Back to Journals » Journal of Hepatocellular Carcinoma » Volume 7
Resection of NAFLD-Associated HCC: Patient Selection and Reported Outcomes
Authors Campani C , Bensi C, Milani S, Galli A , Tarocchi M
Received 2 May 2020
Accepted for publication 10 July 2020
Published 30 July 2020 Volume 2020:7 Pages 107—116
DOI https://doi.org/10.2147/JHC.S252506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Claudia Campani,* Carolina Bensi,* Stefano Milani, Andrea Galli, Mirko Tarocchi
Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence, Italy
*These authors contributed equally to this work
Correspondence: Mirko Tarocchi
Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Viale Morgagni 50, Florence, Italy
Tel +39 0552758115
Email [email protected]
Abstract: Global prevalence of non-alcoholic fatty liver disease (NAFLD) has been growing in the last decades, especially in western countries, due to increased prevalence of diabetes, obesity or other components of metabolic syndrome. NAFLD recently became an important cause of hepatocellular carcinoma (HCC), even in non-cirrhotic patients. Patients with HCC-NAFLD are usually older, with more morbidities (especially cardiovascular diseases and metabolic disorders) and have advanced disease at the diagnosis due to the absence of surveillance, which is considered not cost-effective in patients without advanced fibrosis/cirrhosis, given the large prevalence of NAFLD in the general population. For these reasons, patients with HCC-NAFLD unlikely underwent curative treatments, and have been reported to have lower overall survival (OS) compared to individuals with HCC related to other aetiologies. However, this difference is not confirmed by data of patient subgroups who received curative treatment. In our review, we selected studies published over the past 8 years that analyse characteristics and outcomes of HCC-NAFLD patients who underwent surgery with the aim of identifying features that could predict outcomes and potential selection criteria. All the studies confirm that patients with HCC-NAFLD are older, with many comorbidities and that HCC occurs frequently even in non-cirrhotic livers. There is no agreement about intraoperative and perioperative complications. Regarding outcomes, all papers agree that patients with HCC in NAFLD who undergo surgery have a better OS compared to other aetiologies. Summarizing, surgery is a good curative option for patients with HCC-NAFLD, perhaps even better than transplantation in terms of OS. In this group of patients, it seems to be essential to evaluate cardio-pulmonary and general operative risk, in addition to the normal risk assessment related to liver function to avoid an underestimation, especially for patients without severe underlying fibrosis.
Keywords: hepatocellular carcinoma, non-alcoholic fatty liver disease, surgery
HCC Related to NAFLD
Epidemiology
Non-alcoholic fatty liver disease (NAFLD) includes a large spectrum of features, ranging from simple reversible steatosis (non-alcoholic fatty liver, NAFL) to non-alcoholic steatohepatitis (NASH), with or without fibrosis.
NAFLD is becoming the most common cause of chronic liver disease, especially in developed countries, due to the increased prevalence of metabolic syndrome (MS), obesity and diabetes. The global prevalence of NAFLD is estimated to be approximately 25%1 with the highest prevalence in the Middle East (31%) and South America (30%), and the lowest in Africa (13%). Highest value is described in diabetic patients with rates of 60% to 70%2 and obesity with a rate of 95%.3
This disease is growing in relevance so that NAFLD is surpassing hepatitis C virus (HCV) chronic infection to become the leading indication for liver transplantation in the United States (US), including patients who underwent transplantation for hepatocellular carcinoma (HCC).4,5
During the progression of NAFLD to cirrhosis, the fibrosis stage seems to play a key role due to its direct correlation with liver-related mortality and all-causes mortality.6
Among the possible complication of this disease, there is also the development of HCC: this kind of tumor is the sixth most common cancer and the fourth most frequent cause of cancer-related death globally.7
HCC incidence and prevalence are growing due to population aging, population growth and increase of cause-specific rates, especially the increasing prevalence of NAFLD. In all countries, prevalent HCC cases related to NAFLD range from an increase of 47% in Japan to 130% in the US.8 In NAFLD, the reported HCC incidence is very heterogeneous, ranging from 0.25% to 7.6%. Furthermore, in a relevant proportion of patients, HCC develops in non-cirrhotic livers.9
In particular, HCC incidence in NAFLD-related cirrhosis is estimated in the literature to range between 1% and 3% per year,10 whereas few data are available for HCC in non-cirrhotic NAFLD patients. A study from the Veteran Administration reports incidence values around 13%.11
Risk Factors
Usually, patients with NAFLD-related HCC are older, with more morbidities, especially heart diseases and dysmetabolism.
