Back to Journals » Cancer Management and Research » Volume 14
Research Progress on Radiotherapy Combined with Immunotherapy for Associated Pneumonitis During Treatment of Non-Small Cell Lung Cancer
Authors Zhang A , Yang F, Gao L, Shi X, Yang J
Received 20 May 2022
Accepted for publication 7 August 2022
Published 13 August 2022 Volume 2022:14 Pages 2469—2483
DOI https://doi.org/10.2147/CMAR.S374648
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Anqi Zhang,1,* Fuyuan Yang,2,* Lei Gao,1,* Xiaoyan Shi,3 Jiyuan Yang1
1Department of Oncology, First Affiliated Hospital of Yangtze University, Jingzhou, People’s Republic of China; 2School of Basic Medicine, Health Science Center, Yangtze University, Jingzhou, People’s Republic of China; 3Department of Gynaecology and Obstetrics, First Affiliated Hospital of Yangtze University, Jingzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiyuan Yang, Department of Oncology, First Affiliated Hospital of Yangtze University, Jingzhou, People’s Republic of China, Tel +86 189 7216 1658, Fax +86 716-8062633, Email [email protected]
Abstract: Radiation pneumonitis is a common and serious complication of radiotherapy for thoracic tumours. Although radiotherapy technology is constantly improving, the incidence of radiation pneumonitis is still not low, and severe cases can be life-threatening. Once radiation pneumonitis develops into radiation fibrosis (RF), it will have irreversible consequences, so it is particularly important to prevent the occurrence and development of radiation pneumonitis. Immune checkpoint inhibitors (ICIs) have rapidly altered the treatment landscape for multiple tumour types, providing unprecedented survival in some patients, especially for the treatment of non-small cell lung cancer (NSCLC). However, in addition to its remarkable curative effect, ICls may cause immune-related adverse events. The incidence of checkpoint inhibitor pneumonitis (CIP) is 3% to 5%, and its mortality rate is 10% to 17%. In addition, the incidence of CIP in NSCLC is higher than in other tumour types, reaching 7%– 13%. With the increasing use of immune checkpoint inhibitors (ICls) and thoracic radiotherapy in the treatment of patients with NSCLC, ICIs may induce delayed radiation pneumonitis in patients previously treated with radiation therapy, or radiation activation of the systemic immune system increases the toxicity of adverse reactions, which may lead to increased pulmonary toxicity and the incidence of pneumonitis. In this paper, the data about the occurrence of radiation pneumonitis, immune pneumonitis, and combined treatment and the latest related research results will be reviewed.
Keywords: radiation pneumonitis, immune pneumonitis, pneumonitis after combination therapy, treatment and management of pneumonitis
Introduction
Lung cancer still has the highest mortality rate among all cancers and is the second most common malignant tumour in the world.1,114 Approximately 85% of lung cancers are non-small cell lung cancer (NSCLC), and nearly 50% of the patients have distant metastasis at the time of diagnosis, so the overall prognosis is not ideal.2,3 In recent years, immune checkpoint inhibitors (ICISs) have become the treatment of choice for advanced recurrent or metastatic cancer.4,5 At present, the most widely used drugs are cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed death receptor-1 (PD-1) inhibitors and programmed death receptor ligand-1 (PD-L1) inhibitors. More and more studies have shown that tumor microenvironment is related to immune environment, and immunotherapy can intervene to play a role in it.113
As a traditional treatment method, radiotherapy not only can kill the tumour directly and effectively but can also activate the body’s antitumor immune system and inhibit tumour growth, playing an important role in survival. In recent years, the combined treatment mode of radiotherapy and ICI has attracted great attention in the treatment of NSCLC, and it has been confirmed in clinical trials that there is indeed a synergistic effect between the two treatment strategies. A single-centre, randomized Phase II trial was conducted to evaluate the major pathological response (MPR) in patients with stage I~III A operable NSCLC treated with durvalumab versus SBRT combined with durvalumab neoadjuvant therapy. The results showed that the major pathological response (MPR) of patients treated with SBRT combined with durvalumab was significantly better than that of patients treated with durvalumab monotherapy (53.3% vs 6.7%, P<0.0001).6 However, while playing a synergistic effect, radiotherapy combined with ICIs may also lead to the occurrence of pneumonitis, which not only limits the implementation of the combined treatment plan and reduces the tumour control rate but also leads to systemic, potentially fatal immunosuppressive pneumonitis combined with radiation pneumonitis, which is life-threatening in severe cases.7 In this review, the pneumonitis incidence data and related studies of various treatment options were summarized in terms of different treatment modes of NSCLC: thoracic radiotherapy, ICIs treatment and a combination of the two treatments.
