Back to Journals » Drug Design, Development and Therapy » Volume 10
Resolvins and aliamides: lipid autacoids in ophthalmology – what promise do they hold?
Authors Keppel Hesselink J , Chiosi F, Costagliola C
Received 9 May 2016
Accepted for publication 7 July 2016
Published 27 September 2016 Volume 2016:10 Pages 3133—3141
DOI https://doi.org/10.2147/DDDT.S112389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Frank Boeckler
Jan M Keppel Hesselink,1 Flavia Chiosi,2 Ciro Costagliola2
1University of Witten/Herdecke, Witten, Germany; 2Eye Clinic, Department of Medicine and Health Sciences, University of Molise, Campobasso, Italy
Abstract: Resolvins are a novel class of lipid-derived endogenous molecules (autacoids) with potent immunomodulating properties, which regulate the resolution phase of an active immune response. These modulating factors are locally produced, influencing the function of cells and/or tissues, which are produced on demand and subsequently metabolized in the same cells and/or tissues. This review is focused on certain lipid autacoids with putative relevance for ophthalmology in general and for dry eye more specifically. We also briefly investigate the concept of aliamides and the role of palmitoylethanolamide in ophthalmology, and analyze in more detail the putative role and the preclinical and clinical development of resolvins as emerging treatments for dry eye and related disorders, with a focus on one of the lead resolvin derivatives – RX-10045.
Keywords: resolvins, autacoids, aliamides, dry eye, palmitoylethanolamide, inflammation, ophthalmology, protection
Introduction
More and more, it is realized that drug development has to find new inroads into the treatment of chronic eye disorders. The old recipe of the last century, developing receptor ligands to block certain pathological states or to compensate for deficiencies, has played its role. The idea of treating complex disorders by targeting only one biochemical pathway appears to be of insufficient use for a great many disorders. Most of the chronic eye pathologies need a multitarget approach, and preferably without inducing side effects or drug–drug interactions. To influence a number of pathways using one compound and without inducing side effects, however, is quite difficult. That is where autacoids may enter the stage.
Autacoid pharmacology, developed in the 1970s, started with explorations of therapeutic effects of compounds influencing serotonin and histamine. Autacoid medicine, however, remained in its infancy, although it may lead to the much-sought-after therapies, working via multiple physiological pathways, and free from troublesome side effects. This might be of great use for the treatment of eye disorders. Autacoid drugs are either the body-own compounds themselves or the precursors or other derivatives thereof, preferably based on simple chemistry. We use the word “simple” to characterize precursors, such as 5-hydroxytryptophan, a precursor for serotonin, or for instance galactosylated prodrugs, prodrugs that hydrolyze into the endogenous autacoid and simple sugars, such as galactose or mannose. For such prodrugs, the possibility of unwanted side effects is low. For other (artificial) derivatives, however, this will be less the case, as these molecules are new chemical entities and thus potentially not free from toxicological problems.
Classical medicine focused on blocking one target receptor only; modern autacoid medicine will explore endogenous compounds or derivatives thereof, acting as broad active modifiers via a number of coherent biochemical pathways. While a receptor block will lead to a sudden halt of a physiological process, forcing the targeted system to stop and often leading to collateral damage (statins, nonsteroidal anti-inflammatory drugs), autacoids use natural physiological systems and pathways to modify pathological processes.
How to define autacoids? Autacoids are locally produced modulating factors, influencing the function of cells and/or tissues, which are produced on demand and are subsequently metabolized in the same cells and/or tissues. This review is focused on certain lipid autacoids with putative relevance for ophthalmology in general and for dry eye more specifically. We also discuss the concept of aliamides and the role of palmitoylethanolamide in ophthalmology, and subsequently we analyze the putative roles and the preclinical and clinical development of resolvins (Rvs) as emerging treatments for dry eye and related disorders, with a focus on one of the lead Rv derivatives – RX-10045.
Classes of lipid autacoids
There are many classes of lipid autacoids, among which are the groups of the N-acetylethanolamides, protectins, Rvs, and maresins. The key function of autacoids belonging to these classes is to inhibit hyperactivated immune cascades and thus act like a “stop” signal in inflammation processes otherwise becoming pathological. In 1993, the Nobel laureate Rita Levi-Montalcini (1909–2012) coined the term “aliamides” for such compounds, while working on the inhibiting and modulating role of palmitoylethanolamide (PEA) in overactive mast cells. The concept of aliamides was derived from the acronym ALIA: autacoid local inflammation antagonist. The term found its way into the field of N-acetylethanolamides autacoids, such as PEA, although “aliamide” was defined by Levi-Montalcini as a container concept for all lipid-inhibiting and -modulating mediators. That would also include the Rvs, protectins, and maresins. Rvs are metabolites of the polyunsaturated ω-3 fatty acids: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA). The metabolites of EPA are termed E Rvs (RvEs), those of DHA are termed D Rvs (RvDs), and those of DPA are termed Rvs D (RvDsn-3DPA) and Rvs T (RvTs). Protectins and maresins are derived from the ω-3 fatty acid DHA.
