Back to Journals » Clinical Interventions in Aging » Volume 16
Resistance Training Modulates the Matrix Metalloproteinase-2 Activity in Different Trabecular Bones in Aged Rats
Authors de Sousa Neto IV , Durigan JLQ, Carreiro de Farias Junior G, Bogni FH, Ruivo AL, Araújo JO, Nonaka KO, Selistre-de-Araújo H , Marqueti RC
Received 13 August 2020
Accepted for publication 9 September 2020
Published 8 January 2021 Volume 2021:16 Pages 71—81
DOI https://doi.org/10.2147/CIA.S276518
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Ivo Vieira de Sousa Neto,1 João Luiz Quaglioti Durigan,1,2 Gonçalo Carreiro de Farias Junior,2 Fabio Henrique Bogni,3 Amanda Lima Ruivo,1 Juliana Oliveira de Araújo,1 Keico Okino Nonaka,3 Heloísa Selistre-de-Araújo,3 Rita de Cássia Marqueti1,2
1Laboratory of Molecular Analysis, Graduate Program of Sciences and Technology of Health, Universidade de Brasília, Distrito Federal, Brazil; 2Graduate Program in Rehabilitation Sciences, Universidade de Brasília, Distrito Federal, Brazil; 3Department of Physiological Sciences, Universidade Federal de São Carlos, São Paulo, Brazil
Correspondence: Ivo Vieira de Sousa Neto; Rita de Cássia Marqueti
Laboratory of Molecular Analysis, Graduate Program of Sciences and Technology of Health, Universidade de Brasília, Distrito Federal, Brazil
Email [email protected]; [email protected]
Background: Aging decreases osteogenic ability, inducing harmful effects on the bone extracellular matrix (ECM), while exercise training has been indicated as a tool to counteract bone disorders related to advancing age. The modulation of bone ECM is regulated by several types of matrix metalloproteinase (MMP); however, MMP-2 activity in different trabecular bones in response to resistance training (RT) has been neglected. Remodeling differs in different bones under the application of the same mechanical loading. Thus, we investigated the effects of 12 weeks of RT on MMP-2 activity in the lumbar vertebra (L6), tibia, and femur of young (3 months) and older rats (21 months).
Methods: Twenty Wistar rats were divided into four groups (five animals per group): young sedentary or trained and older sedentary or trained. The 12-week RT consisted of climbing a 1.1-m vertical ladder three times per week with progressive weights secured to the animals’ tails. The animals were killed 48 h after the end of the experimental period. The MMP-2 activity was assessed by the zymography method.
Results: The aging process induced lower MMP-2 activity in the lumbar vertebrae and tibia (p=0.01). RT upregulated pro, intermediate, and active MMP-2 activity in the tibia of young rats (p=0.001). RT also upregulated pro and active MMP-2 activity in the lumbar vertebrae and tibia with advancing age (p=0.01). There was no significant difference (p> 0.05) between groups for MMP-2 of the femur, regardless of age and RT.
Conclusion: The aging process impairs MMP-2 activity, but RT is a potential therapeutic approach to minimize the deleterious effects of ECM degeneration in different aged bones. Distinct MMP-2 responses to exercise training may result in specific remodeling processes.
Keywords: senescent, skeletal system, remodeling, matrix metalloproteinase, exercise
Introduction
Aging is an inevitable process characterized by progressive cellular damage in the different organ systems and tissues, which can lead to pathophysiological conditions.1 Age-related osteoporosis is by far the most common form of the bone disorders in which the individuals are at high risk of suffering fractures.2 Bone deterioration is associated with gait disorders, predisposing the older individual to fall, and functional limitations.3 With age, there is an imbalance between bone resorption and formation, resulting in accelerated bone loss, which could be explained partly by the detrimental changes in the extracellular matrix (ECM).4
Bone ECM is essential for structural support, mechanical stability, and transmission of signals from cells.5 The modulation of bone ECM is regulated by several types of matrix metalloproteinase (MMP).6,7 MMPs are a group of zinc-dependent ECM-degrading enzymes, which have been established to play a crucial role in modeling and bone remodeling.8 These are key endopeptidases responsible for cleaving ECM structural components.9 MMP-2 promotes positive effects during bone repair and regeneration through stimulation of cell adhesion and growth factors to protect them from damage.10,11 It has been reported that MMP-2 is relevant for osteoclast function, osteoblast recruitment and osteocyte viability.12 Age-related decline in MMP-2 activity is associated with deleterious effects in the homeostatic and bone regenerative process, in part as a result of impaired function.13 Therefore, MMP-2 is considered a critical molecule for improving bone turnover.