The association with the development of HCC is described for the following factors (Table 1):
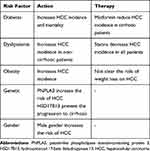 |
Table 1 NAFLD-HCC Risk Factors |
Diabetes
Multiple population-based case-control and cohort studies have confirmed a significant association between type 2 diabetes mellitus (T2DM) and incident HCC in NAFLD patients with and without cirrhosis.12 There is not only an association between T2DM and HCC incidence but also between T2DM and HCC mortality.13
Regarding diabetes therapy, a role in decreasing HCC incidence for metformin has been proved for patients with cirrhosis, whereas its role in NAFLD patients is still under evaluation.12
Dyslipidemia
While there is no clear association between dyslipidemia and HCC incidence, recent studies described a possible connection between dyslipidemia and HCC in non-cirrhotic patients with biopsy-proven NASH.14 Whereas statins do not improve hepatic steatosis, steatohepatitis or fibrosis in NASH patients, various studies demonstrated that statins decrease the risk of HCC.12
Obesity
Obesity is an important risk factor for NAFLD and NAFLD-associated HCC. It is not clear if weight loss reduces the risk of HCC in NAFLD patients, but it certainly improves NAFLD-related outcomes.12
Genetics
Patatin-like phospholipase domain-containing protein 3 (PNPLA3) is a risk allele associated with the progression of fibrosis in NAFLD patients and the development of HCC. Although the role of PNPLA3 has been demonstrated in various studies, it has not yet been introduced into clinical practice to identify patients with a higher risk of developing HCC.15
Unlike PNPLA3, hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) seems to have a role in preventing the progression to cirrhosis due to NAFLD16 and has a protective effect on HCC development in alcohol-related disease,17 so we can speculate that HSD17B13 might be protective also for HCC in NAFLD patients.
Sex
Male gender is a well-known risk factor both for NAFLD and HCC.9
Sarcopenia
Sarcopenia has been identified as a risk factor for NAFLD and it is often associated with significant liver fibrosis.18 Sarcopenia plays also an important role in developing HCC, regardless of the etiology, and an increase of HCC recurrence has been showed in sarcopenic patients after curative treatment.19,20 Based on these data, we can assume that sarcopenia is an important risk factor for HCC on NAFLD and so it must be evaluated in the preoperative assessment especially in these patients.
Pathogenesis
In NAFLD and Hepatitis B Virus (HBV) patients, HCC could arise in non-fibrotic patients. The mechanisms by which HCC develops have not yet been clarified but various observations have been reported in the literature (Figure 1).
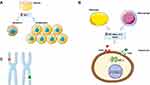 |
Figure 1 NAFLD-HCC pathogenesis. Notes: (A) Obesity is associated with an increase in circulating levels of IGF-I, a hormone that can inhibit apoptosis and stimulate cell division. (B) Obesity promotes the release of various cytokines in the blood including TNF alpha and IL6 which can activate STAT3 leading to cell proliferation. (C) Chromosomal alterations. This figure was made using Servier Medical Art (https://creativecommons.org/licenses/by/3.0/).Abbreviations: IGF-I, insulin-like growth factor 1; IL-6, interleukin 6; TNF alpha, tumour necrosis factor alpha; IL6R, interleukin 6 receptor; TNFR, tumour necrosis factor-alpha receptor; STAT3, Signal Transducer and Activator of Transcription 3. |
In mouse models, obesity promotes hepatocyte proliferation and reduces apoptosis regardless of the presence of fibrosis.21 Obesity promotes the release of various cytokines in the blood including leptin, a proinflammatory molecule which activates the Janus kinase (JAK) pathway that lead cells proliferation,22 in addition to tumour necrosis factor alpha (TNF alpha) and interleukin 6 (IL-6) which activate Signal Transducer and Activator of Transcription 3 (STAT3) promoting the same effect.23 Oncogenic pathways involved in cell proliferation are also activated by insulin-like growth factor (IGF) whose levels are increased in obese and NAFLD-patients due to systemic insulin resistance and compensatory hyper-insulinaemia.24
Moreover, lipid storage implicates the generation of reactive oxygen species (ROS) and saturated free fatty acids (FFAs) which could cause gene transcription alterations.25 Various genetic polymorphisms have been reported in HCC cases developed in NAFLD patients, especially PNLPA3 rs738409 C>G homozygosis polymorphism which is strongly associated with an increased risk of HCC, probably due to the role of PNLPA3 in retinol metabolism.26
Surveillance
The relationship between NAFLD-related cirrhosis and HCC is well known, and surveillance for HCC in this group is generally considered cost-effective.9,12,27
In contrast, the role of surveillance in NAFLD without cirrhosis is unclear, and limited data are available to establish which patients with non-cirrhotic-NAFLD should undergo HCC surveillance.9,12
The incidence of HCC in patients without advanced fibrosis/cirrhosis is insufficiently high to justify universal surveillance, given the large prevalence of NAFLD in the general population.9
For this reason, it is very important to identify high-risk patients with NAFLD who should undergo surveillance.9,28
As reported above, some factors such as diabetes mellitus, older age and concurrent alcohol intake are risk factors for advanced fibrosis/cirrhosis and HCC.29,30
In clinical practice, we cannot use liver biopsy as a primary staging method to identify patients with NAFLD progressive phenotype, due to the large prevalence of NAFLD.31
Non-invasive tests and scores are available to evaluate the presence and degree of liver fibrosis to stratify NAFLD patients. There are three classes of non-invasive tests for fibrosis: point of care tests, specialized blood tests, and imaging-based tests. AGA guidelines recommend combining at least two different non-invasive testing modalities. Individuals in whom both tests are concordant for advanced fibrosis or cirrhosis should be considered for HCC screening.12
Although as reported above a whole body of evidence demonstrates the role of genetic mutations in predisposing development of HCC, eg PNPLA3 polymorphism,26 there are not enough data to justify their clinical use in screening for HCC in patients with NAFLD.