Local modulation of pathological inflammation by aliamides
The first hints of the existence of an inbuilt inhibitory system in inflammation can be found in the work of Melmon et al.1 In 1981, they reported the effects of a number of locally synthesized signaling endogenous compounds, such as histamine and prostaglandins, and pointed out that concentrations of autacoids in tissues during inflammation and immune response are sufficient for modifying the functions of immunocompetent cells that control and express humoral- and cell-mediated immunity. The authors stressed the role of autacoids as “feedback modifiers” for early and late phases of the immune response, and highlighted the importance of such endogenous compounds as potential drugs. This idea of protective endogenous compounds was brought to the next phase by Levi-Montalcini in her work on the endogenous autacoid compound PEA. She explored the relevance of a family of compounds related to the unsaturated lipid messenger PEA in the early 1990s, together with colleagues from a small pharmaceutical company – LifeGroup SPA. In 1993, her group published its first findings.2 In this publication, the first evidence was presented to support the theory that lipid amides of the N-acylethanolamine type (such as PEA) are naturally occurring molecules capable of modulating immune cells, such as mast cells. In 2002, this concept of local anti-inflammatory autacoids was “rediscovered” for the class of lipid-mediators called the Rvs.3 Levi-Montalcini, however, was the first scientist to discover and explore the important concept of inflammation inhibiting lipid mediators. At the beginning of this century, it was generally thought that inflammatory processes phased out. The work on the modulatory effects of PEA on overactive mast cells formed the basis of a new paradigm in immunology and inflammation biology. The leading idea of Levi-Montalcini was that tissue accumulation of N-acylethanolamines occurred in pathological degenerative conditions, and this is a meaningful biological response to control such inflammation. “Aliamide” as a keyword can be found in a number of publications since 1993.4 The aliamide PEA and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons.5 PEA, the prototype of aliamides, was subsequently developed for different indications related to pathological inflammation, including various inflammatory eye disorders. This compound has been available as a nutraceutical since 2005. In a separate paper, we discussed the efficacy and safety of PEA in a number of eye disorders, among which are diabetic retinopathy and glaucoma.6 Recently, new PEA analogs have been developed to use as topical treatment for the skin, which are also promising in terms of eye disorders, when formulated properly as liposomes.7 For the Rvs, the first targeted eye indication for entering preclinical and clinical development was dry eye. We thus first discuss the pathogenesis of dry eye related to the putative role of Rvs in correcting this pathogenesis.
Dry eye: its pathogenesis and the rationale for resolvins as therapy
Dry eye is a common condition that occurs when tears are not able to satisfactorily lubricate the ocular surface. According to the International Dry Eye WorkShop, “Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by an increased osmolarity of the tear film and inflammation of the ocular surface”.8 There are different types of dry-eye syndromes, however, classified into “dry eye with reduced tear production (aqueous-deficient)” and “dry eye with increased evaporation of the tear film (hyperevaporative)”, which have proved useful on practical grounds.9 Recently, clinical and experimental evidence suggests that dry eye is an inflammatory disease.10 In fact, disease or dysfunction of the tear-secretory glands leads to changes in tear composition that stimulate the production of inflammatory mediators on the ocular surface;11 in addition, inflammation may cause dysfunction or disappearance of cells responsible for tear secretion or retention.12 Furthermore, RvD1 was found to block proinflammatory responses in salivary glands and enhance salivary epithelial integrity, while RvD1 biosynthesis remained intact in salivary glands in Sjögren’s syndrome.13 These findings support the use of lipid autacoids in the treatment of dry-eye disease (as well as in Sjögren’s syndrome).
Rvs have potent anti-inflammatory and proresolution actions in several animal models of inflammation.14 As Rvs clearly target an overactivated chronic inflammatory response, such a response needs to be at the core of the pathogenesis of dry eye in order for Rvs to be a therapeutic success.
The resolvins: resolving which issues?
The key question is: What inflammatory problems within the pathology of the eye Rvs are best placed to succeed as a therapy? This seemingly simple question is quite difficult to answer.