Pharmaceutical treatments are commonly used for osteoporosis; however, their deleterious effects are often neglected. Despite the use of anti-resorption and anabolic drugs, osteoporosis cannot be completely cured.14 Minimally invasive interventions including exercise training can be as effective as drugs in restoring bone function and modulating key biomarkers, but without serious side effects.14 Exercise is an economical and effective way to beneficially stress the skeletal bone and stimulate optimal ECM remodeling. Resistance training (RT) is considered an important non-pharmacological agent capable of inducing significant effects on bone development through MMP-2 pathway stimulation. Shiguemoto et al15 showed an improvement in biomechanical properties (stiffness and fracture load) accompanied by an increase in MMP-2 activity induced by RT (12 weeks, three times per week) in the tibia from ovariectomized rats. This indicates that RT exerts important regulatory roles in bone ECM. Similarly, Souza et al16 demonstrated that RT improved bone density, mineral content, and stiffness, besides upregulating MMP-2 activity of the tibia, in a similar hypoestrogenism model. These authors demonstrated that such effects are crucial for bone quality and bone health. Upregulation of MMP-2 activity may represent a potential exercise-induced mechanism to protect aged rats from bone abnormalities and injuries.
Overall, there are bones with different architecture, chemical composition, metabolism, and physical properties, which can promote distinct responses of MMP-2.17 Each bone is presumed to have a different physiological adaptation and biomechanical function, and bone remodeling may not be the same in different bones concerning the same mechanical loading application. Previous data have shown that trabecular bone is more actively remodeled than cortical bone owing to the much larger surface to volume ratio.18 Comparative study between different bones would be relevant to elucidate the specific ECM remodeling process inherent to aging-associated changes, as well as to understand in depth the response of MMP-2 to RT. Advances that have occurred in molecular bone biology in response to exercise could provide fascinating insights for practical diagnostic and/or therapeutic fields related to skeletal function. Therefore, the aim of the present study was to investigate the effects of RT on MMP-2 activity in the lumbar vertebra (L6), tibia, and femur of young and older rats. Our hypothesis is that RT upregulates MMP-2 activity in bones, regardless of the aging process, and that such regulation is different in each bone.
Materials and Methods
Experimental Groups
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, Washington DC, USA). The research protocol received approval from the Animal Research Ethics Committee of the Federal University of Sao Carlos, SP, Brazil (protocol number: 044/2011). Thus, 20 male Wistar rats (Rattus norvegicus albinus) between the ages of 3 and 21 months at the start of the experiment were randomly divided into four experimental groups: YS (young sedentary), YT (young trained), OS (older sedentary), and OT (older trained). The age classification of rats was determined according to Quinn.19 The rats were placed in collective cages with members of the same group (three or four animals/cage) with water and standard rodent chow (Purina®; Descalvado, São Paulo, Brazil) available ad libitum, and exposed to 12-hour light/dark cycles with the temperature maintained at 20–22°C during the experiments.
Resistance Training Protocol
Familiarization
Methods regarding familiarization, determination of maximum carrying capacity, and RT sessions were based on previous studies from our research group.1,18,20 The rats were adapted to an RT of climbing a vertical ladder (Ladder 110cm high, 18 cm wide, 2 cm between grid steps and 80° incline) with no weight on the load apparatus for two non-consecutive days (48-hour rest interval). The load apparatus was fixed to the tail by wrapping its proximal portion with a self-adhesive foam strip. Rats were placed at the bottom of the ladder and familiarized with climbing. Finger pinching was used on the animal’s tail as a stimulus to initiate climbing. At the top of the ladder, the rats reached a house chamber where they rested for 2 minutes. This procedure was repeated until they voluntarily climbed the ladder for three consecutive turns without stimulus, according to Sousa Neto et al.20
Maximum Capacity
Two days after the familiarization procedure, each animal was evaluated to determine its maximum carrying load, which consisted of four to nine ladder climbs with progressively heavier loads. The initial climb was performed with 75% of the animal’s body mass. Upon successful completion of this load, an additional 30 g weight was added to the load apparatus. The highest load that the animal successfully carried for the entire length of the ladder was considered the maximal carrying capacity. Failure was determined when the animal could not progress up the ladder after three successive stimuli to the tail.