Although there is evidence of a higher risk of developing HCC for those with NAFLD also in earlier stages compared to people without NAFLD, the incidence rates and determinants of risk have not been well computed and are probably too low to justify screening.15,32
Ultrasound is the method of choice for HCC surveillance in patients with a good acoustic window, but in overweight or obese patients (often affected by NAFLD), ultrasound quality could be inadequate.33,34
In these cases, we should consider other radiologic techniques such as CT scan or MR.35
In conclusion, according to principal international guidelines, patients with NAFLD and severe fibrosis or cirrhosis should undergo surveillance, whereas for patients without or with an early stage of fibrosis there is too little evidence to perform surveillance.9
HCC Resection
According to EASL guidelines, patients belonging to very early stage (single tumour <2 cm in diameter without vascular invasion/satellites, good performance status Eastern Cooperative Oncology Group [ECOG] 0 and well-preserved liver function classifiable as Child-Pugh [CTP] A class) and to early-stage (single tumours >2 cm or three nodules <3c in diameter, ECOG 0 and preserved liver function) could take advantages from surgery together with patients belonging to different stages who underwent effective downstaging treatment.9
Resection is one of the curative treatments proposed for HCC patients with liver transplantation and local ablation. Transplantation is the best treatment for patients with HCC as it allows to remove other undiagnosed intrahepatic tumor sites and to resolve liver alterations that could lead to the onset of new HCC.
Although recurrences are greater in surgery patients than in transplant patients, overall survival is similar.36 For this reason and given the lack of donors, transplantation is preferably reserved for patients who have no other therapeutic options.
Patients who underwent hepatic resection show a perioperative mortality under 5% and a post-hepatectomy liver failure (PHLF) of 10–12% that increases significantly the mortality after the first postoperative year.36
Selection of patients who undergo surgery involves the analysis of three groups of variables: preoperative liver function, portal hypertension and the extent of resection.
Preoperative Liver Function
Evaluation of preoperative liver function is essential to guarantee a low incidence of PHLF and mortality. The CTP score, proposed in 1973 to assess the severity of cirrhosis in patients with bleeding esophageal varices,37 is the first and the most used classification to define liver function. Patients who undergo surgery usually belong to CTP A class; CTP B patients are not excluded from surgery, but they have a poorer prognosis and must be carefully selected.
In 2014, Johnson et al proposed a new score, the ALBI (albumin-bilirubin) grade, to overcome the limitations of CTP like the interrelation of some parameters (eg ascites and albumin level), the subjectivity in grading ascites and encephalopathy and the fact that HCC could arise on non-cirrhotic liver.38 Pinato, in 2017, demonstrated that ALBI has an overall better discriminatory ability in predicting Overall Survival (OS) compared to CTP class in the early stage HCC39 and Wang et al demonstrated that ALBI grade predicted PHLF and overall survival in patients with HCC undergoing liver resection with curative intent more accurately than the CTP score.40
Model for end-stage liver disease (MELD) is another score used to evaluate liver function, and it is used specifically to refer patients to transplant centers: MELD >9 is associated with a perioperative mortality of 15.3%, a morbidity of 42% and a three-year survival of 30%.41
Asian guidelines recommend indocyanine green kinetics (ICG) to assess liver function and predict the surgical risk.42
ICG is an organic dye that is taken up by the hepatocytes and excreted into the bile without been metabolized and undergoing enterohepatic circulation: the clearance of ICG from systemic circulation is a simple measure of hepatic blood flow and function. The cut-offs reported for ICG are below 20–25% and 30–35% for resection and segmentectomy, respectively.9
In 2011, Cescon et al demonstrated that fibroscan was able to distinguish patients with a higher probability of PHFL reporting a cut off value of 15.7 Kpa.43 Other scores have been proposed to evaluate PHLF but they are not validated in the clinical practice (Table 2).