Radiation Pneumonitis
Radiotherapy is an effective treatment for prolonging survival and improving the local control rate of tumours under certain conditions.8 Radiation-induced lung injury remains the most common lung dose-limiting toxicity. Radiation pneumonitis (RP) is considered an early manifestation of radiation-induced lung injury. Approximately 10% to 30% of patients develop radiation pneumonitis after receiving thoracic radiotherapy.9,10 In addition to chest tumours, breast cancer, oesophageal cancer, rectal cancer, lymphoma and other cancers can also develop after receiving radiotherapy.11,112 The occurrence of pneumonitis not only limits the treatment dose and reduces the probability of tumour control (TCP) but may also develop into radiation fibrosis, reducing the quality of life and even causing death.12,13
Radiation pneumonitis is generally considered to be caused by reactive oxygen species generated by the body after radiotherapy that cause DNA damage, inflammation, fibrosis and other reactions, the exact pathogenesis of which is still unclear.14,15 RP usually occurs within hours to months after irradiation and is characterized by damage to alveolar epithelial cells and vascular endothelial cells as well as the release, infiltration and aggregation of inflammatory cells.16,17
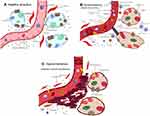
Radiation can damage normal alveolar epithelial and endothelial cells, accelerate cell senescence, destroy alveolar barrier function, and induce increased vascular permeability, the release of macrophages, the production of reactive oxygen species and acute inflammation. The production of reactive oxygen species causes type I and type II alveolar epithelial cell damage and releases cytokines such as transforming growth factor-B (TGF-B), platelet-derived growth factor, and IL, further aggravating the injury. Inadequate synthesis of surfactant in type II alveolar epithelial cells after radiation results in a weakened alveolar barrier effect, inflammatory exudation changes, and radiation pneumonitis (Figure 1).17–19 The damaged tissues enter into a continuous disordered process, which manifests as tissue fibrosis, necrosis, atrophy and vascular damage over time.20 These changes are mainly characterized by extensive proliferation of fibroblasts, matrix proteins and collagen and structural rearrangement of the lung tissue that eventually develops into radioactive pulmonary fibrosis.17
In clinical practice, radiation pneumonitis may be graded in several ways. The following is the Common Terminology Criteria for Adverse Events (CTCAE) 5.0:
Grade 0: No changes
Grade 1: Asymptomatic, radiographic changes only, no intervention
Grade 2: Symptomatic, but not interfering with activities of daily living
Grade 3: Severe symptoms and interfering with activities of daily living, oxygen indicated
Grade 4: Life threatening, ventilator support and urgent intervention are indicated
Grade 5: Death
The typical manifestations of RP on CT are usually related to the stage of the lung injury, and the imaging manifestations may show ground-glass opacities and airspace consolidation within the irradiated field (Table 1).20 The diagnosis of radiation pneumonitis is based on imaging findings and is supported by the associated clinical presentation. Clinical symptoms include low-grade fever and non-productive coughing, dyspnoea (on physical exertion or at rest), pleuritic pain, and chest discomfort. Shortness of breath and cyanosis are signs of disease development. The clinical diagnosis is combined with radiotherapy history, laboratory research and the exclusion of other causes of lung diseases, followed by comprehensive evaluation and timely treatment and intervention.12,13
 |
Table 1 Radiographic Changes of Radiation Pneumonitis in Different Periods |
It was found that female sex,10,19,21,22 lung function23,24 and pre-existing lung diseases25–27 may be related to the occurrence of radiation pneumonitis. Age,28,29 tumour volume,28 primary tumour location,30–33 mean lung dose,34,35 lung V20Gy, V5Gy,36,37 and cardiac radiation dose38,39 are risk factors for RP. However, numerous studies assessed smoking40,41 and IMRT radiotherapy technology,34 and most notably found a reduced inflammatory response and a reduced risk of radiation pneumonitis.
