore, the most likely diagnostic hypothesis was a hemiretinal vein occlusion without macular edema. No systemic or ocular therapy has been introduced (Table 2). At 6th month after the beginning of symptoms, the patient maintained the BCVA on both eyes, without changes at biomicroscopy. At LE fundoscopy, some hemorrhages were observed in the superior retina with some cotton-wool exudates (shown in Figure 5D); SD-OCT showed no macular edema (shown in Figure 5E).
Discussion
Some literature reports that the incidence of thromboembolic complications after COVID-19 infection was significantly higher than in people who received an mRNA vaccine.4,12
There have been some reports of PAMM after infection with COVID-19: Castro et al describe a case that presented with sudden, painless vision loss, with relative afferent pupillary defect, 9 weeks after SARS-CoV-2 infection;8 Padhy et al reported a bilateral case of acute-onset scotoma 2 weeks after SARS-CoV-2 infection;5 Goyal et al reported a paracentral scotoma 4 months after SARS-CoV-2 infection.13 Reports of PAMM have also emerged after vaccination against COVID-19: Pichi et al presented cases of PAMM six days after the first inoculation of the inactivated vaccine for COVID-19 (Sinopharm), in which 2 of 9 eyes in the present series had acute unilateral vision loss associated with acute macular neuroretinopathy and 1 of 9 eyes with PAMM.14
There are also some reports of retinal artery (branch) occlusion and COVID-19 infection/vaccination. Abdin et al report a case of a 76-year-old woman who reported painless vision loss 48 hours after she had received her first dose of the AstraZeneca COVID-19 vaccine;15 Ishibashi et al report a case of a 38-year-old woman with a history of lower visual field defects 15 days after receiving the first dose of the BioNTech/Pfizer – ComiRNATy vaccine;16 Thakar et al report a case of a 44‑year‑old man who presented with sudden painless vision loss 10 days after receiving Covaxin;17 Girbardt et al report a case of a 38-year-old male with a two-day-old painless visual field loss of the inferior hemisphere that had received his second dose of the SARS-CoV-2 vaccination with the BioNTech/Pfizer – ComiRNATy vaccine three days before the event.18
Cases of retinal vasculitis described in the literature after COVID-19 infection or vaccination are rare. However, Mohamed et al described two cases of retinal vasculitis following COVID-19 vaccinations that had been vaccinated with second-dose COVID-19 vaccinations (7 weeks and 4 weeks respectively) and presented with bilateral retinal vasculitis and vitritis.19
In contrast, there are many case reports of central retinal vein occlusion, either post-infection or post-vaccination against COVID-19. Shiroma et al reported 12 cases of retinal vascular occlusion following COVID-19 infections, most of them (85.7%) without previous history of systemic hypertension or thrombotic events: 8 patients with central retinal vein occlusion, 1 patient with hemispheric retinal vein occlusion and 3 patients with branch retinal vein occlusion.3 Endo et al present a case of central retinal vein occlusion 15 days after the first dose of BioNTech/Pfizer – ComiRNATy.11 Sonawane et al also described 2 cases of central retinal vein occlusion after receiving the second dose of the Covishield vaccine.20
Conclusion
The pathogenesis of COVID-19 infection remains poorly understood.3 Studies to date suggest that there is a high incidence of thrombosis in large and small vessels that can be explained by the presence of multiple factors, namely, flow stasis due to prolonged immobilization during the infectious period, vessel wall damage secondary to loss of the endothelium’s normal thromboprotective state, hypercoagulable state caused by endothelial activation in response to the virus itself, thrombophilic inflammation responsible for increased von factor Willebrand and factor VIII and neutrophil/platelet activation.2,3,21 Thus, post-COVID-19 infection status can induce a coagulopathy with extensive micro and macrovascular thromboses, with a similar incidence of venous and arterial thrombosis.21
Some studies report that SARS-CoV-2 infection can induce immunothrombosis, activating neutrophils and monocytes that interact with platelets – assuming that platelets play an important role in coagulation alterations - and the resulting thrombosis leads to platelet consumption. The most frequently reported clotting abnormality is D-dimer elevation, but other changes are reported.21
In addition to coagulation alterations, an exacerbation of pro-inflammatory cytokines such as C-reactive protein, ferritin, interleukin (IL) 2, IL-6, IL 7, IL-10, among others, may be responsible for the activation of the coagulation cascade, with subsequent hypercoagulable state.3,21 This prothrombotic state may last up to 4 months after COVID-19 infection.2 Some authors even report that it was possible to identify coronavirus RNA in the retina of patients with COVID-19.22–24