 |
Table 2 Score Proposed to Evaluate PHLF |
Non-invasive fibrosis scores widely used in NAFLD patients to stage and to identify who have to undergo surveillance for HCC could also play a role in predicting PHFL. Zhou in his paper compared the role of FIB-4 to CPT score in predicting PHLF demonstrating that the first one may be a better predictor.44
Extent of Hepatectomy and Surgical Invasiveness
Hepatic resections include wedge resection, segmental resection, hepatectomy and extended hepatectomy. For patients with HCC, the type of hepatic resection depends upon the number and localization of the lesions, the presence or absence of cirrhosis and the need to save an adequate volume of future liver remnant (FLR). Anatomic resection is preferred to remove either HCC and liver parenchyma where new HCC could arise, especially in cirrhotic patients. Laparoscopic liver resection seems to be superior to laparotomy liver resection in terms of intraoperative blood loss, blood transfusion rate, hospital stay in days, 30-day mortality and morbidity, even if randomized controlled trials are needed to identify the superiority of either strategy.45 Nowadays FLR can be evaluated through a “virtual hepatectomy”.46 Various software programs have been proposed to recreate the 3D liver anatomy to evaluate the liver vascularization and choose the best surgical strategy.
Portal vein embolization (PVE) is performed to promote the hypertrophy of the contralateral lobe in patients with chronic liver disease who should undergo a right hepatectomy in order to increase the amount of the FLR (10–46% within 4–8 weeks).47
Recently, associating liver partition and portal vein ligation for staged hepatectomy (AALPS) has been proposed to reduce the time in which the FLR increases and to expand the percentage of growth, especially for patients where PVE would be insufficient.48
Portal Hypertension Evaluation
Portal hypertension (PH) is an important prognostic factor for patients with cirrhosis and HCC. Although there is no total agreement about the role of PH in predicting post-surgical outcomes, the presence of clinically significant portal hypertension (>10 mmHg) is an important factor in the selection of patients for surgery. An important change is reported in the latest EASL guidelines on the management of HCC. In fact, surgery is not discarded a priori in case of portal hypertension but is allowed if a minor hepatectomy is possible and liver function is classified as MELD <9, even if there is a significant risk of liver decompensation and liver-related mortality (9%).36
Surgery in HCC Related to NAFLD
In the past two decades, the prevalence of MS rose from 25% to 33% and contributed to a rise in NAFLD-related HCC in the US. Patients with NAFLD-HCC have been reported to have lower OS compared with individuals with other HCC related aetiologies,49,50 although this difference is not confirmed in patients who received curative treatment.
In addition, it is difficult for patients with HCC-NAFLD to undergo curative treatments because they are usually older, have greater comorbidities such as cardiovascular diseases; furthermore, at the diagnosis, the tumor is large in size (>5 cm) or metastatic because these patients do not undergo surveillance.51
There are not many studies analysing the selection and outcomes of HCC-NAFLD patients who undergo surgery. In the following paragraphs, we show a selection of papers about this topic published over the past 8 years focusing on the outcomes of these patients and identifying potential selection criteria (Table 3).
 |
Table 3 NAFLD-HCC: Surgery and Outcomes |
Wong et al evaluated the survival after curative treatment for patients with HCC with or without NAFLD.51 NAFLD-HCC patients enrolled had a significantly higher proportion of females (33%), older patients, history of cardiac disease (49%), history of ischaemic stroke (10.2%) and a lower proportion of cirrhosis (43.6%) and decompensated liver disease (31.4%) compared to patients with HCC related to other aetiologies. Regarding tumor features, 57% of HCC was more than 5 cm for the NAFLD-HCC patients vs 32% for HCV, 44% for HBV and 42.1% for alcoholic liver disease (ALD) patients. Moreover, NAFLD-HCC patients had a higher proportion of well-differentiated and moderately differentiated tumors compared to HCV-HCC. Finally, OS patients with NAFLD-HCC had better survival after resection compared to other HCC related to other aetiologies. Pais et al retrospectively included in their study 323 patients with HCC who underwent liver resection in a period between 1995 and 2014 from two tertiary Parisian centers. Twelve percent of the entire cohort had HCC-NAFLD.52 Interestingly, the prevalence of NAFLD-HCC increased from 2.6% in the period 1995–1999 to 19.5% in the period 2010–2014 (p= 0.003) and paralleled the growth in the prevalence of metabolic risk factors.