Exercise Sessions
Exercise was performed three times per week (on Mondays, Wednesdays, and Fridays) for 12 weeks. The length of the ladder permitted the animals to performed eight to 12 dynamic movements per climb. The climbs comprised the carrying of a progressive load of 65, 85, 95, and 100% of the maximum carrying capacity of each animal. RT sessions consisted of five to eight movements per climb over 6–8 seconds. If a rat reached 100% of its carrying capacity, an additional 30 g load was added until a new maximum carrying capacity was determined. The resting period between each climb was 2 minutes. Rats were manually stimulated to climb the ladder. No electrical shock was used to induce the climbing. The RT protocol was adapted from Hornberger and Farrar21 and procedures are described elsewhere.1,20,22,23
Bone Preparation
One at a time, 48 hours after the end of experimental period, the animals were killed using an intraperitoneal injection of xylazine solution (24 mg/kg of body weight) and ketamine (100 mg/kg of body weight). Such a period was determined to avoid the acute effects of the session of resistance exercise. The lumbar vertebra (L6) was immediately dissected, as well as the right diaphyseal femur and tibia from each posterior limb, and then stored at −80°C for zymography analyses. All bones were dissected by an experienced researcher in order to prevent cross-contamination with other tissues, such as tendons, periosteum, and bone marrow.
Gelatin Zymography (MMP-2 Activity)
The techniques for bone MMP-2 activity determination were performed according to the protocol established by Marqueti et al1 and Sousa Neto et al.22 The different bones samples were macerated in liquid nitrogen using a mortar and pestle. To attain complete tissue homogenization, samples were homogenized posteriorly in a tube containing 10 mM cacodylic acid, 1 µM NaCl, 20 mM CaCl2, 1.5 mM NaN3, 0.01% Triton X-100% ZnCl 1 µM with five stainless steel balls (diameter 2.3 mm) (BioSpec Products, Bartlesville, OK, USA) and three silicon carbide sharp particles (1 mm) (BioSpec Products) by being shaken in a FastPrep-24 instrument (MP Biomedicals, Solon, OH, USA). Next, the solution was centrifuged for 20 minutes (13,000 g at 4°C) and the supernatant was reserved. A NanoDrop® spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE, USA) was used to quantify protein concentrations, and 30 µg of total protein was loaded into each lane. Each group was analyzed separately by the same individual and experienced researcher.
The samples were resolved by electrophoresis in polyacrylamide gel containing SDS 10% and gelatin (1 mg/mL). Then, the gels were washed twice in 2.5% Triton X-100 to remove SDS and incubated in substrate buffer (50 mmol/L Tris-HCl (pH 8.0), 5 mmol/L CaCl2, and 0.02% NaN3) at 37°C for 20 hours. The gels were stained with Coomassie Brilliant Blue R-250 (Bio-Rad) for 1.5 hours and destained with acetic acid, methanol, and water. The gelatinolytic activity was manifested as horizontal white bands on a blue background.
Samples of five animals from each experimental group were evaluated to guarantee the precision and linearity of the analysis, and each sample was normalized for the total amount of protein included (30 µg). MMP-2 activity was determined by densitometric scanning of the isoform bands (ImageScanner III, Lab-Scan 6.0). The averages of the band intensities were measured using Image Master 2D Platinum 7.0 software and were conducted by a blinded researcher, reducing possible bias related to this process. Pro, intermediate, and active isoforms bands were identified via standard techniques using molecular weight criteria. The bands found in all groups were 68–62 kDa, as proposed by previous studies.15,16
Statistical Analysis
The results were expressed as the mean ± standard deviation (SD). The Shapiro–Wilk test was applied to check for normality of variables and Levene’s test was used to analyze the homogeneity of variance. All variables presented normal distribution and homogeneity. A two-way independent ANOVA (with training and aging as factors) was used to compare body weight, relative load capacity, and MMP-2 activity in bones between groups. The one-way ANOVA test was used for comparisons between different bones. When a significant difference was detected, a Tukey post hoc test was applied to identify the difference. An alpha level of p≤0.05 was considered significant. The software GraphPad Prism 8.3 (San Diego, CA, USA) was used for statistical analysis and graphic design. The power of the sample size for activity was verified by post hoc tests using G*Power version 3.1.9 (Kiel University, Kiel, Germany) (supplementary Tables S1-S2 and supplementary Figure S1). Importantly, all outcomes revealed a power above 95%.
