Back to Journals » Drug Design, Development and Therapy » Volume 17
Resveratrol Improves Paclitaxel-Induced Cognitive Impairment in Mice by Activating SIRT1/PGC-1α Pathway to Regulate Neuronal State and Microglia Cell Polarization
Authors Liu X , Tang M, He TY, Zhao S , Li HZ, Li Z, Guo YX, Wang XL
Received 23 December 2022
Accepted for publication 29 March 2023
Published 12 April 2023 Volume 2023:17 Pages 1125—1138
DOI https://doi.org/10.2147/DDDT.S400936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Xin Liu,1,* Miao Tang,2,* Tian-Yi He,1 Shuang Zhao,1 Hui-Zhou Li,1 Zhao Li,1 Yue-Xian Guo,3 Xiu-Li Wang1
1Department of Anesthesiology, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050051, People’s Republic of China; 2Department of General Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050000, People’s Republic of China; 3Department of Surgery, The Third Hospital of Hebei Medical University, Shijiazhuang, 050051, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiu-Li Wang, Department of Anesthesiology, The Third Hospital of Hebei Medical University, No. 139 Ziqiang Road, Shijiazhuang, Hebei, 050051, People’s Republic of China, Tel +86-13313019968, Email [email protected]
Objective: This study aimed to investigate the effect of resveratrol (Res) on paclitaxel (PTX)-induced cognitive impairment and elucidate the underlying molecular mechanisms.
Methods: Morris Water Maze (MWM) test was used to evaluate the mice’s spatial learning and memory abilities. Western blotting was applied to detect protein expression of receptor-interacting protein (RIP3), mixed lineage kinase domain-like protein (MLKL), silencing information regulator 2 related enzyme 1 (SIRT1), peroxisome proliferator activated receptor coactivator-1 (PGC-1α), NADPH oxidase 2 (NOX2), NOX4, postsynaptic density zone 95 (PSD95), arginase-1 (Arg-1) and inducible nitric oxide synthase (iNOS). Immunofluorescence of RIP3, MLKL, Arg-1, Iba-1 and iNOS was conducted to observe the apoptosis of hippocampal cells and the polarization of microglia. qRT-PCR was performed to detect BDNF mRNA expressions. DHE staining was used to assess the level of oxidative stress response. Golgi-Cox staining and dendritic spine counting were applied to visualize synaptic structural plasticity. Postsynaptic density was performed by transmission electron microscope. ELISA was used to detect the contents of tumour necrosis factor alpha (TNF-α), IL-1β, IL-4, and IL-10.
Results: PTX-induced cognitive impairment model was constructed after the application of PTX, represented as longer latency to platform and less platform crossing times over the whole period in PTX group. After Res treatment, the above indicators were reversed, indicating that cognitive function was improved. Moreover, Res reduced neuronal apoptosis and oxidative stress through SIRT1/PGC-1α pathway in mice, manifesting as down-regulated expression of RIP3, MLKL, NOX2 and NOX4. Meanwhile, Res increased the density of dendritic spines and the expression of PSD95 and BDNF, thereby ameliorating the PTX induced synaptic damage. Besides, M2 microglia was in the majority, eliciting the expression of anti-inflammatory cytokines IL-4 and IL-10 after Res treatment in PTX+Res group, while immunofluorescence images results demonstrated an decrease in the proportion of M2 microglia a following SIRT1 inhibitor EX-527.
Conclusion: Res improves PTX-induced cognitive impairment in mice by activating SIRT1/PGC-1α pathways to regulate neuronal state and microglia cell polarization.
Keywords: resveratrol, paclitaxel, SIRT1/PGC-1α, microglia, M2 polarization
Introduction
Paclitaxel (PTX) is a commonly used chemotherapeutic agent for the treatment of a variety of cancers in clinical practice,1 killing cancer cells by stabilizing microtubules and inhibiting cell division.2 Chemobrain or chemofog, chemotherapy-induced cognitive dysfunction, is widely recognized as a common adverse reaction following the administration of chemotherapeutic drugs.3 More than 75% of cancer patients suffered from it.4 Its clinical manifestations include forgetfulness, trouble with multi-tasking and executing tasks, learning difficulties, memory problems, and poor attention span.5 Notably, chemobrain may continue for up to 10 years after chemotherapy in 17% to 34% of cancer survivors.6 However, there are no effective interventions to prevent it.
Emerging pharmacotherapeutic approaches to the treatment of cognitive dysfunction experienced by cancer survivors and recognized in murine models include modafinil, lithium, donezepil, methylphenidate, resveratrol (Res), peroxisome proliferator-activated receptors (PPARs) agonists, etc.7 Res is a natural polyphenolic, naturally occurring phenolic compound found in the skin of red grapes, peanuts and cranberries, that is compound with a wide range of biological activities, such as antioxidant, anti-obesity, anticarcinogenic, anti-inflammatory, anti-aging, and immunomodulatory properties.8,9 Of these, Res is widely known for its anti-inflammatory properties.10 It reduces the synthesis of pro-inflammatory mediators and induces anti-inflammatory proteins, which result in anti-inflammatory effects. Accumulating evidence suggests that Res has pharmacological benefits in life-threatening diseases, including cardiovascular disease, cancer, diabetes, and neurodegenerative diseases, while the underlying mechanisms need to be further explored.11
In murine models and cognitive studies in humans, memory impairments have been extensively reported after chemotherapeutic protocols, which have been attributed mainly to oxidative damage and/or neuroinflammation.12,13 Microglia are the resident innate immune cells in the central nervous system (CNS) that are responsible for homeostasis, phagocytosis of cellular debris or pathogens, and the secretion of cytokines and chemokines.14 Inflammatory mediators from microglia amplify the neuroinflammatory responses in CNS via innate immune activation mechanisms.15 In such context, the objective of the present study was to investigate if Res could restore the behavioral and morphological changes found in mice receiving PTX administration, aiming to identify possible agents that could prevent or minimize cognitive deficits and glial changes induced by PTX.