In 2002, Serhan et al had published a milestone paper on the identification of a number of endogenous inflammation-modulating compounds, synthesized in vivo from ω-3 fatty acids.15 They introduced the term “Rvs” for this class of lipid modulators: “Rvs, by definition, are endogenously generated within the inflammatory resolution phase and downregulate leukocytic exudate cell numbers to prepare for orderly and timely resolution”.16 Serhan and Levy filed a patent related to this matter covering new therapeutic di- or trihydroxy derivatives of EPA or DHA (Rvs) that diminish, prevent, or eliminate inflammation. The patent also covers various Rv precursors leading to the production of bioactive novel endogenous products controlling physiologic events in inflammation and resolution in vascular endothelial reactions and neural systems. Furthermore, the patent claims a number of stable analogs of Rvs with enhanced biologic properties that are also targeted to expedite resolution by inhibiting the proinflammatory cascades.17 Rv1,5,12,18R-trihydroxy-EPA is a prototype natural Rv, also known by its codename RX-10001 (Figure 1). There are a number of Rvs and (semi-) synthetic derivatives and prodrugs interesting enough to construct patents for. Indications vary from inflammatory eye disorders and intestine inflammation to bone loss. The key question here is: What indication is the best fit for Rv, based on what pathogenesis and pathology? Sadly, such questions are not often put forward in a straightforward form, and thus development of certain Rvs and their analogs seems often to take a somewhat meandering tour. We now describe a few of the additional patents on Rvs, as far as is relevant for this review. In 2002, the University of Southern California filed a patent, which was subsequently licensed to Resolvyx and covered the use of an Rv1 analog: RX-10045 (US7582785 B2) (Figure 2). Methods for the preparation of naturally occurring trihydroxy polyunsaturated eicosanoids and their structural analogs were described, as well as its use in a variety of diseases associated with inflammation or inflammatory response, such as autoimmune diseases, rheumatoid arthritis, cardiovascular diseases, or abnormal cell proliferation or cancer. In 2007, a patent was filed related to the use of Rvs and protectins to treat pathologies associated with angiogenesis.18 It is interesting to note that around that time, topical administration of ω-3 fatty acids was reported to have beneficial effects in a dry-eye model: signs of inflammation decreased after treatment once daily with 0.2% α-linolenic acid (ω-3 fatty acid) drops in an emulsion.19 Theoretically, α-linolenic acid eye drops could act as a reservoir for localized biosynthesis of Rvs, thus solving the issue of the metabolic instability of the Rvs proper.20 Meanwhile, these drops are available as a microemulsion of polyunsaturated fatty acids and moisturizing polymers.
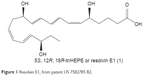  | Figure 1 Resolvin E1, from patent US 7582785 B2. |
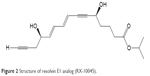  | Figure 2 Structure of resolvin E1 analog (RX-10045). |
Resolvyx Pharmaceuticals’ semisynthetic resolvins
Resolvyx Pharmaceuticals Inc is the first company taking up preclinical and clinical development of RVs for eye disorders. Initially, the inventors targeted pulmonary disorders, such as asthma and cystic fibrosis, as the main indications for the Rvs.21 Why the transition was made from pulmonary target indications to dry eyes is not easy to understand. From the work in asthma models, a concentration-dependent inflammation-inhibiting effect of RvE1 was seen at 3 nM, and in vitro effects were more explicit compared to in vivo. This was interpreted as a hint for an endogenous mechanism via multiple pathways.22 Later, data were presented on the methyl ester prodrug of RvE1/RX-10001, code name RX-10005, in a murine model of desiccating stress, validated for dry eyes. To enhance the penetration of RvE1 through the corneal epithelium, the ester prodrug of RvE1 (RX-10005) was administered. The choice for a prodrug was motivated by earlier studies on the pharmacokinetic behavior of RvE1 and prodrugs in an animal model: for the parent compound, corneal exposure increased around tenfold after topical administration as a prodrug.23 RX-10001 (RvE1) was initially taken into preclinical development for a number of indications – asthma, colitis, rheumatoid arthritis, atherosclerosis, dry eye, and retinopathy – and formulations for parenteral and oral use were developed. Resolvyx in 2009 reported having successfully completed a Phase I trial evaluating the safety of an orally formulated RX-10001, based on 300 mg tablets (https://clinicaltrials.gov/ct2/show/NCT00941018). The results have not been published, so it is possible that the pharmacokinetic profile was suboptimal, and after this trial development seems to have stopped. New development activities were subsequently focused on RX-10045 and no longer on RX-10001. Therefore, we discuss those new activities in the next section.