With the development of radiation technology, intensity-modulated radiation therapy (IMRT) has been widely used in the treatment of lung cancer. IMRT can greatly increase the conformality of the irradiated target, reduce the radiation dose and damage to normal lung tissue, and it has advantages in avoiding the danger to adjacent organs. Chun performed a secondary analysis of the RTOG 0617 trial, which showed a significant reduction in the incidence of grade III or higher RP in NSCLC patients treated with IMRT compared to 3D conventional radiotherapy (3.5% vs 7.9%).42 At present, the exact parameter threshold cannot be determined, so additional prospective experiments are required to explore the dose threshold leading to the occurrence of radiation pneumonitis to guide clinical radiotherapy planning. The main treatment methods of RP include oxygen support therapy and glucocorticoid therapy, but the effect is not good, and there are many complications, which seriously affect the quality of life of patients. At present, there is no effective treatment for RF.
Checkpoint Inhibitor Pneumonitis
Immune-related pneumonitis refers to a syndrome in which the immune defence mechanism of the body is damaged by various factors. There are two kinds of immune-related pneumonitis encountered in the clinic: autoimmune pneumonitis and immune-associated pneumonitis caused by immune checkpoint inhibitors.
Clinical studies have gradually confirmed the remarkable efficacy of ICIs in the treatment of tumours, and the accompanying adverse reactions of immunotherapy have also attracted the attention of clinicians.115 Immune checkpoint inhibitors (ICIs) enhance the antitumor immune response of T cells by blocking the activity of immune checkpoint-related proteins and blocking the immunosuppressive signal to inhibit the immune escape of tumour cells and realize the antitumor effect. ICIs block the negative regulatory signals of T cells to relieve immunosuppression and enhance the antitumor effect of T cells.
Meanwhile, ICIs may also induce overactivation of specific immune responses, resulting in immune regulation imbalance and autoimmune-like adverse reactions in normal tissues. Immune-related adverse events (irAEs) can involve multiple systems and organs, such as the skin, digestive tract, liver, endocrine system and lung.43–45 Most irAEs are mild and reversible, and only a few irAEs, such as immune pneumonitis, may evolve into severe pneumonitis or even a life-threatening condition. Checkpoint inhibitor pneumonitis (CIP), a type of irAE, has an incidence of 2.7% to 3.5%, with a median onset time of 2.3 months,32 but it is found clinically to have a higher incidence, even as high as 20%.33 In addition, the incidence of CIP in NSCLC is higher than that in other tumour types, reaching 7% to 13%, and is positively associated with a decrease in the survival rate.
The specific pathogenesis of CIP is still not completely clear. Studies have found the following potential pathogenesis of irAEs: (1) after overactivation, T cells attack normal tissues expressing the same antigens as cancer cells in the human body, and autoantibody activity is activated along with an increase in inflammatory cytokines, further infiltrating normal tissues and triggering irAEs;46–48 (2) the level of pre-existing antibodies increases, which may trigger irAEs;49 (3) ICI treatment activates the release of inflammatory cells and increases the level of inflammatory cytokines, which may trigger irAEs.50
CIP mainly occurs in the first 6 months after the start of immunotherapy. Compared with other types of cancers, CIP occurs earlier and more frequently in NSCLC and appears to occur earlier in patients treated with multiagent combination therapy.51,52 The main clinical symptoms are dyspnoea, chest distress, cough, and fever. Imaging manifestations are usually nonspecific. The main differential diagnostic diseases are diffuse pulmonary parenchymal diseases, such as nonspecific interstitial pneumonitis and organizing pneumonitis.53,54
Pre-existing lung diseases, such as chronic inflammatory respiratory diseases, interstitial pneumonitis,53 pulmonary fibrosis, or radiation pneumonitis (RP), may affect the accuracy of CIP diagnosis.55–57 The diagnostic criteria of CIP include the following: (1) history of immunotherapy; (2) new respiratory symptoms or persistent aggravation of existing clinical symptoms after immunotherapy; (3) chest X-ray or CT showing new lung consolidation or diffuse ground-glass opacities; and (4) differential diagnosis of disease progression, infection and radiation pneumonitis. CIP can be divided into five subtypes: (1) acute interstitial pneumonitis, characterized by ground-glass opacities involving most of the lungs (69); (2) acute respiratory distress syndrome, characterized by diffuse and patchy lung consolidation;58 (3) nonspecific interstitial pneumonitis, characterized by ground-glass opacities in the peripheral lung and lower lobe;59,60 (4) allergic pneumonitis, characterized by diffuse ground-glass opacities and small centrilobular nodules;59 and (5) occult pneumonitis, characterized by multifocal pulmonary parenchymal changes.60 According to the evaluation criteria for common adverse reactions,61 CIP can be divided into five grades. Patients with no clinical symptoms or imaging changes confined to a single lung lobe or involving less than 25% of the lung parenchyma are grade I, and continuous follow-up and close monitoring and suspension of immunotherapy are recommended. For new respiratory symptoms, imaging changes involving more than one lobe or involving the lung parenchyma up to 25% to 50% are grade II. The clinical symptoms are obvious, involving more than 50% of the lung parenchyma, and the life-threatening grades are Ill-IV.