Materials and Methods
Animals
A total of 60 C57BL6/N male mice, 6–8 weeks of age and weighing 20–24g, purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd, was housed in independent ventilation cages (IVC) (trolled temperature (25°C ± 2°C) and humidity (55–65%)) with artificial lighting (12 h/12 h light/dark cycle, lights on at 8:00 AM). Sterilized and residue-free wood shavings were used for animal bedding. All mice had ad libitum access to standard rodent chow and filtered water and were acclimatized for 1 week before the initiation of the experiment. All procedures were approved by the Laboratory Animal Ethical and Welfare Committee Hebei Medical University (IACUC-Hebmu-2020009) and were in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Drugs and Treatment
PTX (TCI, Japan) was dissolved in Cremophor EL (Sigma, USA), 6 mg/mL of absolute ethanol 1:1 solution, stored at −20°C, diluted to 0.4 mg/mL of working solution with normal saline, 10 mg/kg. Res (TCI, Japan), undiluted DMSO (Sigma, USA) −20°C, diluted to 90 mmol/L in PBS with DMSO 1% in a dose of 5 µL. EX-527 (MCE, USA), stored with undiluted DMSO after −20E, diluted to 1.2 mmol/L in PBS and give DMSO 1% at a dose of 5 µL. Gadolinium trichloride (GdCl3) (Sigma Company, USA), 1 mL of liquid (1000×) was dissolved in PBS, stored at −80°C, working solution was diluted to 270 umol/L with PBS at a dose of 5 µL.
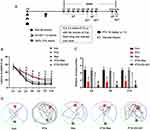
Mice were divided into 6 groups, 1) PTX group (n=10), mice receiving 10 mg/kg PTX; 2) Res group (n=10), mice injected with 90 mmol/L Res; 3) PTX+Res group (n=10), mice injected with PTX and treated with Res; 4) PTX+EX-527 group (n=10), mice injected with 10mg/kg PTX and treated with 1.2 mmol/L EX-527; 5) PTX+GdCl3 group (n=10), mice injected with 10 mg/kg PTX and treated with 270 umol/L GdCl3; 6) Control (Con) group (n=10), mice receiving the same equal amount of normal saline at the same time point. Specifically, PTX (intraperitoneal, i.p.) was administered once daily at the same time point for 7 consecutive days before behavior test. Res, EX-527 and GdCl3 (intra-cerebroventricular injection, ICV) were administered once at the same time point 7 days before behavior test. The experiment schedule is shown in Figure 1A.
ICV
Firstly, the mice were intraperitoneally injected with 1% sodium pentobarbital 50 mg/kg. After successful anesthesia, the mice were located in the right ventricle using a standard brain locator. Specifically, the stereotaxic coordinates were: 0.6 mm posterior to the bregma, 1.2 mm lateral to the midline, and 1.8 mm in depth. The coordinates were as follows: AP, −0.6 mm; ML, 1.2 mm; DV, −1.8 mm, 5 μL of drug or solvent at the rate of 0.5 μL/min. It was allowed to stand for 5 minutes before pulling out the needle. Afterwards, bone wax was used to seal bone pores, and normal saline was applied to clean the incision and then suture the wound.
Morris Water Maze (MWM) Test
MWM test was used to evaluate the spatial learning and memory abilities. Experiments were performed in a circular pool (diameter 150 cm × depth 80 cm) filled with water (22–24°C). In acquisition phase (AP), all mice were trained for 5 days, to find the platform with 4 trials daily (60 s at most, with an interval of more than 20 min). The swimming paths of the mice were tracked using a video recording device. The escape latency and velocity baseline were measured at 24 h after the end of the fifth day of training, and the space exploring test (SET) was conducted at the second 24 h (the platform was removed from the pool during the SET).
Western Blotting Analysis
Mice were sacrificed after pentobarbital sodium anesthesia, and hippocampal tissues were collected on ice, washed with PBS buffer, and put into RIPA lysate (Protech Technology Enterprise Co., Ltd.) mixed with protease inhibitor cocktail (Abcam US). Tissue homogenate was prepared by tissue fragmentation apparatus (60 Hz for 3 times, 2 min each time, ice bathing for 2 min during this period). After centrifugation at 4°C (12,000 rpm/min, 45 min), the supernatant was taken, and the protein concentration was measured through bicinchoninic acid (BCA) method.