RX-10045, a synthetic resolvin derivate for ophthalmological indications
RX-10045 ([5S,8E,10E,12R]-isopropyl 5,12-hydroxypentadeca-8,10-dien-6,14-diynoate) (Figure 2) is Resolvyx’s lead Rv, and this is an isopropyl ester prodrug of the active Rv1-analog compound RX-10008. On the Internet, we find the news: “Resolvyx announces initiation of phase I/II clinical trial of novel resolvin therapeutic RX-10045 in patients with dry eye” (http://www.businesswire.com/news/home/20081218005251/en/Resolvyx-Announces-Initiation-Phase-III-Clinical-trial). We may be a bit critical here by noting that the URL reports the start of Phase III, while the article itself reports the start of Phase I/II. RX-10045 is not an easy molecule to formulate; it is reported to be light sensitive, highly unstable, and easily prone to degradation at room temperature, while its phenotype is a yellow viscous oil with very poor aqueous solubility. An aqueous solution of RX-10045 was prepared using propylene glycol as a solubilizing agent, and this formulation was first tested in murine models for the treatment of dry eye with topical drops. From these experiments, it was reported that RX-10045 was highly efficacious in treating dry-eye syndrome.24 Resolvyx Pharmaceuticals decided that the first selected target indication for RX-10045 was dry-eye syndrome.25 We now review published preclinical studies supporting this indication and related eye indications for the RvE1 analog.
Preclinical data
One of the first issues to address was whether RvE1 penetrates into the ocular tissue and whether the compound is stable enough.23 Ester prodrugs were compared to parent compounds for their pharmacokinetic properties after topical administration to the rabbit eye. Rabbits received a single 30 μL drop of either a 30 μg/mL or 300 μg/mL drug concentration in the right and left eye, respectively. At 15, 30, 60, 120, and 360 minutes, drug levels were measured in the eye tissues. In the case of RX-10008, this resulted in a tenfold increase in corneal concentration when administered topically as a prodrug, a methyl, or isopropyl ester, compared to the concentrations of the parent compound after administration. Following administration of the prodrugs, intact prodrugs could not be measured. The drug half-life (of the parent compound) in the cornea was ~1.5 hours, with peak levels of ~4 ng/mg at 15 minutes. Administration of the prodrug led to significant enhancement of RX-10008 levels in the aqueous humor and vitreous, with peak concentrations at ~1,000 ng/mL and ~3 ng/mL, respectively.
Dry-eye models
In an experimental dry-eye model in mice, subcutaneous scopolamine injection and exposure to an air draft for 5 days, 300 μg/mL of RX-10005 (parent compound RvE1), 300 μg/mL of RX-10065 (probably RX-10045; parent compound RX-10008), and placebo delivered four times per day as 1 μL drops were compared.26 One week after air-draft exposure, the animals were treated with 5 μL of 0.001% RX-10065, 0.001% RX-1005, or vehicle topically four times daily for an additional week. The efficacy of compounds was verified in vivo and in vitro on corneal specimens through Western blot analysis and immunofluorescence examination. In vivo tests showed a significant decrease in tear production in exposed animals (4.8±1.5 mm) compared with the control group (7.1±1.8 mm) (P<0.001). There was no change at 2 days and 4 days after treatment with the vehicle (4.7±1.2 mm, 6.2±2.1 mm), but a significant increase was observed in the group treated with RX-10005 at 2 days (5.6±1.3 mm) and 4 days (7.4±1.2 mm). The density of the superficial epithelial cells showed a significant decrease after exposure (838.75±86 cells/mm2) compared with controls (1,072±9,025 cells/mm2), which increased after RX-10005 and RX-10065 treatment (1,043.75±138 cells/mm2 and 1,006.5±98 cells/mm2, respectively). Western blot analysis showed that α-SMA and COX2 were strongly upregulated after exposure and decreased after administration of both drugs. Immunofluorescence showed strong positive staining in stroma and/or the epithelium after exposure and decreased with treatment. These findings strongly supported the theory that RX-10065 and RX-10005 significantly increase tear production and inhibit keratocyte transformation to myofibroblasts and the inflammation-inducible COX2.27
Decreased density of mucin-filled goblet cells is a well-recognized feature of aqueous-deficient dry eye: goblet-cell density correlates with severity of ocular surface disease in patients with aqueous tear deficiency.28 RvE1 reduced corneal epithelial barrier disruption and protected against goblet-cell loss in a murine model of dry eye.29 Topical treatment of eyes with RX-10005 significantly reduced Oregon green dextran staining compared to the vehicle control-treated group (122.2±5.9 vs 135.1±17.04 gray levels, respectively; P<0.05) and also significantly maintained goblet-cell density (6.29±0.47 vs 5.1±0.55 cells/100 μm, respectively; P<0.05). In RX-10065, there was a slight decrease in Oregon green dextran staining (128.5±17.7 gray levels), but a significant preservation of goblet-cell density compared to the vehicle group (5.72±0.5, P<0.05), indicating the potential utility of endogenous Rvs and Rv analogs in the treatment of dry eye.