It was found that the incidence of CIP may be closely related to smoking history, advanced age (≥70 years), and female sex.62–64 Lung diseases in NSCLC patients prior to ICI treatment, including interstitial lung disease (ILD), chronic obstructive pulmonary disease (COPD), asthma, pneumothorax, pleural effusion, and pulmonary fibrosis, may increase the incidence of CIP.65–68 Ryota conducted a retrospective analysis of the relationship between prior ILD and pneumonitis in NSCLC patients receiving anti-PD-1 antibody inhibitors, showing that among 331 patients, 17 had prior interstitial lung disease.66 Among 331 patients, 17 had prior interstitial lung disease. The incidence of pneumonitis in patients with a prior ILD history was nearly three times higher than that in patients without an ILD history (29% vs 10%, P = 0.027). Multidrug therapy with different immune checkpoint inhibitors and combined immunosuppressants may also be associated with CIP incidence.69
Compared with PD-L1 inhibitors, patients treated with PD-1 inhibitors appeared to have a higher probability of developing any grade of pneumonitis (3.6% vs 1.3%, P =0.001) and were also associated with a higher incidence of grade 3–4 pneumonitis (1.1% vs 0.4%, P =0.02). Patients who received immunotherapy for the first time were more likely to develop grade 1–4 pneumonitis than those who had previously received immunotherapy (4.3% vs 2.8%, P =0.03). Different ICIs reflect the toxicity specificity, with CTLA-4 inhibitors (ipilimumab and tremelimumab) showing higher toxicity than PD-1 and PD-L1 inhibitors. In addition, the incidence of CIP in NSCLC patients treated with a combination of multiple inhibitors increased by two to three times compared to treatment with a single inhibitor.69,70
Currently, corticosteroids are the mainstay treatment, according to the CIP treatment guideline recommendation issued by the American Society of Clinical Oncology. Grade 1 CIP generally does not require intervention and suspension of ICI treatment; however, low doses of steroids (0.5 to 1 mg/(kg·d)) should be given when the condition worsens. Grade 2 CIP requires suspension of ICI treatment, oral medium dose of prednisone 1~2 mg/(kg·d), tapering within 4 to 6 weeks, giving 5 to 10 mg/(kg·7d). ICIs should be stopped immediately and permanently with grade 3–4 CIP, and methylprednisolone (1~2 mg/(kg·d)) should be given intravenously. Additional immunizing agents, such as infliximab and cyclophosphamide, should be considered if the disease is not in remission within 2 days. Most patients with mild immune pneumonitis have a good prognosis after hormone withdrawal, while a few patients with severe CIP have a poor prognosis, which may be related to secondary infection or tumour disease progression. Some studies have reported that the incidence of recurrent pneumonitis in CIP patients after restarting ICI treatment is approximately 25%. At present, restarting ICI treatment after CIP should be done with great caution.71
Pneumonitis Occurring After Radiotherapy Combined with Immunotherapy
Immune checkpoint inhibitors have significantly improved the outcomes of patients with non-small cell lung cancer (NSCLC). Patients with advanced NSCLC who had previously received first-line nivolumab plus ipilimumab showed lasting efficacy even after at least 2 years with no immunotherapy. The results of the PEMBRO-RT Phase 2 randomized clinical trial showed that radiotherapy combined with ICI treatment further improved the response rate compared with ICI monotherapy. At present, there is no clear conclusion as to whether radiotherapy combined with immunotherapy superposes toxic reactions, but an increasing number of studies and experimental studies have shown that radiotherapy combined with immune checkpoint inhibitors has an increasing trend of pneumonitis incidence in NSCLC patients.