Similarly to other studies, patients with NAFLD-HCC were older (mean age in years: 70 for NAFLD, 61 for chronic hepatitis C, 51 for chronic hepatitis B and 64 for ALD, p < 0.001), had a higher BMI, have larger tumor size and with a high prevalence of moderately differentiated tumors from a histologic point of view. The paper underlines that a greater percentage of patients belonging to the NAFLD-HCC group (49%) underwent a greater hepatectomy (25% ALD, 31% HCV and 48% HBV; p<0.02). In addition, patients belonging to NAFLD-HCC had a lower mortality rate after surgery (36% NAFLD, 48% ALD, 45% HCV and 36% HBV; p=0.601) whereas the rate of recurrence did not significantly differ from other groups (50% NAFLD, 50% ALD, 45% HCV and 60% HBV; p = 0.363). Regarding strictly the patients belonging to the NAFLD-HCC group, no difference between patients without significant fibrosis (F0-F2) and significant fibrosis/cirrhosis (F-F4) has been demonstrated except for the presence of a greater probability of having a single nodule in patients with F0-F2 (95% vs 54%, p< 0.01). A propensity towards a higher frequency of microvascular invasion was observed in non-cirrhotic vs cirrhotic NAFLD (52% vs 33%).
In a more recent paper, Koh et al53 compare perioperative and long-term outcomes after liver resection for NAFLD-related and non-NAFLD-related HCC, analysing retrospectively data from a prospectively collected database of all patients who underwent liver resection for HCC in two hospitals in Singapore between 2000 and 2015 (152 vs 996). Similarly to the two previous papers reported this study showed that patients with NAFLD HCC were older (p < 0.0001) and had more common comorbidities such as diabetes mellitus (p < 0.0001), hypertension (p < 0.0001), hyperlipidemia (p < 0.0001), ischemic heart disease (p<0.0092), and congestive cardiac failure (p < 0.0001). Patients with non-NAFLD had larger median tumor size (40 mm vs 7 mm; p < 0.0001) and more frequently liver cirrhosis (p < 0.0001). Patients with NAFLD-HCC had more common intraoperative and perioperative complications such as liver failure of all grades, cardiorespiratory complications, pulmonary embolism and blood loss also requiring intraoperative transfusions. Consequently, total hospitalization was longer in the NAFLD group (p < 0.0001). Despite this, the long-term survival outcomes are favourable compared with non-NAFLD etiologies, with 5-year OS rates of 70.1% vs 60.9% (p< 0.0355). These results suggest that patients with NAFLD HCC eligible for surgical treatment would have optimal outcomes, so, surgical options should be considered preferentially, whenever possible. The authors underline that PHFL in NAFLD HCC group should be related to the underlying hepatocellular dysfunction due to steatosis and the pro-inflammatory status.
Cauchy et al54 retrieved retrospectively data for patients with metabolic syndrome (but not specifically NAFLD) as a unique risk factor for HCC from a prospectively collected database of all patients who underwent liver resection for HCC between 2000 and 2011 (Beaujon Hospital, Clichy, France). Metabolic syndrome was a unique risk factor in 11.1% of patients (61% with abnormal underlying liver and 39% with normal liver). The median number of metabolic syndrome factors was 3. The proportion of MS-HCC increased continuously during the study, from 2.5% in the early part of the study to more than 15% of all resected HCCs in more recent years. Ninety-four percent of patients were male and elderly patients (44% aged 70 years or more). In this group, 58% of HCC was diagnosed incidentally, during follow-up of diabetes or investigation for abnormal liver function tests. Patients with normal underlying liver had larger tumours and more frequently associated with satellite nodules and microvascular invasion, probably as a consequence of the delayed diagnosis in the absence of specific symptoms.
Patients with abnormal underlying liver (including patients with severe underlying fibrosis and patients with stage F0–F2 fibrosis and a NAS of 2 or more, with no significant difference between these two groups) had more frequent postoperative complications (p=0.010), compared with patients with normal underlying liver and had increased rates of mortality (p =0.026).
Finally, patients with MS-HCC showed a good long-term prognosis: both 1- and 3-year overall and disease-free survival rates compared positively with data reported recently on resection of HCC in chronic liver disease of other aetiology.