In an in vitro stress model of human corneal epithelial cells, it was tested whether RvE1 and its analog RX-10008 (the metabolite of RX-10045) in a concentration range of 10−11–10−7 M could suppress a hypertonicity-induced increase in proinflammatory cytokine release, administered 30 minutes prior to starting the hyperosmolar stress.30 Such stress leads to an increase in IL-6 levels from a basal level of approximately 2,000 pg/mL to 4,000 pg/mL, and for IL-8 an increase from 3,700 pg/mL to 9,000 pg/mL. At 10−7 M, M RvE1 reduced IL-6 release by 75% and IL-8 release by 70%. RX-10008 reduced these releases by 70% and 65%, respectively. The inhibition was concentration-dependent. The no-effect dose was 10−11 M. Altogether, these findings demonstrate that RvE1 induces increases in migration in human corneal epithelial cells and strongly contributes to preserving corneal integrity, stimulating the continuous renewal of epithelial cells; in fact, the improvement in ocular surface health observed in these experiments may be attributed to the fact that inflammation suppression can hasten reepithelialization.31
In an immunopathological model of the cornea, mice were infected ocularly with herpes simplex virus 1, and RvE1 (500 ng per eye; 5 μL drop from 100 μg/mL solution) was topically applied to the cornea twice daily starting from day 1 until day 10 postinoculation. A significant reduction in the severity and incidence of stromal keratitis was demonstrated in the treated animals compared to the control animals. Furthermore, neutrophil infiltration and pathogenic CD4+ T cells into the cornea were reduced, as well as the concentration of proinflammatory cytokines, such as IL-6, IFNγ, and IL-17, was reduced, while the concentration of the anti-inflammatory cytokine IL-10 increased. This experiment could have had more impact if more doses were tested in order to obtain a dose–response curve.32
Li et al investigated the role of RvE1 administrated as the ester prodrug RX-10005 in a dry-eye mouse model.33 One week after desiccating stress exposure, animals were treated with topical drops of a 0.01% concentration of RX-10005 or vehicle four times per day for an additional week. Only one dose was tested. Tear production increased by 60% compared to control. Active treatment also completely and significantly restored epithelial cell density. Unregulated COX2 expression and infiltration of dendritic cells/macrophages and T cells were significantly decreased compared to the control group.
Other models
In in vitro models, Rvs and derivatives can be administered relatively easily, without the ADME (absorption, distribution, metabolism, and elimination) problems of in vivo models. Such models in general are positive for Rvs as proof-of-principle screening models. In a monolayer culture of a rat parotid-cell line, positive results on all selected outcome parameters were described, in line with protection against inflammatory damage by RvD1.34
In a rabbit model, the effect of 0.1% RX-10045 on haze and myofibroblast generation was tested after haze-producing corneal injury via photorefractive keratectomy.35 RX-10045 was tested in a 0.01% and 0.1% mixed polymeric micelle formulation. Rabbits were treated with 30 μL of 0.1% RX, 0.01% RX, and placebo immediately after the lesion and every 4 hours for 5 days. The corneal epithelium closed before the last application of the test drug in all animals. Corneal opacity was significantly lower in the 0.1% RX-10045 group compared to the placebo. Myofibroblast cell densities at 1 month after the lesion were not significantly different between 0.1% RX-10045 and placebo.