Pathogenesis and Influencing Factors
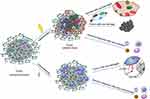
Ionizing radiation damages tumour cells to produce reactive oxygen species (ROS), which further interact with tumour cell DNA and other cell components to cause radiation-induced damage and indirectly damage normal human cells. Subsequently, antigen-presenting cells remove the damaged tumour cells, further promoting the activation and recruitment of T cells by antigen-presenting cells. ICI therapy, by disabling immunosuppressive signals such as CTLA-4 and PD-1/PDL1, continuously releases T cells that are overactivated. By targeting the negative feedback loop of T cells, ICIs can impair tumour immune tolerance and induce immune cell infiltration into normal tissues, leading to adverse toxic reactions such as pneumonitis (Figure 2).72
Cell damage caused by radiotherapy and T-cell reactivation caused by immunotherapy both lead to a common event endpoint: the activation and release of a large number of cytokines. These cytokines can not only directly damage lung tissue through the TGF-β/Smad and TNF-α/NF-κβ signalling pathways but also induce lung injury indirectly by recruiting neutrophils, macrophages and lymphocytes. Among these cytokines, IL-4, IL-6, IL-10, and IL-17 have been shown to be associated with radiation pneumonitis and immune checkpoint inhibitor pneumonitis, which may cause concurrent superposition damage to normal tissue.73–75 Other studies have revealed the potential mechanism of the increased risk of pneumonitis after combined therapy. For example, Deng observed synergistic promotion of antitumor immunity in mice by radiotherapy combined with ICls.76 Combined therapy plays an antitumor role by activating cytotoxic T-cell-derived TNF to mediate the reduction of myeloid suppressor cells (MDSCs) in the tumour microenvironment. However, TNF, as an inflammatory mediator, can reverse mediate inflammation and participate in the occurrence and development of pneumonitis when it is overactivated. Naqash reported a case of immune pneumonitis in a patient receiving immunotherapy.77 After receiving PD-L1 inhibitors, the IL-6 and CRP levels were significantly increased in this NSCLC patient. While IL-6 is a risk factor for radiation pneumonitis, it is thought that patients with pneumonitis after receiving immunotherapy also have an increased risk of pneumonitis after receiving radiation therapy. Voong found that among 188 subjects, 36 (19.1%) developed pneumonitis, of which 53% (19/36) had received thoracic radiotherapy, and patients who received radical thoracic RT (17/19, 89%) were more likely to develop pneumonitis than those who received palliative THORACIC RT (2/19, 11%).78 This shows that the occurrence of pneumonitis is closely related to the radiation dose. However, the results of the study showed that pneumonitis mainly occurred in the areas of medium and low doses of lung radiation, rather than the area of high doses of radiation, suggesting that pneumonitis that occurs after combined treatments may be a specific type of pneumonitis, and it is likely that ICIs combined with RT weakens the tolerance of lung tissues in areas of low doses, resulting in toxic superposition.
The timing of combination therapy may also be a potential factor in the development of pneumonitis. It has been reported in the literature that NSCLC patients with irAEs receiving immunotherapy have a significantly increased risk of pneumonitis following thoracic radiotherapy.79 Shaveprdian evaluated the safety of chest radiotherapy in patients with immune-related adverse events caused by immune checkpoint inhibitors. The study included 496 patients, 41 of whom had a history of irAEs, and 25 (61%) developed grade ≥2 radiation pneumonitis after radiotherapy. Among the 25 patients with pneumonitis, 5 had ≥2 immune pneumonitis. This may indicate that patients with a history of irAEs who have previously received immunotherapy are at a very high risk of developing pneumonitis following thoracic radiation therapy.
However, in KEYNOTE-001 Phase I80 and Phase III PACIFIC,81 the treatment sequence was reversed, with patients receiving radiotherapy followed by immunotherapy. In the KEYNOTE-001 trial, a secondary analysis was performed on 97 patients. Radiation pneumonitis occurred after radiotherapy in 15 of the 24 patients (63%) who had previously received chest radiotherapy and it recurred after sequential immunotherapy in 3 patients (13%) who had previously received chest radiotherapy. The incidence of pneumonitis of all grades was significantly higher among patients receiving combination therapy (13% versus 1%, P =0.046). The phase III PACIFIC trial assessed the safety and toxicity of immunotherapy in 713 patients treated within 2 months after radiotherapy. The incidence of full-scale pneumonitis was significantly higher in the combination therapy group than in the radiotherapy group alone (33.9% vs 24.8%).