Results
Body Weight and Relative Load Capacity
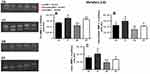
The initial body weight was higher in old rats compared to young animals (p=0.01). With respect to body weight after the training period, the young groups displayed a significant increase (p=0.01); however, old animals showed a significant decrease (p=0.01) (Figure 1A). Both trained groups presented increased final relative maximal load capacity (p=0.01); however, the relative load was greater in young rats than in older animals in the 12th week of training (p=0.01) (Figure 1B).
Effects of Aging and RT on MMP-2 Activity in Different Bones
Lumbar Vertebra
Supplementary Table S3 shows the p-values of factors (aging and exercise training) and interactions. There was a statistically significant interaction between training and age for pro and active MMP-2 activity in the lumbar vertebra (p=0.003 and p=0.02, respectively). All isoforms were modified by age status and exercise training, which showed a decrease in the OS compared to the YS group (p=0.001) (Figure 2A–C). The YT showed higher activity values compared to the YS group (p=0.01) (Figure 2A–C) for all isoforms. The pro-MMP-2 activity was higher in the OT group compared to the OS group (p=0.01) (Figure 2A). No MMP-9 activity was detected by zymography.
Femur
There was no difference between groups in pro, intermediate, and active MMP-2 in the femur (p>0.05) (Figure 3A and B). No MMP-9 activity was detected by zymography.
Tibia
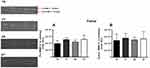
Regarding the tibia, training significantly increased the pro-MMP-2 activity in the YT compared to the YS group for all isoforms (p=0.01) (Figure 4A–C). The pro isoform was modified by age status, which showed a decrease in the OS group compared to the YS and YT groups (p=0.001). The OT group demonstrated a decrease compared to the YT group for pro and intermediate isoforms (p=0.001) (Figure 4A and B).
Finally, the active MMP-2 activity increased in the right tibia in both trained groups (YT and OT) compared to the sedentary groups (p=0.001 and p=0.04, respectively). Moreover, the OT group showed lower activity compared to the YT group (p=0.001) (Figure 4C). No MMP-9 activity was detected by zymography.
Comparison of MMP-2 Activity Between Distinct Bones
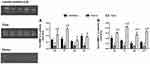
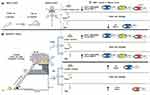
The pro-MMP-2 activity was lower in the femur compared to the lumbar vertebrae in all experimental groups (p=0.001). The young groups demonstrated higher activity values in the tibia compared to the other bones (p=0.001). Furthermore, older groups showed the highest activity values in the tibia compared to the femur (p=0.01) (Figure 5A). With respect to active MMP-2, young groups and the OT group showed lower activity in the femur compared to the lumbar vertebrae (p=0.01). All groups displayed higher activity values in the tibia compared to the other bones analyzed (p=0.001) (Figure 5B). A schematic representation of the MMP2 activity during the aging process and RT in different bones is presented in Figure 6.
Discussion
This study confirmed our hypothesis that the aging process impairs a key enzyme that regulates bone ECM. Downregulated MMP-2 activity in the lumbar vertebrae and tibia of old rats can result in dysfunctional remodeling and compromise bone structure, leading to weakness. RT upregulated MMP-2 activity in the lumbar vertebrae and tibia during aging, but not in the femur, indicating that comprehensive assessment of bone structure may be required to improve our understanding of bone health and clinical implications. Different MMP-2 responses to RT may result in distinctive remodeling processes. RT has the potential to positively affect bone remodeling and this approach presents an innovation in the current knowledge on bone. Those findings regarding bone plasticity may have crucial implications for the treatment of osteoporosis.