After corneal surgery in rabbits, treatment with NGF or PEDF in conjunction with DHA increases nerve density and corneal epithelial cell proliferation. Increased synthesis of neuroprotectin D1 was found in corneas after the animals were treated with PEDF and DHA. Topical application of these lipids derived from ω-3 fatty acids prevented clinical complications, such as cornea erosion and ulcerations.36 Recently, Aragona et al, applying a topical microemulsion containing EPA and DHA (patent W02008-IB3381 20081107) to patients having undergone photorefractive keratectomy, observed a significant improvement in the corneal nerve pattern (unpublished data). A mouse model was used to evaluate the putative value of RX-10001 (RvE1) and RX-10008 (an RvE1 analog) in macular degeneration, resulting in choroidal neovascularization.37 Four lesions were positioned at 3, 6, 9, and 12 o’clock around the optic nerve. RX-10001 (RvE1) and RX-10008 (the RvE1 analog) (from a 50 nM stock, 18.7 and 14.3 μg/kg, respectively) or a placebo were administered at days 1, 2, 4, 6, and 8. In controls, 75% of the lesions displayed leakage at 7 days and 56% at day 14. RX-10001- and RX-10008-treated animals demonstrated only 7% and 26% leakage, respectively, at day 7, and 4% and 6% leakage for these treatments at day 14. The no-leakage figures were 86% for RX-10001 and 72% for RX-10008 compared to 15% in controls at day 14. Choroid lesions were 14 μm and 43 μm in diameter in RvE1 and the RvE1 analog, respectively, compared to 105 μm in the placebo. The conclusion was that the reduction in leakage in the first week suggested that RX-10001 administered IP protected early in the cascade of events, and while the RX-10008 reduced leakage, it was not as efficient by day 7. However, by 14 days, the effect of RX-10008 approached that of RvE1. The lesion was reduced by 70% with RvE1, but remained unchanged with the analog, while lesions of the controls increased, suggesting that RvE1 was more efficient than its analog in reducing choroidal neovascularization.
Inflammatory stimulation increases the biosynthesis of Rvs E1 and D1 from EPA and DHA in a coculture of chorioretinal endothelial cells and leukocytes. Rvs inhibit the expressions of VCAM1, IL-8, MIP1β, and TNFα. In other words, inflammatory stimuli-activated chorioretinal endothelial cells and leukocytes biosynthesize RvE1 and RvD1 from EPA and DHA. These Rvs inhibit inflammatory signaling from chorioretinal endothelial cells and leukocytes. Therefore, these findings further confirm that Rvs and their biosynthesis pathways are potential targets for novel treatment of inflammatory ocular diseases.38
Rvs seem also to be potent anti-inflammatory compounds and responsible for anti-inflammatory actions by suppressing the production of TNFα, IL-6, and other proinflammatory cytokines.39 It is evident that Rvs act especially against VEGF and TNFα, supporting the hypothesis that these bioactive molecules could be useful in the prevention and management of macular edema, proliferative diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration.40,41
Formulation issues
Cholkar et al described the effects of an aqueous micellar formulation of RX-10045 in a rabbit-eye model to explore tolerance, intraocular pressure, and electroretinography and to determine ocular tissue drug concentrations, as well as whole blood. RX-10045 levels were measured following topical ocular instillation of a placebo or 0.1% and 0.05% RX-10045 encapsulated inside the lipid core of micelles.24 The goal of this micelle formulation was to increase RX-10045 aqueous solubility and its stability, to prevent interactions with influx and efflux proteins, and to deliver high drug concentrations in the cytoplasm. Topically, 35 μL of the formulation (and placebo) was applied via a pipette in one eye four times daily at 2-hour intervals for 5 days. The mean size of the micelles was around 10 nm. The tolerability and stability of this formulation were excellent. In ocular tissues, concentrations measured indicated a rapid hydrolysis of RX-10045 into the active metabolite (RX-10008). Active-metabolite concentration was highest in the cornea (17,725 ng/mL) > aqueous humor (3,205 ng/mL) > iris ciliary body (2,655 ng/mL) > conjunctiva (879 ng/mL). In the sclera, lens, retina, choroid, and vitreous humor, concentrations were smaller (700 ng/mL and less). These drug levels indicate that this formulation could be relevant for inflammatory states of the cornea and the ciliary body, but not for retinitis.
RX-10045 clinical development program
In 2009, it was enthusiastically communicated by Resolvyx that RX-10045 had successfully passed a Phase II clinical trial for treating chronic dry-eye syndrome (http://www.eurekalert.org/pub_releases/2009-08/ypr-rap081909.php). In this Phase II, 4-week, dose-finding, randomized, placebo-controlled study, 232 patients were said to be entered. RX-10045 was claimed to have produced a dose-dependent, statistically significant improvement on the primary end points of signs and symptoms of dry eye. However, other sources pointed out that the results were equivocal. So far, the data seem not to have been published, which might be regarded as an indication that the initial analyses were mistakenly reported as positive for the primary end point. Based on the Phase II results, it was said that a Phase III study was initiated in 2011.42 This Phase III program was probably aborted. To date, only three animal-study results have been published describing the pharmacological effects of RX-10045.24,35,43 It is quite strange that the result of the said positive Phase II dry-eye intervention study has not yet been published, while in the database of ClinicalTrials.gov, the completion date for the study is 2009. A new intervention study in a new indication was started in 2014, and meanwhile (in 2016) is reported to be completed.44
Resolvyx’s RX-10045 was subsequently licensed to Celtic Therapeutics in 2010, and this company intended to advance RX-10045 through Phase III development in the dry-eye indication and to broaden the indications of Rvs to treat more eye diseases. Auven (formerly Celtic) Therapeutics subsequently licensed the compound in 2015, and stated that it had entered Phase II clinical development for inflammatory diseases of the anterior segment in 2015 and in two noneye indications: in postoperative inflammation and postoperative pain. Furthermore, the compound entered late preclinical development for posterior-segment diseases, including age-related macular degeneration and diabetic macular edema (http://www.auventx.com/auven/products/RX10045.php). No further development was reported for allergic conjunctivitis, dry-eye, or retinal disorders (http://adisinsight.springer.com/drugs/800029389).