Equal amounts of protein were loaded and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred and then transferred to a polyvinylidene fluoride (PVDF) membrane (Merck Millipore, ISEQ00010, USA). After blocking with 5% skim milk (0.1% Tween dissolved in TBS solution) for 2 h at room temperature, the cells were incubated with primary antibody overnight at 4 °C. Primary antibodies included: receptor-interacting protein 3 (RIP3) (1:1000, ADI-905-242, Enzolifescience, USA), mixed lineage kinase domain-like protein (MLKL) (1:1000, 3137756, Millipore, USA), β-actin (1:5000, AC026, ABclonal, USA), silencing information regulator 2 related enzyme 1(sirtuin1, SIRT1) (1:1000; Abcam; cat. no. ab110304), peroxisome proliferator-activated receptor Gamma Coactivator-1α (PGC-1α) (1:1000, Abcam, ab54481), NADPH oxidase 2 (NOX2) (1:1000, Abcam, ab80897), NADPH oxidase 4 (NOX4) (1:1000; Abcam; cat. no. ab154244), postsynaptic density zone 95 (PSD95) (1:1000, Abcam; R, ab238135), arginase-1 (Arg-1) (1:1000, Abcam; ab269541) and inducible nitric oxide synthase (iNOS) (1:1000, Abcam, ab178945). After washing with Tris buffered saline tween (TBST) for 3 times, the fluorescent secondary antibody corresponding to the primary antibody was incubated in the dark. After 2 h, Odyssey was used to develop the target bands, and Image J software (version 6.0) was used to analyze the gray values of protein bands.
Immunofluorescence
Mice were submitted to intracardiac perfusion with 0.9% saline, and then submitted to intracardiac perfusion with 4% paraformaldehyde (Solarbio, China). The brains were removed, fixed in 4% paraformaldehyde for 24 h, and then dehydration in graded sucrose concentrations between 20–30% were performed. After that, it was embedded in optimal cutting temperature compound (OCT) for sectioning on a microtome (VT1000S, Leica Microsystems) of 10 µm thickness. Nonspecific antibody-binding sites (brain tissue) were blocked with 3% goat serum in 0.01 M PBS for 30 min. Then, the sections were incubated with the corresponding primary antibodies against RIP3 (1:1000, ADI-905-242, Enzo life science, USA), MLKL (1:1000, 3137756, Millipore, USA) and SIRT1 (1:1000, ADI-905-242, Abcam, USA) overnight at 4°C. Subsequently, these sections were incubated with secondary antibody for 2 h at room temperature. Thereafter, fluorescence intensity was observed under confocal microscope, 3 of which from each animal were averaged and then statistically analyzed.
Dihydroethidium (DHE) Staining
DHE staining was applied to measure the expression level of reactive oxygen species (ROS) to assess the level of oxidative stress response. The brain was carefully and quickly isolated, cut into 10 μm and placed on chilled microscope slides. After returning to room temperature, ROS staining was added droppingly. After incubated at 37°C for 30 min, the cell nucleus were counterstained with DAPI (4’,6-diamidino-2-phenylindole), washed with 0.1 M PBS, and sealed, which were observed under a fluorescence microscope.
Golgi-Cox Staining and Dendritic Spine Counting
Golgi-Cox staining and dendritic spine counting were used to visualize synaptic structural plasticity. Mouse hippocampal tissues were immersed in Golgi-Cox staining solution and stored in the dark for 2 weeks according to Servicebio reagent instructions. Rinse with distilled water and then put 80% glacial acetic acid for overnight. The tissue was cut into 100 μm by an oscillating microtome. Nikon Eclipse E100 was used for imaging dendrites and dendritic spines in hippocampus. Scanning and analysis were performed using the Pannoramic 250 and Nikon DS-U3, respectively.
Transmission Electron Microscopy
The mice were anesthetized with 1% sodium pentobarbital and killed at day 15. The hippocampal tissues from the mice were fixed in 2% paraformaldehyde plus 2% glutaraldehyde (pH 7.4) for 2 h, and then was sectioned (1×1×1 mm3). Post-fixed tissue was incubated in 1% osmium tetroxide (0.1 M, pH 7.4) and dehydrated in ethanol and acetone at room temperature, then embedded in resin. Ultrathin sections (60 nm) were obtained using an ultramicrotome (UC-7; Leica, Germany) and then stained with lead citrate and uranyl acetate. Samples were detected using transmission electron microscope (H-7500; HITACHI, JAPAN) for analysis at 15,000× magnifications.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Following the manufacturer’s instructions provided by Promega (Beijing, China), the total RNA was extracted from the hippocampus using RNA extraction kit. 1 μg total RNA was reversely transcribed into cDNA. The quantitated expression of brain-derived neurotrophic factor (BDNF) mRNA in the hippocampus of mouse was used as qRT-PCR with the ABI 7300 real-time PCR system. RNA quantities of target genes were calculated using the 2−ΔΔCt method. The primers are as follows: BDNF F: 5’-TGGCCCTGCGGAGGCTAAGT-3’, R: 5’-AGGGTGCTTCCGAGCCTTCCT-3’; β-actin F: 5’- CTACCTCATGAAGATCCTGACC-3’, R: 5’-CACAGCTTCTCTTTGATGTCAC-3’. The final results were normalized and were expressed as the fold change compared to the target gene/GAPDH.
Enzyme-Linked Immunosorbent Assay (ELISA)
The well-mixed homogenate was centrifuged for 15 min at 12,000 rpm at 4°C, and the supernatant was taken for detection according to the specific steps of tumor necrosis factor alpha (TNF-α) (No. CSB-E04741m, CUSABIO), interleukin-1beta (IL-1β) (No. CSB-E08054m, CUSABIO), IL-4 (No. CSB-E04634m, CUSABIO) and IL-10 (No. CSB-E04594m, CUSABIO) as per the manufacturer’s instructions. The optical density of each well was determined using a multi-function microplate reader (BioTeK, Epoch, USA) at 405 nm.
