Back to Journals » Cancer Management and Research » Volume 14
Response to Pyrotinib in a Patient with Metastatic Bladder Urothelial Carcinoma Harboring HER2 V842I Mutation: A Case Report
Authors Li S , Liu Q, Liu M, Liu T
Received 6 May 2022
Accepted for publication 10 August 2022
Published 29 September 2022 Volume 2022:14 Pages 2927—2932
DOI https://doi.org/10.2147/CMAR.S365951
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Suyao Li,1,* Qing Liu,1,2,* Mengling Liu,1 Tianshu Liu1,2
1Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Cancer Center, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianshu Liu, Department of Medical Oncology, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Shanghai, 20032, People’s Republic of China, Email [email protected]
Background: The highest incidence of human epidermal growth factor receptor 2 (HER2) mutations has been observed in bladder cancer (BC). However, the function of HER2 mutation in tumor progression and metastasis remains unclear. Currently, no responses to the pan-HER kinase inhibitor were observed in HER2-mutant BC.
Case Presentation: We described a patient with metastatic bladder urothelial carcinoma (BUC) carrying a HER2 V842I mutation both in circulating tumor DNA (ctDNA) and biopsy sample. The patient was then treated with a HER2 tyrosine kinase inhibitor, pyrotinib, and responded well. However, the targeting treatment was terminated due to G3 diarrhea. Reduced dose of pyrotinib was later added to late-line treatment, the patient’s tumor again responded with a significant decrease in CA199.
Conclusion: This is the first reported case of HER2 V842I mutation successfully treated with pyrotinib in BUC, suggesting pyrotinib therapy might serve as a therapeutic option for BUC patients harboring HER2 activating mutation.
Keywords: bladder cancer, pyrotinib, V842I mutation, human epidermal growth factor receptor 2, next-generation sequencing
Background
Bladder cancer (BC) is the tenth most common cancer in the world, with an estimation of 81,400 new cases and 17,980 deaths in the USA in 2020.1 Currently, multiple new non-chemotherapeutic drugs (such as checkpoint inhibitors, enfortumab, erdafitinib, and sacituzumab) are available for use.2 Considering that BC is one of the cancers with the highest somatic mutation frequencies, targeted therapy is believed to hold great promise.3 Notably, BC represents the histology with the highest incidence of human epidermal growth factor receptor 2 (HER2, encoded by ERBB2) mutations, ranging from 9 to 12%, followed by bile duct, stomach, and breast.4,5 However, the functional role of HER2 mutation in tumor progression and metastasis remains unclear. In a multicenter “basket” trial using the pan-HER kinase inhibitor neratinib targeting patients with HER2- and HER3-mutant cancers, no responses were observed in BC.6 Here, we reported a HER2 mutated BC case that clinically responded to pyrotinib, a pan-HER kinase inhibitor, who was refractory to multiple lines of treatment.
Case Presentation
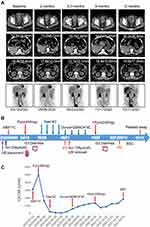
A 64-year-old male initially presented with painless hematuria in September 2017 and was treated by a partial cystectomy as stage I BC. The patient showed local recurrence in August 2018 and underwent transurethral resection of bladder tumor (TURBT) followed by regular intravesical chemotherapy with epirubicin after TURBT. In March 2020, the patient approached to our hospital complaining about severe anorexia and fatigue. Subsequent FDG-PET/CT scan showed bladder recurrence with local invasion of the adjacent right lower ureter, resulting in the upper urinary tract dilation. Multiple lymph node metastasis, liver metastasis and lumbar metastasis (L5) were also identified (Figure 1A). Ureteral stent placement was performed due to obstructive renal insufficiency and hepatic biopsy confirmed metastatic high-grade urothelial carcinoma with positive PD-L1 expression on 1% of the tumor cells. Then, next-generation sequencing (NGS) by a commercial laboratory (Genecast Biotechnology Co., Ltd, Jiangsu, China) identified ERBB2 p.V842I in both circulating tumor DNA (ctDNA) and biopsy sample with an allele frequency of 3.94% and 26.07%, respectively (Figure 2).