In 2018, Yang et al were the first to compare the curative liver resection for NAFLD-HCC versus HBV-HCC in a large multicenter study.55 Patients were divided into two groups: the NAFLD group (patients with MS, history or evidence of fatty liver at the ultrasound and an alcohol intake minor than 30g/day) and the HBV group (patients with histopathological features or positive serology of HbsAg). The authors observed that patients with NAFLD-HCC were generally older, more often had diabetes mellitus, dyslipidemia, higher BMI values and a major proportion of female patients. Regarding tumor features, NAFLD-HCC patients had a large tumor size (7.2 vs 6.2, p <0.05) and less poor tumor differentiation (72.9% vs 82.4%, p<0.05) at HCC diagnosis; most patient had no evidence of cirrhosis (69.8% vs 27.5%). According to this study, no differences in terms of intraoperative blood loss, incidence of intraoperative blood transfusion, major liver resection, anatomical resection, perioperative mortality (according to Clavien-Dindo classification) and morbidity (postoperative hepatic failure, biliary complications, sepsis of any aetiology, pulmonary, renal, cardiac and wound complications) were found. HCC-NAFLD patients had similar perioperative outcomes as long-term OS (more than 50%) and recurrence free survival (RFS) (40%) compared to HBV-HCC patients.
Reddy et al56 conducted a retrospective study comparing HCC-NASH patients and HCC-HCV and/or ALD patients. Patients belonging to the first group were older, more often female, had larger BMI and more often components of metabolic syndrome in addition to less-severe background liver disease at HCC diagnosis with the evidence of less bridging fibrosis or cirrhosis at histopathologic evaluation. According to this study, NASH patients with HCC have better OS after curative treatment compared to corresponding patients with HCV and/or ALD, with discrepancies in OS not correlated to differences in postoperative mortality.
Wakai et al57 compared the surgical outcomes of three distinct groups: NAFLD-HCC patients (n=17), HCV-HCC patients (n = 147), and HBV-HCC patients (n = 61). They demonstrated that patients belonging to the NAFLD-HCC group had less recurrences than patients belonging to HCV-HCC and HBV-HCC groups.
Liang et al58 in their study evaluated retrospectively surgical outcomes in patients with T2DM who underwent curative hepatectomy for HCC, comparing patients with T2DM and/or NASH-related HCC and patients with T2DM and viral or alcoholic hepatitis (VAH)-related HCC. The median tumor size was significantly larger in the DM and/or NASH-related HCC group than in the T2DM and VAH-related HCC group. Cirrhosis was significantly more frequent in the DM and VAH-related HCC group than in the T2DM and/or NASH-related HCC group whereas 5-year OS and RFS rates were significantly higher in the T2DM and/or NASH-related HCC group.
Conclusion
Summarizing all the studies above, it is clear that patients with HCC-NAFLD are usually older and with many comorbidities such as diabetes, overweight/obesity, dyslipidemia and cardiovascular diseases. Although male is the predominant gender in this group of patients, Wang and Yang observed a greater proportion of women within their NAFLD-HCC subgroup.51,55
Regarding the tumor features, all the studies agree that HCC occurs frequently even in the absence of cirrhosis or decompensated liver disease and that at the diagnosis, the tumor is generally larger in size, with the exception of Koh’s paper53 which shows a smaller median size for NAFLD-HCC compared to other aetiologies.
There is no agreement on the degree of differentiation of the neoplasm, as Wang et al and Pais et al40,52 report well or moderately differentiated tumors for NAFLD-HCC while Yang55 describes a lower degree of differentiation.
Differences in terms of vascular invasion based on different aetiologies are not described in the various studies; however, Pais et al52 and Cauchy et al54 describe a higher percentage of microvascular invasion for patients with non-cirrhotic HCC-NAFLD compared to HCC-NAFLD patients with advanced liver disease.
Regarding intra and perioperative complications, there is no agreement between the various studies. Koh et al53 describe a greater number of intraoperative and perioperative complications for resected HCC in NAFLD patients, whereas Yang et al55 do not observe differences between HCC in NAFLD or in HBV subgroups who undergo surgery. Furthermore, Cauchy et al54 do not observe differences in terms of percentage of intra and perioperative complications among patients with HCC in NAFLD without and with advanced fibrosis even though the latter seem to have a higher rate of postoperative complications.
In terms of outcomes, all studies agree that patients with HCC in NAFLD who undergo surgery have a better OS compared to other aetiologies. Additionally, Wong et al51 report a better outcome after surgery than after transplantation for HCC-NAFLD patients. There is not a greater recurrence of HCC in the group of patients with NAFLD; indeed, the previous papers describe a lesser or equal recurrence rate than other aetiologies.
In conclusion, surgery for patients with HCC-NAFLD is a good therapeutic option, even better than transplantation in this group of patients in terms of OS. Due to the advanced age and the numerous comorbidities of the patients, in addition to the normal risk assessment related to the degree of liver disease and liver function, anaesthesiologic and general operative risk stratification (mainly for cardio-pulmonary risk), is extremely important to avoid an underestimation, especially for patients without severe underlying fibrosis.