Statistical Analysis
Continuous data were tested for normality using the Shapiro–Wilk method. If the normality criteria were satisfied, data were described as the mean ± standard deviation. One-way ANOVA is used for the completely randomized design of multiple groups of quantitative data of normal distribution and equal variance, and Kruskal–Wallis test is used for the quantitative data that do not conform to the normal distribution and/or unequal variance. LSD test was used for post-hoc tests for ANOVA and Kruskal–Wallis test. Statistical significance was defined as P< 0.05. Statistical analysis and image processing were performed by using IBM SPSS 26.0 (IBM Corp., Armonk, USA), GraphPad Prism 8 (GraphPad Prism Software Inc., San Diego, CA) and Adobe Illustrator 2020 (Adobe Systems Inc, San Jose, CA, USA).
Results
Res Ameliorated PTX-Induced Cognitive Impairment in Mice
MWM was conducted to evaluate the impairment and improvement of cognitive ability of mice. As shown in Figure 1B and C, there was no significant difference in latency to platform and platform crossing times between the Res group and the Con group. However, compared with the Con group, the PTX group had longer latency to platform and less platform crossing times at D2–D14, respectively, indicating that the PTX-induced cognitive impairment model was successfully constructed. Surprisingly, after Res treatment, the mice in the PTX+Res group had shorter latency to platform and more platform crossing times at D2–D14, respectively. On the contrary, after the selective inhibition of SIRT1 by EX-527 injected, the mice in the PTX+EX-527 group had longer latency to platform at D7, D14 and less platform crossing times at D6, D8 and D15. Meanwhile, it could also be seen from the movement track of mice in each group on the 15th day that the cognitive ability of mice receiving Res had been improved, while the damage of mice receiving EX-527 had been aggravated compared with the PTX group (Figure 1D).
Res Down-Regulated the Expression of RIP3 and MLKL Proteins, and Reduced PTX-Induced Apoptosis of Hippocampal Neuron
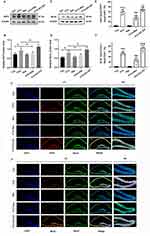
RIP3 and MLKL are specific molecules of necrotic apoptosis. We further compared the degree of necrotic apoptosis in hippocampal neuron of mice in each group by Western blotting and immunofluorescence double-label staining. As shown in Figure 2, the expression levels of RIP3 and MLKL protein in PTX group were significantly higher than those in the Con group and they were down-regulated after Res treatment. When EX-527 was added, the increased expression of RIP3 and MLKL was further intensified (Figure 2A–D). Consistently, this phenomenon was more intuitively observed via the co-labeled staining of neurons and RIP3/MLKL. The results showed that compared with the Con group, the number of RIP3+NeuN+ and MLKL+/NeuN+ cells in the hippocampal neuron in group PTX was significantly increased. However, the number of RIP3+NeuN+ and MLKL+/NeuN+ cells Res was observed to be significantly reduced in the Res intervention and increased after the EX-527 intervention. Compared with PTX group, the number of RIP3+NeuN+ and MLKL+/NeuN+ cells in the PTX+Res group was decreased, and the number of RIP3+NeuN+ and MLKL+/NeuN+ cells in the PTX+EX-527 group was increased (Figure 2E–H).
Res Exerted Hippocampal Neuron Anti-Apoptotic Effect by Reducing PTX-Induced Oxidative Stress Through SIRT1-PGC-1α Pathway
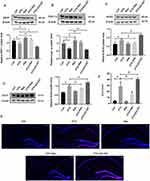
The results in Figure 3A and B confirmed that the protein expressions of SIRT1 and PGC-1α were significantly increased in hippocampal neuron of Res group compared with Con group, while the protein expressions of SIRT1 and PGC-1α were significantly decreased in PTX group. Compared with the PTX group, the protein expressions of SIRT1 and PGC-1α in the PTX+Res group were significantly increased. Meanwhile, the expression of SIRT1 protein in the PTX+EX-527 group was significantly decreased compared with the PTX group.
We further detected the protein expressions of NOX2 and NOX4 in the tissues to determine the level of oxidative stress in the hippocampal neuron after drug intervention. As is shown in Figure 3C and D, the expression of NOX2 and NOX4 in PTX group increased significantly compared with Con group. Compared with PTX group, the expression of NOX2 and NOX4 decreased in PTX+Res group, and further increased in PTX+EX-527 group. DHE staining of hippocampal neuron showed the same trend (Figure 3E and F). Compared with Con group, ROS+/DAPI+ in PTX group increased significantly, Res could significantly inhibit the expression of ROS+/DAPI+, while EX-527 further promoted the increase of ROS+/DAPI+ expression, significantly increasing the level of oxidative stress in hippocampal neuron.