The first COVID-19 vaccine, outside of a clinical trial setting, was administered in December 2020.25 With the exception of rare cases of anaphylaxis, no episodes of thrombosis have been reported in early Phase III clinical trials.21 In March 2021, some episodes of thrombosis in unusual localizations began to be described, after immunization with the Oxford vaccines - AstraZeneca and Janssen in healthy individuals, mostly women under 50 years of age with very rare cases of thrombosis related to mRNA vaccines.11,21 So hypercoagulability is observed after vaccination, however the pathogenesis is poorly understood.21 Several hypotheses have been able to justify this hypercoagulable state: (a) the fact that there is a stage of thrombosis and thrombocytopenia led to the hypothesis of a condition like heparin-induced thrombocytopenia (HIT), in which vaccination may induce the formation of antibodies against platelet antigens as part of the inflammatory reaction and immune stimulation. The formation of these autoantibodies would explain the appearance of adverse reactions between 4 and 14 days after vaccination;21,26 (b) after vaccination, there is a marked immune response that mimics an acute infection by active COVID-19, with subsequent activation of neutrophils and monocytes, which interact with platelets and the coagulation cascade, with subsequent intravascular formation of thrombi in large and small vessels;21,27 (c) finally, it is known that SARS-CoV-2 interact with ACE-2, with loss of activity and a subsequent lower inactivation of angiotensin, with an increased thrombotic risk.21,23
Evaluation of retinal vascular structures with OCT-A to more accurately assess the presence of local ischemia was performed in only one case. The literature has shown that patients with a recent history of COVID-19 have a lower density of vessels, among others, in the superficial capillary plexuses (at the level of the nasal quadrants).24
Regarding the cases presented above, it is difficult to ensure the causal relationship between the development of retinal vascular pathology and the post-COVID-19 vaccination and post-COVID-19 infection status, respectively.4 However, the presence of symptoms and retinal changes in a patient without cardiovascular risk factors, without known prothrombotic pathologies, without therapy with antiangiogenic drugs or with estrogenic systemic anticoagulation, and given the temporal gap, suggests a possible relationship between vaccination (in the first cases) and infection (in later cases) with the development of retinal vascular pathology.
Abbreviations
COVID-19, Coronavirus Disease 2019; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; PAMM, Paracentral Acute Middle Maculopathy; SD-OCT, Spectral Domain, Optical Coherence Tomography; FAF, Fundus Autofluorescence; FA, Fluorescein Angiography; OCT-A, Optical Coherence Tomography Angiography; BCVA, Best Corrected Visual Acuity.
Data Sharing Statement
The clinical data that support the findings of this clinical case are available in the electronic hospital register of CHUPorto. All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Statement of Ethics
The study was conducted under the tenets of the National Council of Ethics for the Life Sciences and the Declaration of Helsinki and its latest amendment (Brazil, 2013). Ethical approval is not required for this study in accordance with the local IRB (“Departamento de Ensino, Formação e Investigação” of Centro Hospitalar Universitário de Santo António). The patient’s informed consent was obtained during the assessment visits.
Consent for Publication
Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this study.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
2. Ashkenazy N, Patel NA, Sridhar J, Yannuzzi NA. Hemi- and central retinal vein occlusion associated with COVID-19 infection in young patients without known risk factors. Ophthalmol Retina. 2020;6(6):520–530.
3. Shiroma HF, Lima LH, Shiroma YB, et al. Retinal vascular occlusion in patients with the Covid-19 virus. Int J Retin Vitr. 2022;8(1):4–9. doi:10.1186/s40942-022-00371-7
4. Le NX, Betzler BK, Ng S, et al. The Eye of the Storm: COVID-19 Vaccination and the Eye. Ophthalmol Ther. 2022;11(1):81–100. doi:10.1007/s40123-021-00415-5
5. Padhy SK, Dcruz RP, Kelgaonkar A. Paracentral acute middle maculopathy following SARS-CoV-2 infection: the D-dimer hypothesis. BMJ Case Rep. 2021;14(3):1–2. doi:10.1136/bcr-2021-242043
6. Venkatesh R, Reddy NG, Agrawal S, Pereira A. COVID-19-associated central retinal vein occlusion treated with oral aspirin. BMJ Case Rep. 2021;14(5):1–3. doi:10.1136/bcr-2021-242987
7. Afonso MG, Marques JH, Monteiro S, Lume M, Abreu AC, Maia S. Acute macular neuroretinopathy following sars-CoV-2 Vaccination. Retin Cases Br Reports. 2022;17(4):438–440.