Our findings demonstrated that RT increased relative load capacity, regardless of age, suggesting a beneficial adaptation to mitigate age-related osteosarcopenia. The rationale to use the RT model was based on previous studies that suggest that this modality results in osteogenesis and consequently has the ability to delay the onset of osteoporosis.24,25 This may occur as a result of the existing relationship between the bone mineral density and the level of muscular strength,26,27 the major physical quality reported in the present study. Importantly, muscle strength exerts a dominant effect over bone’s structural adaptation.27 In clinical studies, there is evidence that muscle strength can reduce the risk of vertebral fracture and the development of kyphosis in older women with osteoporosis,28 which shows the relevance of increasing MMP-2 activity in lumbar vertebrae. The young and old rats displayed different adaptations in relative load capacity in response to 12 weeks of RT, which may have contributed to lower active MMP-2 activity in the OT group compared to the YT group.
Age-related bone loss is caused by increases in resorptive activity and reduced bone formation. Accumulating evidence suggests that dysregulation of MMP activity is responsible for the pathological matrix found in many diseases associated with the aging process, such as osteoporosis and osteoarthritis.10,12 Specifically in bone tissue, it has been demonstrated that MMP-2 deficiency reduced tibia and femur length in adult mice.10 The novelty of this study was that the aging process downregulated the activity of pro and active MMP-2 in the tibia and lumbar vertebrae, respectively, which may have compromised bone integrity.18 An imbalance of MMP-2 leads to abnormal bone remodeling.12 Lower MMP-2 activity disrupts tissue repair and results in decreased bone mineralization and defects in osteoblast and osteoclast survival, which compromises optimal bone turnover.10
Elsewhere, Nyman et al17 evaluated the effects of MMP-2 knockout (Mmp2−/−) on architectural, compositional, and biomechanical properties in different bones (tibia, femur, and L5 vertebral body) of female mice at 16 weeks of age. Mmp2−/− did not affect stiffness, yield, or peak force. The lack of a difference in MMP-2 activity of the femur during aging may be associated with the preservation of mechanical properties. Additionally, these authors reported that a lower body weight could be associated with a reduction in the strength of the femur via an indirect bone functional adaptation.17 However, rats in the OS and OT groups had higher body weights than those in the YS and YT groups. These factors may explain the absence of difference in MMP-2 activity of the femur.
The MMPs are regulated at the transcriptional level, post-transcriptionally, as well as via activity through their inhibitors.29 This may suggest that other adjacent molecular pathways were involved in MMP-2 activity. One possibility is that RT also modulates tissue inhibitors of metalloproteinases (TIMPs), because TIMPs must be well managed to optimize damage bone repair following RT.30 It has been demonstrated that low TIMP-2 activity stimulates the activation of pro-MMP-2 in bone.31 The balance between MMP-2 and TIMP-2 is crucial for assuring bone quality, since MMP-2 depletion leads to bone loss, but TIMP-2 stimulates the bone-resorbing activity of the mature osteoclasts by protecting the cells from apoptosis.31 These mechanisms may clarify the distinct MMP-2 isoforms presented here.
An RT program leads to an increase in mechanical signals such as fluid flow dynamics, tension, and compression.15 These mechanical signals modulate MMP-2 activity, which may be one of the main reasons why exercise training attenuates the development of bone disorders.15 In the present study, RT upregulated MMP-2 in the lumbar vertebrae and tibia of older animals, representing a phenomenon of major clinical significance. Our results concur with previous studies indicating that exercise programs exert important regulatory roles in bone ECM remodeling. Souza et al16 and Shiguemoto et al15 reported that RT (12 weeks, three times per week) contributed to an increase in MMP-2 activity in tibia of ovariectomized rats, which prevented the deleterious effects of menopause. Gelatinase activity in bone is important for aging, since it is associated with biological pathways related to bone protection and damage recovery,18 besides playing a role in maintaining canalicular networks.32 Considering these molecular findings, we suggest that RT could be a relevant intervention capable of inducing significant effects on lumbar vertebrae and tibia, which is necessary for structural arrangement, mechanical function, and consequently cellular longevity.
On the other hand, a higher activity of MMP-2 in the trained animals may also be attributed to other factors. MMP-2 is expressed on both osteocytes and osteoblasts,33 but osteocyte density is reduced with aging,34 which is, at least partly, rescued by exercise training.35 This could explain the differences encountered between old and young animals. Also, it has been demonstrated that acute exercise induces osteoblast activation, which may have influenced the contrast between sedentary and trained animals over time.36
In a review, Chen et al28 demonstrated that trabecular bone mineral density of both women and men tended to decrease by around 30% in the first thoracic vertebra to the third lumbar vertebra with advancing age. Impairment in vertebral trabecular remodeling is significantly correlated with bone fracture.28 Most significantly, the current study suggests positive effects of RT on the pro-MMP-2 activity in the L6 vertebra during aging, likely as a protective effect. The local increase in MMP-2 activity in L6 is fundamental to assisting force development after mechanical loading and tissue homeostasis, which are considered substantial responses to bone adaptation.37 The present results are important because they suggest an additional mechanism to prevent the detrimental effects with advancing age and show novel contributions to the basic mechanisms related to the cellular aging process.