Recent studies have found that some serological indicators may have predictive effects. Schoenfeld reported a patient with advanced melanoma who developed pneumonitis after radiotherapy combined with the PD-1 inhibitor nivolumab and found that increased levels of CXCR2, IL-1 receptor antagonists, and IL-2 receptor antagonists were consistent with the occurrence and development of pneumonitis.82 Jing also found that four cytokines, GM-GSF, CD62E, IL-6 soluble receptor and stem cell factor, were associated with the occurrence of pneumonitis in inoperable stage III NSCLC patients treated with concurrent chemoradiotherapy and durvalumab.83
Radiation recall pneumonitis (RRP), which is considered a radiation-related lung injury characterized by acute inflammation limited to previously irradiated lung fields, occurs weeks, months or even years after the completion of radiation therapy. It is a kind of delayed radioactive pulmonary toxicity caused by systemic drugs, usually anticancer drugs. Immune checkpoint inhibitors (ICIs) have recently been identified as potential causal agents of RRP, but their actual incidence, potential risk factors, and mechanisms of action remain unclear.84–86 For example, in the Pacific trial, the safety of 713 patients treated with ICIs between Days 1 and 42 after completion of chemoradiotherapy was analysed.81 Compared with the radiotherapy alone group, the incidence of grade ≥3 pneumonitis (3.4% vs 2.6%) and the incidence of pneumonitis of any grade were significantly higher in the combined treatment group than in the radiotherapy alone group (33.9% vs 24.8%), suggesting that patients who had previously received chest radiotherapy and then received immunotherapy had an increased risk of pneumonitis. It has been suggested that radiotherapy may play a negative role in eliciting an immune response, and there is a possibility of toxicity superposition in the later stages of combined therapy, which should be closely monitored and followed up.
The difference in the incidence of pneumonitis caused by different combination treatment sequences may be related to the difference in the immune and radiation sensitivity and tolerance of individual patients, as well as the degree of disease progression and different immune checkpoint inhibitors of each patient. In general, irradiated lungs exhibit an acute radiation-induced inflammatory response, or latent inflammatory response, for a period of time after radiotherapy, and ICI treatment after radiotherapy may enhance the immune system while increasing the risk of all levels of toxicity in the combination therapy group. Therefore, pneumonitis after combined therapy may not be a simple superposition mode of RP and CIP. In the future, large clinical studies are needed to compare the imaging features of patients with CIP alone, RP alone and RP+CIP after combined therapy to help clinicians identify and more comprehensively treat patients. The possibility of toxic reactions in patients at different times needs to be analysed, and the types and incidence of pneumonitis should be predicted by evaluating the tumour characteristics, underlying pulmonary diseases, clinical symptoms, imaging characteristics and autoimmune status of the patients.
Morbidity
Radiotherapy Combined with CTLA-4 Inhibitors
Radiotherapy combined with CTLA-4 inhibitors has shown good efficacy and tolerance in melanoma and prostate cancer, but there are relatively few studies on the application of CTLA-4 inhibitors in non-small cell cancer.87,88 Chen retrospectively analysed two prospective trials of radiotherapy combined with anti-CTLA-4 or anti-PD-1 for the treatment of metastatic NSCLC patients.89 SBRT was administered in combination with the CTLA-4 inhibitor ipilimumab (n=17) and the PD-1 inhibitor pembrolizumab (n=16). The results showed that in the SBRT combined with CTLA-4 inhibitor group, there were 2 cases of any-grade pneumonitis (n = 17, 11.8%) and 1 case of grade ≥3 pneumonitis (n= 17, 5.9%). However, in the SBRT combined with PD-1 inhibitor group, there were 5 cases of any grade pneumonitis (n = 16, 31.3%) and 3 cases of ≥ 3 grade pneumonitis (n=16, 18.8%). Previous studies have proven that the risk of CIP caused by PD-1 inhibitors is higher than that of CTLA-4 inhibitors during the treatment of NSCLC.90–92 To prove that the incidence of pneumonitis during radiotherapy combined with PD-1 inhibitors/PD-L1 inhibitors is higher than that in combination with CTLA-4 inhibitors, further exploration in large studies and additional prospective experiments are required to compare the incidence of pneumonitis in different radiotherapy techniques combined with PD-1 inhibitors, PD-L1 inhibitors and CTLA-4 inhibitors.