 |
Figure 2 Dynamic profiling of somatic mutations in ctDNA. |
The patient received one cycle of palliative reduced-dose albumin-bound paclitaxel due to elevated serum creatinine level (Scr 204μmol/L). The tumor biomarker CA199 was even higher after first cycle chemotherapy (Figure 1C) without any relief of his symptoms. Based on his NGS result, pyrotinib (400mg orally daily) was started from April 10, 2020. Within one month, his tumor markers dramatically improved with remission of clinical symptoms (Figure 1C). Due to renal dysfunction, the patient performed FDG-PET/CT scan for treatment efficacy assessment, which showed significantly decreased FDG uptake of multiple metastatic sites in May 2020 (Figure 1A). Even though the overall response was considered stable based on the RECIST 1.1 criteria, the decrease in FDG uptake and remission of clinical symptoms indicated the patient responded well to the HER2 targeting therapy. However, he developed G3 diarrhea and terminated the medication on May 20, 2020 (The pyrotinib treatment lasted for 40 days). The third-line treatment with the PD-1 inhibitor tislelizumab (200mg every three weeks) was next started but the disease progressed after 2 months (Figure 1A). ctDNA was collected in August 2020 and ERBB2 p.V842I with an allele frequency of 2.06% was identified by NGS. Ureteral stent removal was performed with improved renal function. Four cycles of PD-L1 inhibitor durvalumab, oxaliplatin and gemcitabine were administered, but level of CA199 gradually increased. NGS was performed again showing the same ERBB2 mutation with an allele frequency of 1.58%. Reduced dose of pyrotinib (240mg orally daily) was added, the patient’s tumor again responded with significant improvement of CA199 within 20 days. Dynamic monitoring of the ctDNA again confirmed ERBB2 p.V842I by NGS with a lower allele frequency of 0.41% (Figure 2). The regimen was generally tolerated this time but the tumor marker gradually increased in this patient. PET/CT evaluation revealed disease progression in March 2021, and the treatment was then discontinued (Figure 1A). The length of time for reduced pyrotinib therapy was 4.5 months. The patient passed away on July 1st, 2021, and the post-recurrence survival was 16 months.
Discussion and Conclusion
In this case, we described a patient with metastatic urothelial carcinoma of the bladder harboring HER2 V842I mutation, who was treated with pyrotinib and responded well to the anti-HER2 therapy. The post-recurrent survival was 16 months in this patient, longer than the median overall survival of advanced bladder cancer patients (12–14 months).7 To our knowledge, this is the first reported case of HER2 V842I mutation successfully treated with pyrotinib in bladder urothelial carcinoma (BUC).
ctDNA has emerged as an invaluable tool in the development of personalized medicine. However, ctDNA assay is much less widely used in BC than that in other cancer types like lung cancer.8,9 In our case, targeting HER2 V842I mutation achieved satisfactory benefits under the guidance of ctDNA monitoring, suggesting ctDNA detection has a potential role in selecting cancer patients for targeted treatment. For newly diagnosed and relapsed patients, NGS of tissue and ctDNA should be performed at the same time. However, considering the difficulty of obtaining biopsy samples, ctDNA monitoring was recommended during treatment. In addition, studies have shown that CA199 was a serum marker of poor prognosis in urothelial carcinoma.10 In this case, the patient showed clinical symptom remission after pyrotinib administration, associated with a decrease of CA199, which might provide important clues on the disease diagnosis and treatment.
With a frequency of 0.09%,5 V842I is an activating HER2 mutation located in tyrosine kinase domain (TKD).11 V842I mutation has been confirmed to strongly increase the phosphorylation of signal proteins and activate specific PI3K/Akt and MAPK pathways in vivo.12–14 However, contradictory results have also been reported based on in vitro studies.15 Besides pre-clinical findings, clinical evidence for targeting HER2 V842I is also inconclusive. In a “basket” trial using the pan-HER kinase inhibitor neratinib targeting patients with HER2- and HER3-mutant cancers, three colorectal cancer patients and one endometrial cancer patient with the V842I mutation suffered progressive disease after anti-HER2 therapy.6 Therefore, there is still a lack of pre-clinical and clinical evidence on the role of HER2 V842I mutation in BC.
Anti-HER2 therapies have been found to be effective in HER2-mutated lung cancer and breast cancer patients. However, no responses were observed in BC or colorectal cancer, suggesting lineage-dependent resistance to certain single-agent pan-HER kinase inhibition in these tumor types.6,16 Therefore, it is urgent to explore suitable anti-HER2 therapies for HER2-mutated BC patients. Pyrotinib is an oral, irreversible, pan-ErbB tyrosine kinase inhibitor against HER1, HER2, and HER4. In August 2018, pyrotinib obtained its first global conditional approval in China for HER2-positive, advanced or metastatic breast cancer patients.17 Pyrotinib plus capecitabine significantly improved patients’ objective response rate and progression-free survival compared with lapatinib plus capecitabine in metastatic breast cancer.18,19 Additionally, previous studies have demonstrated favorable antitumor activity of pyrotinib in the patient-derived HER2-mutant cancer xenograft model.20 Clinical trials also have shown that pyrotinib exhibited high efficacy in NSCLC patients harboring activating HER2 mutations.21,22 Therefore, pyrotinib therapy was considered as an option for the late-line treatment in HER2 mutant cancer patients. Large clinical trials should be conducted to verify the treatment efficacy of pyrotinib in HER2 amplified or mutant cancers. Diarrhea was the most common adverse effect for patients using pyrotinib with a probability of 40%. Compared with other tyrosine kinase inhibitors, pyrotinib was more lipophilic, providing better passive absorption with less gastrointestinal adverse effects. Most diarrhea events caused by pyrotinib were minor, only 10.7–15.4% reached grade 3 and were generally manageable.18,23,24 In our case, the patient developed severe diarrhea, resulting in the dose reduction of pyrotinib. Hence, future work includes exploring the best treatment efficacy with manageable toxicity in targeted therapy.