Res Played Neuroprotective Role by Improving PTX-Induced Synaptic Plasticity Damage and Increasing Dendritic Spine Density
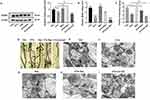
Res showed a significant neuroprotective effect by inhibiting the down-regulation of PSD95 protein expression, which was increased in the PTX+Res group, as compared with the PTX group (Figure 4A). BDNF is one of the regulated factors of SIRT1/PGC-1α pathway. qRT-PCR results showed that the expression of BDNF mRNA in the hippocampus in PTX group was significantly decreased, which was increased in PTX+Res group compared with PTX group. It was further decreased in PTX+EX-527 group (Figure 4B). Golgi-Cox staining showed that the density of dendritic spines in PTX group was significantly decreased, while Res increased the density of dendritic spines, and EX-527 further decreased the density of dendritic spines in mice neurons (Figure 4C and D). The effect of Res/EX-527 on synapses was observed more directly by the transmission electron microscopy. It showed that Res effectively alleviated the widening of synaptic cleft and the reduction of the length and thickness of postsynaptic dense material, while EX-527 aggravated this phenomenon (Figure 4E–I).
Res Promoted the Polarization of Microglia to M2 Phenotype Through SIRT1-PGC-1α Pathway and Play an Anti-Inflammatory Role
Since microglial polarization was also one of the mechanisms involved in PTX-induced cognitive dysfunction, we hypothesized that Res may also have microglial regulatory effects. Several studies reveals that GdCl3 could protect the ganglion cells by eliminating M1 microglia selectively, which provided a theoretical basis for further localizing different types of microglia in neurodegenerative disease.16,17 In the present study, the hippocampal CA1 area in mice was detected. Compared with the PTX group, the expression of iNOS was significantly inhibited in the PTX+Res group, while the expression of Arg-1 was increased. In contrast, only the expression of iNOS was decreased in the hippocampal tissue of mice treated with GdCl3 (Figure 5A and B). The results of immunofluorescence double-label staining showed that microglia in PTX group were mainly polarized to M1 microglia expressing iNOS, and M2 microglia expressing Arg-1 had very few polarized forms. However, in PTX+Res group, M2 type microglia was dominant, and M1 type was significantly reduced. Both M1 and M2 microglia were decreased in PTX+GdCl3 group, while IF images of PTX+EX-527 group demonstrated an increase in the proportion of M1 microglia and a decrease in the proportion of M2 microglia following SIRT1 inhibitor EX-527 (Figure 5C–E).
The same trend was found in the expression products of microglia with different polarization types: the concentrations of anti-inflammatory factors IL-4 and IL-10 were significantly increased in the PTX+Res group, while the release of pro-inflammatory factors TNF-α and IL-1β were decreased. These results indicated that Res could inhibit the polarization of microglia toward the M1 type and promote its polarization toward the M2 type (Figure 5F).
Discussion
Learning and spatial memory abilities are important indicators to evaluate the function of the central nervous system in animals. The earlier stage of cognitive dysfunction is a loss of learning/memory abilities, and the executive dysfunction at the later stage18 Therefore, the learning and memory ability can reflect the changes in cognitive function. The MWM test, developed by Richard G Morris in the 1980s, is a commonly used method to evaluate the cognitive function of animal models of neurodegenerative diseases, and it is currently recognized as one of the gold standards for the comprehensive ability of the mouse central nervous system in neuroscience research.19 Therefore, the MWM test was used to observe the learning and memory ability of mice receiving PTX to evaluate their cognitive function. We found that continuous intraperitoneal injection of PTX for 1 week led to a decline in the cognitive level of mice. After Res treatment, the mice were also tested for the above behavioral indicators, and it was found that Res treatment significantly improved the cognitive impairment. Similar results were also verified in rats submitted to doxorubicin-induced cognitive impairment, and the results showed that Res treatment significantly improved doxorubicin-induced cognitive impairment of rats.20 Necroptosis is associated with a variety of neurodegenerative diseases, such as Parkinson’s disease21 and Alzheimer’s disease22 and amyotrophic lateral sclerosis,23 among others. Hippocampus is an important part related to learning, memory and other cognitive functions. Some studies have found that PTX can induce apoptosis in hippocampal neurons, leading to learning and memory dysfunction.24 RIP3 is a switch molecule for cell necrosis and plays an important role in the necroptosis pathway. In recent years, it has been shown that RIP3 causes necroptosis independently of RIP1 and induces mixed lineage kinase domain-like protein (MLKL) phosphorylation, leading to changes in cell membrane permeability and even cell membrane rupture.25 In the present study, after PTX treatment, RIP3 and MLKL protein expression was increased, indicating increased apoptosis in the hippocampus and impairment of cognitive function. Similarly, a study has found that polydatin reduced the protein expression of RIP3 and MLKL, and inhibited hippocampal apoptosis and adriamycin-induced cognitive dysfunction in rats.26 Delightingly, our study found that when RES was added, the expression of RIP3 and MLKL protein was significantly reduced, the apoptosis of hippocampal cells was reduced, and the cognitive dysfunction of mice was improved. Conversely, when EX-527 pathway inhibitor was added, the expression of RIP3 and MLKL protein was significantly increased, and the apoptosis of hippocampal cells was increased. These results indicate that RES inhibits necroptosis of hippocampal cells through SIRT1/PGC-1α pathway so as to improve PTX-induced cognitive impairment of mice.