Author Contributions
CC and CB contributed equally to this work. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
2. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
3. Sasaki A, Nitta H, Otsuka K, et al. Bariatric surgery and non-alcoholic Fatty liver disease: current and potential future treatments. Front Endocrinol (Lausanne). 2014;5:164. doi:10.3389/fendo.2014.00164
4. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. doi:10.1053/j.gastro.2014.11.039
5. Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755.e743. doi:10.1016/j.cgh.2018.05.057
6. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi:10.1002/hep.29085
7. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
8. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi:10.1016/j.jhep.2018.05.036
9. [email protected] EAftSotLEa, Liver EAftSot. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
10. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
11. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124–131.e121. doi:10.1016/j.cgh.2015.07.019
12. Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158(6):1822–1830. doi:10.1053/j.gastro.2019.12.053
13. El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4(3):369–380. doi:10.1016/j.cgh.2005.12.007
14. Phan J, Ng V, Sheinbaum A, et al. Hyperlipidemia and nonalcoholic steatohepatitis predispose to hepatocellular carcinoma development without cirrhosis. J Clin Gastroenterol. 2019;53(4):309–313. doi:10.1097/MCG.0000000000001062
15. Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109(3):325–334. doi:10.1038/ajg.2013.476
16. Abul-Husn NS, Cheng X, Li AH, et al. A protein-truncating HSD17B13 variant and protection from chronic liver DISEASE. N Engl J Med. 2018;378(12):1096–1106. doi:10.1056/NEJMoa1712191
17. Yang J, Trépo E, Nahon P, et al. A 17-beta-hydroxysteroid dehydrogenase 13 variant protects from hepatocellular carcinoma development in alcoholic liver disease. Hepatology. 2019;70(1):231–240. doi:10.1002/hep.30623
18. Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008–2011). Hepatology. 2016;63(3):776–786. doi:10.1002/hep.28376
19. Feng Z, Zhao H, Jiang Y, et al. Sarcopenia associates with increased risk of hepatocellular carcinoma among male patients with cirrhosis. Clin Nutr. 2020. doi:10.1016/j.clnu.2020.01.021
20. Kamachi S, Mizuta T, Otsuka T, et al. Sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment. Hepatol Res. 2016;46(2):201–208. doi:10.1111/hepr.12562
21. Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition? Cancer Res. 2001;61(13):5016–5023.
22. Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67(6):2497–2507. doi:10.1158/0008-5472.CAN-06-3075
23. Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi:10.1016/j.cell.2009.12.052
24. Chettouh H, Lequoy M, Fartoux L, Vigouroux C, Desbois-Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int. 2015;35(10):2203–2217. doi:10.1111/liv.12903
25. Vinciguerra M, Carrozzino F, Peyrou M, et al. Unsaturated fatty acids promote hepatoma proliferation and progression through downregulation of the tumor suppressor PTEN. J Hepatol. 2009;50(6):1132–1141. doi:10.1016/j.jhep.2009.01.027
26. Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81. doi:10.1016/j.jhep.2014.02.030
27. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367
28. Kolly P, Dufour JF. Surveillance for hepatocellular carcinoma in patients with NASH. Diagnostics (Basel). 2016;6:2.
29. Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol. 2014;20(36):12945–12955. doi:10.3748/wjg.v20.i36.12945
30. Ajmera VH, Terrault NA, Harrison SA. Is moderate alcohol use in nonalcoholic fatty liver disease good or bad? A critical review. Hepatology. 2017;65(6):2090–2099. doi:10.1002/hep.29055
31. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281.e1264. doi:10.1053/j.gastro.2018.12.036
32. Salameh H, Raff E, Erwin A, et al. PNPLA3 gene polymorphism is associated with predisposition to and severity of alcoholic liver disease. Am J Gastroenterol. 2015;110(6):846–856. doi:10.1038/ajg.2015.137
33. Del Poggio P, Olmi S, Ciccarese F, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(11):1927–1933.e1922. doi:10.1016/j.cgh.2014.02.025
34. Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–177. doi:10.1111/apt.13841
35. Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6(12):1418–1424. doi:10.1016/j.cgh.2008.08.005
36. Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020;72(2):262–276. doi:10.1016/j.jhep.2019.11.017
37. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi:10.1002/bjs.1800600817
38. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
39. Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–346. doi:10.1016/j.jhep.2016.09.008
40. Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. doi:10.1002/bjs.10095
41. Delis SG, Bakoyiannis A, Dervenis C, Tassopoulos N. Perioperative risk assessment for hepatocellular carcinoma by using the MELD score. J Gastrointest Surg. 2009;13(12):2268–2275. doi:10.1007/s11605-009-0977-5
42. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370.