Surprisingly, no prominent adaptations in MMP-2 of the femur were observed in the trained groups. Although both femur and tibia are characterized as long bones, they may have different biomechanical components and radiological characteristics. Prodinger et al38 showed that the femur and humerus displayed higher stiffness compared with the tibia in male Wistar rats. A plausible explanation for this is that optimal bone remodeling is distinct between bones and a mechanical–molecular set point may exist for MMP-2 induction. Another possible explanation is that the femur is surrounded by a thick muscle, which demands a higher mechanical load for remodeling. Furthermore, the thin soft tissue mantle around the tibia facilitates responsiveness to mechanotransduction.38 Such an anatomical structural characteristic may have influenced the increased pro and active MMP-2 activity in the YT group, as well as higher activity values in the tibia compared to the other bones.
In addition, Hughes et al39 demonstrated a potential mechanism for limited osteogenic signal cellular accommodation, in which mechanosensitive cells adjust cytoskeletal stiffness in response to fluid flow and consequently bone mechanoadaptation processes, thereby inhibiting the release of important pro-osteoblastic signaling enzymes, such as MMP-2. It has been demonstrated that these adaptations may explain cellular saturation, in which bones become less mechanically sensitive with RT, and why a small number of loading cycles seems to have the same osteogenic potential as an exercise program.39,40 These previously proposed aspects may be linked to the absence MMP-2 modulation observed in the femur. Additionally, it is important to emphasize that the femur is a long and strong bone, and it usually needs a higher loading rate to be remodeled owing to its larger surface to volume ratio, besides the greater trabecular bone mass accompanied by lower cortical porosity,41 which may explain the lower MMP-2 activity compared to the other bones analyzed.
There have been no previous studies comparing MMP-2 activity in different bones. In the current study, the tibia showed greater MMP-2 activity compared to the other bones, regardless of age and RT, implying that remodeling is not the same in different bones concerning the same mechanical loading application and aging process. Galea et al42 investigated the transcriptomic changes associated with the aging process and mechanical loading on the tibia. The authors reported that the tibia is extremely responsive to regulation of gene expression related to primary metabolism, cell-matrix interactions, and cell cycle-independent aging and mechanical loading, which are essential for cellular functioning.42 Therefore, it is possible that these numerous biological pathways also modulate MMP-2 activity. Galea et al42 explained that high-impact exercises such as weighted stair climbing may be most promising at distal sites, such as bones closer to the ground, including the tibia.42
Finally, a mechanistic approach in the different bones could provide crucial clues for the development of therapeutic approaches to mitigate bone remodeling disorders, as well as mapping of the relevant biomarkers in physiological responses inherent to aging, and understanding of the complete picture of cellular adaptation in response to exercise in aged bone. Despite this, it is important to point out some limitations of the present study. It was not possible to assess biomechanical factors, which could add information about bone strength. Another limitation was the absence of histological analysis of the ECM. It should be noted that only the diaphyseal portion of the femur was considered. However, there is little evidence to illuminate the relationship between MMP-2 and bone regions. Future studies should investigate the ability of RT to modulate different bone regions, and we state this limitation here and look forward to the results of future similar studies.
Conclusion
In summary, RT promotes different levels of MMP-2 activity in the lumbar vertebra, tibia, and femur of young and older rats. RT induced higher MMP-2 activity in the lumbar vertebrae and tibia of older rats, while positive effects in the femur were limited. The present study showed that bone extracellular remodeling can be modulated by RT as an important component in adaptive responses during the aging process.
Data Sharing Statement
The de-identified data sets generated during the study are available from the corresponding author.
Ethics Approval
The present study was approved by the Animal Research Ethics Committee of the Federal University of Sao Carlos, SP, Brazil (protocol number: 044/2011). The research has been carried out in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, Washington DC, USA).