Oxidative stress refers to the excessive production of ROS in the body or cells, and the weakening of endogenous antioxidant defense function, resulting in an imbalance between the two and causing tissue or cell damage. Several studies have shown that oxidative stress plays an important role in the occurrence and development of neurodegenerative diseases (NDs) such as Parkinson’s disease (PD) and Alzheimer’s disease (AD).27 Both NOX2 and NOX4 are important sources of ROS.28 In this study, the protein expression levels of NOX4 and NOX2 were significantly increased in PTX treated mice, indicating that PTX could cause excessive oxidative stress in the body, and oxidative damage may activate a large number of harmful pathways, eventually leading to cell death. However, oxidative stress levels were significantly reduced in RES-treated mice. Further, SIRT1 is a nicotinamide adenine dinucleotide (NAD+) dependent histone deacetylase that upregulates PGC-1α,29 and then regulates mitochondrial function, participate in energy metabolism and oxidative stress.30 According to previous reports, SIRT1 plays a crucial role in preserving typical cognitive function. Knockout mice that lack SIRT1 display impaired hippocampal-dependent memory.31 Nicotinamide adenine dinucleotide (NAD+), also known as coenzyme I, is a significant signaling molecule that governs intermediary metabolism.32 The master transcription factor in the regulation of mitochondrial antioxidant and clearance systems, as well as its biogenesis, is PGC-1α.33 In the CNS, PGC-1α has been shown to regulate mitochondrial biogenesis, mitochondrial function in neurons and the expression of myelin basic protein in oligodendrocytes.34 Yao Zhao et al, found that NAD+ administration rescued cognitive deficits and inhibited neuroinflammation by protecting mitochondria and decreasing ROS production in CCH rats.35 Correspondingly, in this study, we found that RES reduced the oxidative stress level in hippocampus through SIRT1/PGC-1α pathway, and the oxidative stress level was significantly increased when the SIRT1/PGC-1α pathway inhibitor EX-527 was added. These results suggested that RES inhibited oxidative stress in the hippocampus through SIRT1/PGC-1α pathway, thereby improving cognitive dysfunction in mice.
Synaptic plasticity, the activity-dependent change in neuronal connection strength, has long been considered an important component of learning and memory.36 BDNF and PSD95 are key factors with multipotent impact on brain signaling and synaptic plasticity.37 PSD95 is an important neurocytoskeletal protein in the postsynaptic density zone, which can interact with ion channels, membrane receptors and intracellular signaling molecules to regulate synaptic plasticity.38 An increase in the level of PSD95 expression at synaptic sites specific during learning induced elevated synaptic plasticity.39 BDNF is regulated by levels PGC-1α,30 and increased expression of BDNF can regulate synaptic plasticity and improve cognition, learning and memory formation.40 Similarly, in the present study, the expression levels of PSD95 and BDNF in the hippocampus of the mice in the PTX group was markedly decreased, which was similar to previous reports.41 The production and release of a large number of ROS in neurons necroptosis process can regulate synaptic and non-synaptic information transmission between neurons and glial cells, and promote neuronal degeneration in the form of neuroinflammation and cell death, leading to memory loss and cognitive impairment.42 Likewise, in this study, after the application of Res to PTX-induced cognitive impairment mice, the expression of BDNF and PSD95 increased significantly, speeding up the efficiency of synaptic transmission, and improving the cognitive function of mice. These results clearly demonstrated the neuroprotective efficacy of Res in preventing synaptic plasticity damage from PTX. Besides, when EX-527 was added, the protein level of BDNF and PSD95 protein was significantly decreased, and the synaptic plasticity of hippocampal cells was decreased. It suggested that RES improved the synaptic plasticity of hippocampal cells through SIRT1/PGC-1α pathway, thereby improving cognitive dysfunction in mice.
Microglia is the major cellular element with immune function inside the CNS, which is considered to play an important role in the pathogenesis and progression of neurodegenerative diseases.43 It is now well recognized that microglia bear functional plasticity and dual phenotypes, M1 and M2. Depending on the predominance of secreted factors, microglia have been characterized to express the classical activation phenotype (M1, pro-inflammatory) or the alternative activation phenotype (M2, anti-inflammatory).44 The M1 state causes the release of pro-inflammatory cytokines with increased expression of cluster of differentiation markers iNOS, cluster of differentiation (CD) 86, CD16 and CD32. The M2 state causes the release of the anti-inflammatory cytokines as well as Arg-1, transforming growth factor β1 (TGF-β1), and CD206.45 Jha et al proposed that alterations in microglia M1/M2 polarization have been associated with neurodegenerative diseases.46 However, most reported compounds simply suppress M1 microglia, while few compounds have been demonstrated to promote microglia polarization toward the M2 phenotype.47
This study not only confirmed that GdCl3 improved cognitive impairment induced by PTX by inhibiting M1 microglia clearance, but the data also showed a significant increase in M2 microglia after RES intervention. This suggests that Res promotes microglia polarization to the M2 phenotype while inhibiting microglial polarization to the M1 phenotype. The reduction of the M2 marker Arg-1 after the addition of EX-527 in this study implies that Res may encourage microglial polarization towards the M2 phenotype through the SIRT1/PGC-1α pathway, which further validates previous research. Besides, the M2 microglia produced a large amount of cytokines IL-4 and IL-10 to play an anti-inflammatory role, which is consistent with previous studies.48
There are some limitations in this study that need to be acknowledged. Firstly, the cognitive function in mice was measured using a limited number of behavioral tests. Secondly, the association of SIRT1/PGC-1α pathway and M1/M2 microglia polarization in vitro is not elucidated directly, due to the limited funds and conditions. This limits the scope and depth of the study, and further research is needed to fully explore these potential links.