Here, we have presented a patient with recurrent metastatic BUC with HER2 V842I mutation who showed clinical benefits when treated with pyrotinib. To date, clinical evidence on the efficacy of anti-HER2 therapy in BC is minimal. BC patients with HER2 activating mutation represent a population with significant unmet needs. In this case, pyrotinib therapy might be considered an optional treatment for BUC patients harboring HER2 activating mutation, the mechanism of which needs further exploration.
Data Sharing Statement
All data generated and analyzed during this study are included in the published article.
Ethics Approval and Consent to Participate
This case report was approved by the institutional ethical committee in Zhongshan Hospital, and written informed consent was obtained from the patient.
Consent for Publication
Written informed consent was obtained from the patient for publication.
Acknowledgments
The authors thank the patient and his family for their invaluable contribution to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81802356).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi:10.3322/caac.21590
2. Lenis AT, Lec PM, Chamie K, et al. Bladder cancer: a review. JAMA. 2020;324:1980–1991. doi:10.1001/jama.2020.17598
3. Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171:540–556 e525. doi:10.1016/j.cell.2017.09.007
4. Robichaux JP, Elamin YY, Vijayan RSK, et al. Pan-cancer landscape and analysis of erbb2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of t-dm1 activity. Cancer Cell. 2019;36:444–457.e7. doi:10.1016/j.ccell.2019.09.001
5. Cousin S, Khalifa E, Crombe A, et al. Targeting erbb2 mutations in solid tumors: biological and clinical implications. J Hematol Oncol. 2018;11:86. doi:10.1186/s13045-018-0630-4
6. Hyman DM, Piha-Paul SA, Won H, et al. Her kinase inhibition in patients with her2- and her3-mutant cancers. Nature. 2018;554:189–194. doi:10.1038/nature25475
7. Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475. doi:10.1016/j.eururo.2016.06.020
8. Xu S, Lou F, Wu Y, et al. Circulating tumor DNA identified by targeted sequencing in advanced-stage non-small cell lung cancer patients. Cancer Lett. 2016;370:324–331. doi:10.1016/j.canlet.2015.11.005
9. Thompson JC, Yee SS, Troxel AB, et al. Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor DNA. Clin Cancer Res. 2016;22:5772–5782. doi:10.1158/1078-0432.CCR-16-1231
10. Sashide K, Isobe H, Wakumoto Y, et al. Ca19-9 as a serum marker for poor prognosis in urothelial carcinoma. Urol Int. 2004;72:112–117. doi:10.1159/000075963
11. Kovacs E, Zorn JA, Huang Y, et al. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem. 2015;84:739–764. doi:10.1146/annurev-biochem-060614-034402
12. Gaibar M, Beltran L, Romero-Lorca A, et al. Somatic mutations in her2 and implications for current treatment paradigms in her2-positive breast cancer. J Oncol. 2020;2020:6375956. doi:10.1155/2020/6375956
13. Bose R, Kavuri SM, Searleman AC, et al. Activating her2 mutations in her2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi:10.1158/2159-8290.CD-12-0349
14. Kavuri SM, Jain N, Galimi F, et al. Her2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5:832–841. doi:10.1158/2159-8290.CD-14-1211
15. Ng PK, Li J, Jeong KJ, et al. Systematic functional annotation of somatic mutations in cancer. Cancer Cell. 2018;33:450–462 e410. doi:10.1016/j.ccell.2018.01.021
16. Cocco E, Lopez S, Santin AD, et al. Prevalence and role of her2 mutations in cancer. Pharmacol Ther. 2019;199:188–196. doi:10.1016/j.pharmthera.2019.03.010
17. Blair HA. Pyrotinib: first global approval. Drugs. 2018;78:1751–1755. doi:10.1007/s40265-018-0997-0
18. Ma F, Ouyang Q, Li W, et al. Pyrotinib or lapatinib combined with capecitabine in her2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, Phase II study. J Clin Oncol. 2019;37:2610–2619. doi:10.1200/JCO.19.00108
19. Gourd E. Pyrotinib versus lapatinib in her2-positive breast cancer. Lancet Oncol. 2019;20:e562. doi:10.1016/S1470-2045(19)30568-6
20. Wang Y, Jiang T, Qin Z, et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol. 2019;30:447–455. doi:10.1093/annonc/mdy542
21. Zhou C, Li X, Wang Q, et al. Pyrotinib in -mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase ii study. J Clin Oncol. 2020;38:2753–2761. doi:10.1200/JCO.20.00297
22. Song Z, Li Y, Chen S, et al. Efficacy and safety of pyrotinib in advanced lung adenocarcinoma with HER2 mutations: a multicenter, single-arm, phase II trial. BMC Med. 2022;20:42. doi:10.1186/s12916-022-02245-z
23. Ma F, Li Q, Chen S, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-erbb receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2017;35:3105–3112. doi:10.1200/JCO.2016.69.6179
24. Li Q, Guan X, Chen S, et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in her2-positive metastatic breast cancer patients: a phase i clinical trial. Clin Cancer Re. 2019;25:5212–5220. doi:10.1158/1078-0432.CCR-18-4173
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.