Radiotherapy Combined with PD-1/PD-L1 Inhibitors
In recent years, an increasing number of clinical trials have focused on the efficacy and safety of new models of NSCLC using PD-1/PD-L1 inhibitors in combination with RT.93 The early KEYNOTE 00180 and Pacific trial81 showed that the combination therapy was well tolerated and that the toxicity was within an acceptable range. For example, the results of the Phase 3 PACIFIC94 study confirmed that CRT sequential ICI (duvalizumab) treatment had a better 3-year survival rate and established the role of RT combined with ICIs in the clinical treatment of unresectable stage III non-small cell lung cancer patients without progression after radiotherapy and chemotherapy. Data from current clinical trials suggest that adjuvant duvalizumab is the standard treatment after concurrent chemotherapy (CRT) for stage III non-small cell lung cancer (NSCLC) and it does not significantly increase the incidence of pneumonitis. Chang conducted a phase II trial to study the efficacy and safety of SABR combined with nivolumab in patients with early inoperable NSCLC, while the control group received SABR alone.95 The incidence of grade ≥2 pneumonitis was similar in both groups (4.4% vs 2.1%). The incidence of grade ≥2 pneumonitis was approximately 6.7% in previously reported studies of SBRT for the treatment of early NSCLC.96 SABR combined with ICIs did not significantly increase the risk of pneumonitis in patients with early NSCLC.
However, when RT combined with ICIs was used to treat locally advanced NSCLC patients, the results were less favourable. Moore analysed 39 stage III NSCLC patients who received sequential CRT and durvalumab, and up to 54% developed grade ≥2 pneumonitis, suggesting that the incidence of pneumonitis might be higher when CRT followed by durvalumab was used to treat locally advanced NSCLC patients.97 It seems safe to restart durvalumab therapy after high-dose steroid treatment of pneumonitis, but due to the small number of clinical studies and their small sample sizes, the safety of restarting immunotherapy has not been fully proven. The safety of adjuvant duvalizumab combined with radiotherapy and whether it will continue to be a useful ICI treatment in the later stages needs further exploration.
Barron retrospectively analysed and assessed the development of pneumonitis in patients with prior radiotherapy for non-small cell lung cancer who received second-line immunotherapy.98 The results showed that sequential PD-1 inhibitors significantly increased the risk of pneumonitis in patients who had previously received radiotherapy at any site. The incidence of pneumonitis of any grade (40% vs 9.8%) and ≥3 grade (10% vs 0%) between the combined radiotherapy group and the single immunization group was statistically significant (all P < 0.01). Similarly, Botticella assessed the effect of previous chest radiotherapy on the occurrence of pneumonitis after combined therapy in 318 NSCLC patients and found that the incidence of grade ≥3 pneumonitis was significantly higher in patients who received chest radiotherapy than in those who did not (11.1% vs 0.4%, P < 0.01).99
There seems to be some difference in the incidence of pneumonitis in NSCLC patients treated with CRT concurrently or with sequential PD-1/PD-L1 inhibitors. Jabbour performed a Phase 1 nonrandomized controlled trial of PD-1 inhibitors combined with radiotherapy and chemotherapy for the treatment of locally advanced non-small cell lung cancer.100 The overall incidence of grade ≥2 immune-related adverse events was 67% (n=14), among which 7 patients had pneumonitis (n=14, 33%), and patients with CRT concomitant treatment with PD-1 had an increased risk of pneumonitis. In a phase II clinical trial study of atezolizumab combined with chemoradiotherapy for the treatment of unresectable non-small cell lung cancer, the experimental group was set as CRT synchronous atezolizumab, and the control group was set as CRT sequential atezolizumab.101 The incidence of any grade and grade ≥3 pneumonitis was 23% and 3%, respectively, in the synchronous group and 30% and 0%, respectively, in the sequential group. The results showed that there was no significant difference in the incidence of grade ≥3 pneumonitis between the two groups, while the incidence of grade ≤2 pneumonitis was relatively high after sequential treatment. More prospective studies are needed to explore the effect of timing on the incidence of pneumonitis during combination therapy.
All of the above were retrospective studies, and more prospective trials and clinical trials need to be conducted in the future to analyse the mechanisms of increased pulmonary toxicity during combined treatment with different radiotherapy techniques, the interval between radiotherapy and ICI treatment, the treatment sequence, and different ICI drugs.