43. Cescon M, Colecchia A, Cucchetti A, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256(5):
44. Zhou P, Chen B, Miao XY, et al. Comparison of FIB-4 index and child-pugh score in predicting the outcome of hepatic resection for hepatocellular carcinoma. J Gastrointest Surg. 2020;24(4):823–831.
45. Xiangfei M, Yinzhe X, Yingwei P, Shichun L, Weidong D. Open versus laparoscopic hepatic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc. 2019;33(8):2396–2418. doi:10.1007/s00464-019-06781-3
46. Mise Y, Hasegawa K, Satou S, et al. How has virtual hepatectomy changed the practice of liver surgery?: experience of 1194 virtual hepatectomy before liver resection and living donor liver transplantation. Ann Surg. 2018;268(1):127–133. doi:10.1097/SLA.0000000000002213
47. Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009;197(5):686–690. doi:10.1016/j.amjsurg.2008.04.022
48. Chan A, Zhang WY, Chok K, et al. ALPPS versus portal vein embolization for hepatitis-related hepatocellular carcinoma: a changing paradigm in modulation of future liver remnant before major hepatectomy. Ann Surg. 2019.
49. Weinmann A, Alt Y, Koch S, et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. doi:10.1186/s12885-015-1197-x
50. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730. doi:10.1002/hep.28123
51. Wong CR, Njei B, Nguyen MH, Nguyen A, Lim JK. Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs without non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;46(11–12):1061–1069. doi:10.1111/apt.14342
52. Pais R, Fartoux L, Goumard C, et al. Temporal trends, clinical patterns and outcomes of NAFLD-related HCC in patients undergoing liver resection over a 20-year period. Aliment Pharmacol Ther. 2017;46(9):856–863. doi:10.1111/apt.14261
53. Koh YX, Tan HJ, Liew YX, et al. Liver resection for nonalcoholic fatty liver disease-associated hepatocellular carcinoma. J Am Coll Surg. 2019;229(5):467–478.e461. doi:10.1016/j.jamcollsurg.2019.07.012
54. Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100(1):113–121. doi:10.1002/bjs.8963
55. Yang T, Hu LY, Li ZL, et al. Liver resection for hepatocellular carcinoma in non-alcoholic fatty liver disease: a multicenter propensity matching analysis with HBV-HCC. J Gastrointest Surg. 2020;24(2):320–329. doi:10.1007/s11605-018-04071-2
56. Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55(6):1809–1819. doi:10.1002/hep.25536
57. Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15(8):1450–1458. doi:10.1007/s11605-011-1540-8
58. Liang J, Ariizumi SI, Nakano M, Yamamoto M. Diabetes mellitus and/or nonalcoholic steatohepatitis-related hepatocellular carcinoma showed favorable surgical outcomes after hepatectomy. Anticancer Res. 2019;39(10):5639–5643. doi:10.21873/anticanres.13760
59. Okuda Y, Taura K, Ikeno Y. Usefulness of Mac-2 binding protein glycosylation isomer for prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma. Ann Surg. 2017;265:1201–1208. doi:10.1097/SLA.0000000000001836
60. Kubo S, Tsukamoto T, Hirohashi K. Correlation between preoperative serum concentration of Type IV Collagen 7s domain and hepatic failure following resection of hepatocellular carcinoma. Ann Surg. 2004;239:186–193. doi:10.1097/01.sla.0000109152.48425.4d
61. Donadon M, Costa G, Cimino M. Safe hepatectomy selection criteria for hepatocellular carcinoma patients: a validation of 336 consecutive hepatectomies. The BILCHE Score World J Surg. 2015;39:237–243. doi:10.1007/s00268-014-2786-6
62. Dong J, Zhang X, Zhu Y. The value of the combination of fibrosis index based on the four factors and future liver remnant volume ratios as a predictor on posthepatectomy outcomes. J Gastrointest Surg. 2015;19(4):682–691. doi:10.1007/s11605-014-2727-6
63. Zhou P, Chen B, Miao XY. Comparison of FIB-4 index and child-pugh score in predicting the outcome of hepatic resection for hepatocellular carcinoma. J Gastrointest Surg. 2020;24(4):823–831.
64. Ichikawa T, Uenishi T, Takemura S. A simple, noninvasively determined index predicting hepatic failure following liver resection for Hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:42–48. doi:10.1007/s00534-008-0003-4
65. Hsu H, Yu M, Lee C. RAM score is an effective predictor for early mortality and recurrence after hepatectomy for Hepatocellular carcinoma. BMC Cancer. 2017;17:742. doi:10.1186/s12885-017-3748-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