8. Castro CS, Ferreira AS, Silva NP, Lume MR, Furtado MJ. Paracentral acute middle maculopathy after COVID-19 disease: multimodal evaluation. Retin Cases Br Reports. 2022;2022:10–97.
9. Peters MC, Cheng SSH, Sharma A, Moloney TP. Retinal vein occlusion following COVID −19 vaccination. Clin Exp Ophthalmol. 2022;50:459–461. doi:10.1111/ceo.14056
10. Dehghani A, Ghanbari H, Houshang‐Jahromi M, Pourazizi M. Paracentral acute middle maculopathy and COVID-19 vaccination: causation versus coincidence finding. clinical Case Reports. 2022;10(3):1–4. doi:10.1002/ccr3.5578
11. Endo B, Bahamon S, Martínez-Pulgarín DF. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: a case report. Indian J Ophthalmol. 2021;69:2865–2866. doi:10.4103/ijo.IJO_1477_21
12. Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. E Clin Med. 2021;39:101061. doi:10.1016/j.eclinm.2021.101061
13. Goyal M, Murthy SI, Annum S. Retinal manifestations in patients following COVID-19 infection: a consecutive case series. Indian J Ophthalmol. 2021;69:5.
14. Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139(10):1131–1135. doi:10.1001/jamaophthalmol.2021.3477
15. Abdin AD, Gärtner BC, Seitz B. Central retinal artery occlusion following COVID-19 vaccine administration. Am J Ophthalmol Case Reports. 2022;26(July 2021):2021–2023. doi:10.1016/j.ajoc.2022.101430
16. Ishibashi K, Yatsuka H, Haruta M, Kimoto K, Yoshida S, Kubota T. Branch retinal artery occlusions, paracentral acute middle maculopathy and acute macular neuroretinopathy after COVID-19 vaccinations. Clin Ophthalmol. 2022;16(March):987–992. doi:10.2147/OPTH.S357359
17. Thakar M, Bhattacharya S. Central retinal artery occlusion after vaccination with whole virion inactivatedSARSCoV- 2 vaccine Covaxin. Indian J Ophthalmol. 2022;70:3716–3718. doi:10.4103/ijo.IJO_1148_22
18. Girbardt C, Busch C, Al-Sheikh M, et al. Retinal vascular events after mRNA and adenoviral-vectored covid-19 vaccines—a case series. Vaccines. 2021;9(11):1349. doi:10.3390/vaccines9111349
19. Mohamed S, Chan CKM, Tsang CW, et al. Case Report: retinal vasculitis in two adolescents after COVID-19 vaccination. Ocul Immunol Inflamm;2022. 1–5. doi:10.1080/09273948.2022.2129694
20. Sonawane NJ, Yadav D, Kota AR, Singh HV. Central retinal vein occlusion post-COVID-19 vaccination. Indian J Ophthalmol. 2022;70(1):308. doi:10.4103/ijo.IJO_1757_21
21. Abrignani MG, Murrone A, De Luca L, et al. COVID‐19, vaccines, and thrombotic events: a narrative review. J Clin Med. 2022;11(4):1–25. doi:10.3390/jcm11040948
22. Casagrande M, Fitzek A, Püschel K, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020;28(5):721–725. doi:10.1080/09273948.2020.1770301
23. Amin MA, Nahin S, Dola TA, Afrin S, Hawlader MDH. Retinal hemorrhage of late post‐COVID‐19 and post‐vaccine‐related pathogenic mechanisms: a new challenge for ophthalmologist in COVID era. Clin Case Reports. 2022;10(2):1–7. doi:10.1002/ccr3.5471
24. Li M, Wang Z, Xie C, Xia X. Free Information in English and Mandarin on the Novel Coronavirus COVID- Advances in mRNA Vaccines. Academic Press; 2020.
25. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–1302. doi:10.1016/S1473-3099(22)00320-6
26. Terpos E, Politou M, Ntanasis-Stathopoulos I, et al. High prevalence of anti-PF4 antibodies following ChAdOx1 nCov-19 (AZD1222) vaccination even in the absence of thrombotic events. Vaccines. 2021;9(7):712. doi:10.3390/vaccines9070712
27. Elalamy I, Gerotziafas G, Alamowitch S, et al. SARS-CoV-2 vaccine and thrombosis: an expert consensus on vaccine-induced immune thrombotic thrombocytopenia. Thromb Haemost. 2021;121(8):982–991. doi:10.1055/a-1499-0119
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.