Acknowledgments
The authors are grateful for the financial support provided by Fundação de Apoio a Pesquisa do Distrito Federal (FAPDF) (grant numbers 193.001.452/2016; 193.000.653/ 2015, 00193.00000641/2018-45 and 00193.0000168/2019-87) and FAPESP (2013/00798-2 and 2011/11229-3), the Decanato de Pesquisa e Inovação e de Pós-Graduação (grant number DPI/DPG N.º 02/2020), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES Finance Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant numbers 445069/2014-7 and 312136/2018-8) from Brazil. We also thank Robin Hambly for reviewing the English.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Marqueti RC, Durigan JLQ, Oliveira AJS, et al. Effects of aging and resistance training in rat tendon remodeling. FASEB J. 2018;32(1):353–368. doi:10.1096/fj.201700543r
2. Ukon Y, Makino T, Kodama J, et al. Molecular-Based Treatment Strategies for Osteoporosis: A Literature Review. Int J Mol Sci. 2019;20(10):10. doi:10.3390/ijms20102557
3. Vashishth D. The role of the collagen matrix in skeletal fragility. Current Osteoporosis Reports. 2007;5(2):62–66. doi:10.1007/s11914-007-0004-2
4. Kular JK, Basu S, Sharma RI. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 2014;5:2041731414557112.
5. Haj J, Haj Khalil T, Falah M, Zussman E, Srouji S. An ECM-Mimicking, Mesenchymal Stem Cell-Embedded Hybrid Scaffold for Bone Regeneration. Biomed Res Int. 2017;2017:8591073.
6. Kok MO, Rodrigues A, Silva AP, de Haan S. The emergence and current performance of a health research system: lessons from Guinea Bissau. Health Res Policy Syst. 2012;10:5.
7. Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15(12):1334–1348.
8. Krane SM, Inada M. Matrix metalloproteinases and bone. Bone. 2008;43(1):7–18.
9. Freitas-Rodriguez S, Folgueras AR, Lopez-Otin C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 Pt A):2015–2025.
10. Paiva KBS, Granjeiro JM. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog Mol Biol Transl Sci. 2017;148:203–303.
11. Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi:10.1038/nrm2125
12. Paiva KBS, Granjeiro JM. Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch Biochem Biophys. 2014;561:74–87. doi:10.1016/j.abb.2014.07.034
13. Becerikli M, Jaurich H, Schira J, et al. Age-dependent alterations in osteoblast and osteoclast activity in human cancellous bone. J Cell Mol Med. 2017;21(11):2773–2781. doi:10.1111/jcmm.13192
14. Yuan Y, Chen X, Zhang L, et al. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog Biophys Mol Biol. 2016;122(2):122–130. doi:10.1016/j.pbiomolbio.2015.11.005
15. Shiguemoto GE, Prestes J, Leite RD, et al. Effects of resistance training on matrix metalloproteinase-2 activity and biomechanical and physical properties of bone in ovariectomized and intact rats. Scand J Med Sci Sports. 2012;22(5):607–617. doi:10.1111/j.1600-0838.2010.01284.x
16. Souza MVC, Lino ADS, Ruffoni LGD, et al. Resistance training and hormone replacement increase MMP-2 activity, quality and quantity of bone in ovariectomized rats. Motriz: Revista De Educação Física. 2017;23:4.
17. Nyman JS, Lynch CC, Perrien DS, et al. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J Bone Miner Res. 2011;26(6):1252–1260. doi:10.1002/jbmr.326
18. Liang HPH, Xu J, Xue M, Jackson C. Matrix metalloproteinases in bone development and pathology: current knowledge and potential clinical utility. <![CDATA[Metalloproteinases in Medicine]]>. 2016;3:93–102. doi:10.2147/MNM.S92187
19. Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21(6):775. doi:10.1016/j.nut.2005.04.002
20. de Sousa Neto IV, Tibana RA, da Cunha Nascimento D, et al. Effects of Resistance Training Volume on MMPs in Circulation, Muscle and Adipose Tissue. Int J Sports Med. 2017;38(4):307–313. doi:10.1055/s-0042-123192
21. Hornberger Jr. TA, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16–31. doi:10.1139/h04-002
22. de Sousa Neto IV, Durigan JLQ, Guzzoni V, et al. Effects of Resistance Training on Matrix Metalloproteinase Activity in Skeletal Muscles and Blood Circulation During Aging. Front Physiol. 2018;9:190. doi:10.3389/fphys.2018.00190
23. de Sousa Neto IV, Carvalho MM, Marqueti RC, et al. Proteomic changes in skeletal muscle of aged rats in response to resistance training. Cell Biochem Funct. 2020;2:12–68.