Conclusion
Res significantly improved PTX-induced cognitive impairment by activating SIRT1/PGC-1α pathway to regulate neuronal state. Specifically, neuronal necrotic apoptosis were significantly reduced, as well as oxidative stress reaction, and synaptic plasticity was tremendously improved. In addition, Res promoted the M2 polarization of microglia via SIRT1/PGC-1α pathway playing an anti-inflammatory role. Therefore, Res-based neuroprotection measures provide new ideas and methods for the treatment and prevention of chemotherapy-induced brain.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
Animal research was approved by the Laboratory Animal Ethical and Welfare Committee Hebei Medical University (No. IACUC-Hebmu-2020009) and carried out in accordance with the code of ethics and animal welfare.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by the National Natural Science Foundation of China (81971001), and Natural Science Foundation of Hebei Province (H2021206109, H2021206149).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Ojima I, Lichtenthal B, Lee S, Wang C, Wang X. Taxane anticancer agents: a patent perspective. Expert Opin Ther Pat. 2016;26(1):1–20. doi:10.1517/13543776.2016.1111872
2. Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9(10):790–803. doi:10.1038/nrd3253
3. Raghu SV, Kudva AK, Rao S, Prasad K, Mudgal J, Baliga MS. Dietary agents in mitigating chemotherapy-related cognitive impairment (chemobrain or chemofog): first review addressing the benefits, gaps, challenges and ways forward. Food Funct. 2021;12(22):11132–11153. doi:10.1039/D1FO02391H
4. Ding K, Zhang X, Zhao J, Zuo H, Bi Z, Cheng H. Managing Cancer and Living Meaningfully (CALM) Intervention on Chemotherapy-Related Cognitive Impairment in Breast Cancer Survivors. Integr Cancer Ther. 2020;19:1534735420938450. doi:10.1177/1534735420938450
5. Taillibert S, Le Rhun E, Chamberlain MC. Chemotherapy-related neurotoxicity. Curr Neurol Neurosci Rep. 2016;16(9):81. doi:10.1007/s11910-016-0686-x
6. Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol. 2011;38(3):431–438. doi:10.1053/j.seminoncol.2011.03.014
7. Davis J, Ahlberg FM, Berk M, Ashley DM, Khasraw M. Emerging pharmacotherapy for cancer patients with cognitive dysfunction. BMC Neurol. 2013;13:153. doi:10.1186/1471-2377-13-153
8. Zhao YN, Li WF, Li F, et al. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun. 2013;435(4):597–602. doi:10.1016/j.bbrc.2013.05.025
9. Orsu P, Murthy BV, Akula A. Cerebroprotective potential of resveratrol through anti-oxidant and anti-inflammatory mechanisms in rats. J Neural Transm. 2013;120(8):1217–1223. doi:10.1007/s00702-013-0982-4
10. Thiel G, Rössler OG. Resveratrol stimulates cyclic AMP response element mediated gene transcription. Mol Nutr Food Res. 2016;60(2):256–265. doi:10.1002/mnfr.201500607
11. Tufekci KU, Eltutan BI, Isci KB, Genc S. Resveratrol Inhibits NLRP3 inflammasome-induced pyroptosis and miR-155 expression in microglia through Sirt1/AMPK pathway. Neurotox Res. 2021;39(6):1812–1829. doi:10.1007/s12640-021-00435-w
12. Cardoso CV, de Barros MP, Bachi ALL, et al. Chemobrain in rats: behavioral, morphological, oxidative and inflammatory effects of doxorubicin administration. Behav Brain Res. 2020;378:112233. doi:10.1016/j.bbr.2019.112233
13. Barry RL, Byun NE, Tantawy MN, et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging Behav. 2018;12(1):87–95. doi:10.1007/s11682-017-9674-2
14. Walter J, Kemmerling N, Wunderlich P, Glebov K. γ-Secretase in microglia - implications for neurodegeneration and neuroinflammation. J Neurochem. 2017;143(4):445–454. doi:10.1111/jnc.14224
15. Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33(7):333–342. doi:10.1016/j.it.2012.03.001
16. Yang P, Wei L, Tian H, Yu F, Shi Y, Gao L. Gadolinium chloride protects neurons by regulating the activation of microglia in the model of optic nerve crush. Biochem Biophys Res Commun. 2022;618:119–126. doi:10.1016/j.bbrc.2022.05.088
17. Li T, Xu T, Zhao J, Gao H, Xie W. Depletion of iNOS-positive inflammatory cells decelerates neuronal degeneration and alleviates cerebral ischemic damage by suppressing the inflammatory response. Free Radic Biol Med. 2022;181:209–220. doi:10.1016/j.freeradbiomed.2022.02.008
18. Hoshi H, Hirata Y, Kobayashi M, et al. Distinctive effects of executive dysfunction and loss of learning/memory abilities on resting-state brain activity. Sci Rep. 2022;12(1):3459. doi:10.1038/s41598-022-07202-7
19. Zhu Z, Yang T, Zhang L, et al. Inhibiting Aβ toxicity in Alzheimer’s disease by a pyridine amine derivative. Eur J Med Chem. 2019;168:330–339. doi:10.1016/j.ejmech.2019.02.052
20. Moretti RL, Dias EN, Kiel SG, et al. Behavioral and morphological effects of resveratrol and curcumin in rats submitted to doxorubicin-induced cognitive impairment. Res Vet Sci. 2021;140:242–250. doi:10.1016/j.rvsc.2021.09.009
21. Iannielli A, Bido S, Folladori L, et al. Pharmacological inhibition of necroptosis protects from dopaminergic neuronal cell death in parkinson’s disease models. Cell Rep. 2018;22(8):2066–2079. doi:10.1016/j.celrep.2018.01.089
22. Cao LL, Guan PP, Zhang SQ, Yang Y, Huang XS, Wang P. Downregulating expression of OPTN elevates neuroinflammation via AIM2 inflammasome- and RIPK1-activating mechanisms in APP/PS1 transgenic mice. J Neuroinflammation. 2021;18(1):281. doi:10.1186/s12974-021-02327-4
23. Ito Y, Ofengeim D, Najafov A, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353(6299):603–608. doi:10.1126/science.aaf6803
24. Abu Samaan TM, Samec M, Liskova A, Kubatka P, Büsselberg D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules. 2019;9(12):789. doi:10.3390/biom9120789
25. Su Z, Yang Z, Xie L, DeWitt JP, Chen Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016;23(5):748–756. doi:10.1038/cdd.2016.8
26. Tong Y, Wang K, Sheng S, Cui J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci Biotechnol Biochem. 2020;84(6):1201–1210. doi:10.1080/09168451.2020.1722057
27. Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med. 2005;257(2):156–166. doi:10.1111/j.1365-2796.2004.01442.x
28. Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19(14):1711–1747. doi:10.1089/ars.2012.4530
29. Chandrasekaran K, Anjaneyulu M, Choi J, et al. Role of mitochondria in diabetic peripheral neuropathy: influencing the NAD(+)-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 2019;145:177–209.