Conclusion
Autacoids are promising new therapeutic inroads into chronic inflammatory eye diseases. Oral and topical formulations of natural autacoids, as well as derivatives and prodrugs, seem possible. For topical administration, one needs to take into account the penetration degree of the active compound in the various compartments of the eye. Both anatomical and physiological aspects, such as the movements of the various fluids in the eye, need to be taken into account when selecting compounds for certain eye indications. Furthermore, for the new class of Rvs, it is still quite unclear what compound or prodrug would be fittest for what indication. The preclinical and clinical development of RX-10045 illustrates this critical development aspect. First selected as a compound targeted for pulmonary disorders, the target indication of RX-10045 quickly changed in dry eyes without clear arguments. Next, the preclinical development based on a number of paradigms seemed to support the clinical development of RX-10045 in dry eyes, although there seems to be no perfect fit between the pathogenesis of dry eyes in general and the mode of action of RX-10045. It seems that the exploration of such fit needs more attention, while one should pay somewhat less attention to positive preclinical findings in dry-eye-related animal models, unless there are clear dose–response relations found. In order to optimize clinical development, one should select disease-specific surrogate parameters and test these extensively in animal models, followed by the implementation of these surrogate parameters in human Phase I and II trials. This will assist in more elaborate dose-finding. Dose-finding to date is often a chapter leading to cutting corners and ultimately to failed drug development. The results of the claimed pivotal positive dose-finding trial of RX-10045 did not support the drug development going into further Phase III trials, and this was probably due to an overenthusiastic interpretation of the dose-finding data. Furthermore, the results of the Phase II trial finalized in 2009 had still not been published at the time of writing. Autacoids, such as the Rvs, might very well be of great use for certain inflammatory eye disorders, but one needs to be more careful when selecting the target indication. For the time being, Rvs still seem to be drugs looking for a disease. Too little attention seems to have been given to innovative formulations based on high-tech liposomes of the natural autacoids. With such formulations, one can possibly reach the same effect, or even more, with prodrugs from autacoid derivatives. Moreover, the side-effect profiles of such natural autacoid formulations are expected not to induce troublesome side effects. Autacoid eye medicine is still in its infancy, and will only mature if the correct development steps are taken.
Disclosure
The authors report no conflicts of interest in this work.
References
Melmon KL, Rocklin RE, Rosenkranz RP. Autacoids as modulators of the inflammatory and immune response. Am J Med. 1981;71:100–106. | ||
Aloe L, Leon A, Levi-Montalcini R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions. 1993;39:C145–C147. | ||
Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. | ||
Skaper SD, Buriani A, Dal Toso R, et al. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc Natl Acad Sci U S A. 1996;93:3984–3989. | ||
Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514–520. | ||
Keppel Hesselink JM, Costagliola C, Fakhry J, Kopsky DJ. Palmitoylethanolamide, a natural retinoprotectant: its putative relevance for the treatment of glaucoma and diabetic retinopathy. J Ophthalmol. 2015;2015:430596. | ||
Tronino D, Russo R, Ostacolo C, et al. Improvement of topical palmitoylethanolamide anti-inflammatory activity by pegylated prodrugs. Mol Pharm. 2015;12:3369-3379. | ||
[No authors listed]. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5:75–92. | ||
Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112:71–81. | ||
Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32:19–41. | ||
Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4230. | ||
Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren’s syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. | ||
Leigh NJ, Nelson JW, Mellas RE, Aguirre A, Baker OJ. Expression of resolvin D1 biosynthetic pathways in salivary epithelium. J Dent Res. 2014;93:300–305. | ||
Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. | ||
Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. | ||
Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. | ||
Serhan CN, Levy BD. Brigham and Women’s Hospital, assignee. Use of docosatrienes, resolvins, and their stable analogs in the treatment of airway diseases and asthma. United States patent US8569542 B2. 2013 Oct 29. | ||
Smith L, Connor K, Serhan CN. Brigham and Women’s Hospital, assignee. Use of resolvins and docosatrienes and analogues thereof for the treatment of angiogenesis and ocular neovascularization. United States patent US20100105773 A1. 2010 Apr 29. | ||
Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225. | ||
Aleo D, Barabino S, Mangiafico S, Rolando M, Saita MG. TRB Chemedica International, assignee. Ophthalmic compositions based on polyunsaturated omega-3 and omega-6 fatty acids. United States patent US8957110 B2. 2015 Feb 17. | ||
Haworth O, Levy BD. Lipoxins, resolvins and protectins: new leads for the treatment of asthma. Expert Opin Drug Discov. 2008;3:1209–1222. | ||
Haas MJ. New resolve in inflammation. Science-Business eXchange. 2008:1–3. Available from: http://www.nature.com/scibx/journal/v1/n23/full/scibx.2008.543.htm. Accessed September 6, 2016. | ||
Schwartz CE, Savinainen A, Gjörstrup P. Resolvin analogs with pharmacokinetic properties suitable for topical administration to treat ocular diseases. Invest Ophthalmol Vis Sci. 2008;49:3179. | ||
Cholkar K, Gilger BC, Mitra AK. Topical delivery of aqueous micellar resolvin E1 analog (RX-10045). Int J Pharm. 2016;498:326–334. | ||
Colligris B, Pintor J. Dry eye disease compounds currently under evaluation in clinical trials. An Real Acad Nacl Farm. 2014;80:151–178. | ||
Gjorstrup P, Pflugfelder SP, Pangelinan S, De Paiva CS. Resolvins protect against goblet cell loss and reduce corneal epithelial barrier disruption in a murine model of KCS. Invest Ophthalmol Vis Sci. 2008;49:122. | ||
Li N, He J, Gjorstrup P, Bazan H. The resolvin E1 analogs, RX-10065 and RX-10005 improve tear production and decrease inflammation in a mouse dry eye model. Invest Ophthalmol Vis Sci. 2008;49:121. | ||
Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose Bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–235. | ||
de Paiva CS, Schwartz CE, Gjörstrup P, Pflugfelder SC. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31:1299–1303. | ||
Pan Z, Gjorstrup P, Reinach P. Resolvins inhibit hypertonicity-induced proinflammatory cytokine release by suppressing MAPK pathway activation in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:125. | ||
Zhang F, Yang H, Pan Z, et al. Dependence of resolvin-induced increases in corneal epithelial cell migration on EGF receptor transactivation. Invest Ophthalmol Vis Sci. 2010;51:5601–5609. | ||
Rajasagi NK, Reddy PB, Suryawanshi A, Gjorstrup P, Rouse BT. Application of resolvin E1 modulates the severity of HSV-induced corneal immunopathology. Invest Ophthalmol Vis Sci. 2010;51:3867. | ||
Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26:431–439. | ||
Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol. 2012;302:C1331–C1345. | ||
Torricelli AA, Santhanam A, Agrawal V, Wilson SE. Resolvin E1 analog RX-10045 0.1% reduces corneal stromal haze in rabbits when applied topically after PRK. Mol Vis. 2014;20:1710–1716. | ||
He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2010;82:319–325. | ||
Tian H, Zhou Y, Elison J, Gordon WC, Gjorstrup P, Bazan NG. Resolvin E1 or a resolvin E1 analog inhibits vascular leakage in experimental choroidal neovascularization (CNV). Invest Ophthalmol Vis Sci. 2008;49:5414. | ||
Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50:3613–3620. | ||
Das UN. Lipoxins, resolvins, and protectins in the prevention and treatment of diabetic macular edema and retinopathy. Nutrition. 2013;29:1–7. | ||
Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of ω-3 polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;7:868–873. | ||
Das UN. Polyunsaturated fatty acids in pathological retinal angiogenesis. Curr Nutr Food Sci. 2009;5:94–111. | ||
Lee CH. Resolvins as new fascinating drug candidates for inflammatory diseases. Arch Pharm Res. 2012;35:3–7. | ||
Cholkar K, Trinh HM, Vadlapudi AD, Wang Z, Pal D, Mitra AK. Interaction studies of resolvin E1 analog (RX-10045) with efflux transporters. J Ocul Pharmacol Ther. 2015;31:248–255. | ||
AT Resolve SARL. Efficacy and safety of RX-10045 ophthalmic solution for ocular inflammation and pain in cataract surgery. Available from: https://clinicaltrials.gov/ct2/show/NCT02329743. NLM identifier: NCT02329743. Accessed August 10, 2016. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