24. Hong AR, Kim SW. Effects of Resistance Exercise on Bone Health. Endocrinology and Metabolism. 2018;33(4):435–444. doi:10.3803/EnM.2018.33.4.435
25. Ikedo A, Kido K, Ato S, et al. The effects of resistance training on bone mineral density and bone quality in type 2 diabetic rats. Physiol Rep. 2019;7(6):e14046. doi:10.14814/phy2.14046
26. Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12(10):1547–1551. doi:10.1359/jbmr.1997.12.10.1547
27. Hart NH, Nimphius S, Rantalainen T, Ireland A, Siafarikas A, Newton RU. Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017;17(3):114–139.
28. Chen H, Zhou X, Fujita H, Onozuka M, Kubo K-Y. Age-related changes in trabecular and cortical bone microstructure. Int J Endocrinol. 2013;2013:213234. doi:10.1155/2013/213234
29. Chen J, Zhou J, Li F, et al. Expression of MMP-2 and TIMP-1 during rapid maxillary expansion in rats. Arch Oral Biol. 2017;76:30–35. doi:10.1016/j.archoralbio.2017.01.002
30. Lo Presti R, Hopps E, Caimi G. Gelatinases and physical exercise: A systematic review of evidence from human studies. Medicine. 2017;96(37):e8072. doi:10.1097/MD.0000000000008072
31. Sobue T, Hakeda Y, Kobayashi Y, et al. Tissue inhibitor of metalloproteinases 1 and 2 directly stimulate the bone-resorbing activity of isolated mature osteoclasts. J Bone Miner Res. 2001;16(12):2205–2214. doi:10.1359/jbmr.2001.16.12.2205
32. Wang I-NE, Mitroo S, Chen FH, Lu HH, Doty SB. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24(8):1745–1755. doi:10.1002/jor.20149
33. Hatori K, Sasano Y, Takahashi I, Kamakura S, Kagayama M, Sasaki K. Osteoblasts and osteocytes express MMP2 and −8 and TIMP1, −2, and −3 along with extracellular matrix molecules during appositional bone formation. Anat Rec a Discov Mol Cell Evol Biol. 2004;277(2):262–271.
34. Vashishth D, Verborgt O, Divine G, Schaffler MB, Fyhrie DP. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone. 2000;26(4):375–380.
35. Fonseca H, Moreira-Goncalves D, Esteves JL, et al. Voluntary exercise has long-term in vivo protective effects on osteocyte viability and bone strength following ovariectomy. Calcif Tissue Int. 2011;88(6):443–454.
36. Tong X, Chen X, Zhang S, et al. The Effect of Exercise on the Prevention of Osteoporosis and Bone Angiogenesis. Biomed Res Int. 2019;2019:8171897.
37. Kawamata H, Nakashiro K, Uchida D, Harada K, Yoshida H, Sato M. Possible contribution of active MMP2 to lymph-node metastasis and secreted cathepsin L to bone invasion of newly established human oral-squamous-cancer cell lines. Int J Cancer. 1997;70(1):120–127.
38. Prodinger PM, Foehr P, Burklein D, et al. Whole bone testing in small animals: systematic characterization of the mechanical properties of different rodent bones available for rat fracture models. Eur J Med Res. 2018;23(1):8.
39. Hughes JM, Charkoudian N, Barnes JN, Morgan BJ. Revisiting the Debate: does Exercise Build Strong Bones in the Mature and Senescent Skeleton? Front Physiol. 2016;7:369.
40. Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res. 1997;12(9):1480–1485.
41. Jimenez MJ, Balbin M, Alvarez J, et al. A regulatory cascade involving retinoic acid, Cbfa1, and matrix metalloproteinases is coupled to the development of a process of perichondrial invasion and osteogenic differentiation during bone formation. J Cell Biol. 2001;155(7):1333–1344.
42. Galea GL, Meakin LB, Harris MA, et al. Old age and the associated impairment of bones’ adaptation to loading are associated with transcriptomic changes in cellular metabolism, cell-matrix interactions and the cell cycle. Gene. 2017;599:36–52.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.