30. El Hayek L, Khalifeh M, Zibara V, Abi Assaad R. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369–2382. doi:10.1523/JNEUROSCI.1661-18.2019
31. Michán S, Li Y, Chou MM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695–9707. doi:10.1523/JNEUROSCI.0027-10.2010
32. Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208–1213. doi:10.1126/science.aac4854
33. Kaarniranta K, Uusitalo H, Blasiak J, et al. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog Retin Eye Res. 2020;79:100858. doi:10.1016/j.preteyeres.2020.100858
34. Tsunemi T, La Spada AR. PGC-1α at the intersection of bioenergetics regulation and neuron function: from Huntington’s disease to Parkinson’s disease and beyond. Prog Neurobiol. 2012;97(2):142–151. doi:10.1016/j.pneurobio.2011.10.004
35. Zhao Y, Zhang J, Zheng Y, et al. NAD(+) improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J Neuroinflammation. 2021;18(1):207. doi:10.1186/s12974-021-02250-8
36. Magee JC, Grienberger C. Synaptic Plasticity Forms and Functions. Annu Rev Neurosci. 2020;43:95–117. doi:10.1146/annurev-neuro-090919-022842
37. Xu T, Liu J, Li XR, et al. The mTOR/NF-κB pathway mediates neuroinflammation and synaptic plasticity in diabetic encephalopathy. Mol Neurobiol. 2021;58(8):3848–3862. doi:10.1007/s12035-021-02390-1
38. Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10(7):274–280. doi:10.1016/S0962-8924(00)01783-9
39. Chugh D, Nilsson P, Afjei SA, Bakochi A, Ekdahl CT. Brain inflammation induces post-synaptic changes during early synapse formation in adult-born hippocampal neurons. Exp Neurol. 2013;250:176–188. doi:10.1016/j.expneurol.2013.09.005
40. Pardon MC. Role of neurotrophic factors in behavioral processes: implications for the treatment of psychiatric and neurodegenerative disorders. Vitam Horm. 2010;82:185–200.
41. Reddy PH, Yin X, Manczak M, et al. Mutant APP and amyloid beta-induced defective autophagy, mitophagy, mitochondrial structural and functional changes and synaptic damage in hippocampal neurons from Alzheimer’s disease. Hum Mol Genet. 2018;27(14):2502–2516. doi:10.1093/hmg/ddy154
42. Al-Rawaf HA, Alghadir AH, Gabr SA. Molecular changes in circulating microRNAs’ expression and oxidative stress in adults with mild cognitive impairment: a biochemical and molecular study. Clin Interv Aging. 2021;16:57–70. doi:10.2147/CIA.S285689
43. Xu L, He D, Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol. 2016;53(10):6709–6715. doi:10.1007/s12035-015-9593-4
44. Park J, Ha HJ, Chung ES. O-GlcNAcylation ameliorates the pathological manifestations of Alzheimer’s disease by inhibiting necroptosis. Sci Adv. 2021;7:3. doi:10.1126/sciadv.abd3207
45. Yang X, Xu S, Qian Y, Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun. 2017;64:162–172. doi:10.1016/j.bbi.2017.03.003
46. Jha MK, Lee WH, Suk K. Functional polarization of neuroglia: implications in neuroinflammation and neurological disorders. Biochem Pharmacol. 2016;103:1–16. doi:10.1016/j.bcp.2015.11.003
47. Tao Y, Li L, Jiang B, et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav Immun. 2016;58:118–129. doi:10.1016/j.bbi.2016.05.020
48. Sun ZQ, Liu JF, Luo W, et al. Lycium barbarum extract promotes M2 polarization and reduces oligomeric amyloid-β-induced inflammatory reactions in microglial cells. Neural Regen Res. 2022;17(1):203–209. doi:10.4103/1673-5374.314325
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.