SBRT Combined with ICIs
Tian evaluated and analysed the safety and toxicity reports of SBRT combined with ICIs in multicentre treatment, including acute (within 30 days) and subacute (within 180 days) toxicity reports.102 Compared with monotherapy of ICIs or SBRT combined with immunotherapy of SBRT, the incidence of ≥3 adverse reactions was 2.9% and 26.8%, respectively. However, the incidence of ≥3 pneumonitis cases treated with SBRT combined with ICIs was significantly different and statistically significant (10.7% vs 0%, P <0.01). Miyamoto reported 6 patients with advanced NSCLC who received pulmonary SBRT and maintenance therapy with nivolumab (PD-1), of whom 1 patient (n=6, 16.7%) developed grade 3 pneumonitis.103 A recent meta-analysis also found an association between the combination therapy pattern with an anti-CTLA-4 inhibitor (ipilimumab) and radiation pneumonitis in patients with brain metastases treated with ICI and SRS.104 Qin conducted a prospective study of low-fraction, image-guided radiotherapy (HIGRT) combined with atezolizumab (PD-L1) for patients with metastatic non-small cell lung cancer.105 The results showed that 2 patients (n=12 16.7%) developed grade 3 pneumonitis, and the incidence of grade 3 adverse events was similar to that of atezolizumab alone. There was no significant increase. According to the above results, the incidence of lung toxicity of SBRT combined with ICIs is not significantly increased in NSCLC, but radiotherapy combined with CTLA-4 may increase the risk for some specific tumours compared to the risk of immunization or radiotherapy alone. More clinical studies are needed to analyse the optimal mode of SBRT and ICI combination therapy to improve the tumour control rate, reduce the toxicity and side effects during combination therapy, and accurately grasp the most likely occurrence of pneumonitis for timely intervention and treatment.
Low-Dose Radiotherapy Combined with ICIs
Low-dose radiation (LDR) refers to a dose of low linear energy conversion (LET) radiation < 0.2 Gy or a dose of high LET radiation < 0.05 Gy, while the radiation dose rate is higher than 0.05Gy/min.
Low-dose radiation can promote the growth of normal human cells but has no stimulating effect on the growth of tumour cells.106 The cytological basis of low-dose radiation is to activate immune organ cells; activate the T-cell signal transduction pathway; increase the expression of cytokines such as IL-2, IFN-γ, TNF-α and GM-CSF; enhance the role of NK cells, cytotoxic T lymphocytes and macrophages; accelerate the apoptosis of tumour cells; and enhance the antitumor immune effect in the body. To date, there are few studies on low-dose irradiation combined with anti-PD-1/PD-L1 therapy, and they have primarily been conducted on mice. In studies of tumour-bearing mice, PD-1 knockout T cells combined with low-dose radiotherapy produced highly effective antitumor effects.107 When low-dose radiotherapy was combined with ICIs, low-dose radiation promoted the body’s immune system, and the ICIs played a role in synergistic treatment at the same time. T cells were excessively activated and released, leading to immune adjustment. In normal tissue, the damage triggers an inflammatory reaction, but there are too few studies. This speculation needs to be further explored by medical workers in future experiments and clinical studies to control the incidence of adverse events while ensuring the synergistic effect of the two to improve the tumour control rate and increase the overall survival of patients, thus providing new methods and ideas for the treatment of NSCLC.
Identification and Treatment
CIP is a type of systemic immune system overactivation that causes potentially fatal systemic immunotoxic adverse reactions. CIP above grade 3 indicates an urgent need to stop ICI treatment permanently, while CIP below grade 3 can be treated with temporary interruptions of ICI to allow for the recovery of patients followed by restarting treatment. Patients with RP can continue to receive ICI and RT treatment after recovering from hormone therapy. In cases of pneumonia after combined therapy, close attention should be given to the changes in lung imaging and clinical symptoms before making a differential diagnosis. First, the pneumonia caused by RP is mostly confined to the radiation field of the lung, and any pulmonary inflammatory changes in the radiation field can be considered CIP caused by ICIs. The differential diagnosis of patients with pneumonia after RT combined with ICIs is not only CIP and RP but also infection (bacteria, fungi, virus, tuberculosis, etc.) and tumour progression.
The treatment process of radiotherapy combined with immunotherapy for pneumonia is similar to that of RP or CIP, and radiotherapy or immunotherapy should be stopped immediately if pneumonia develops. Second, cough relief, expectorants, bronchodilators and other treatments can be applied (Table 2). For pneumonia patients with mild symptoms below grade 2, especially those with small pneumonia lesions, symptomatic treatment and close monitoring and observation can be given first. Some patients will be relieved and recover, while glucocorticoid therapy should be given to those who do not respond or worsen. Patients with grade ≥3 are recommended to be given oxygen inhalation or mechanical support ventilation to ensure airway patency and remove life-threatening risks.
 |
Table 2 Classification, Symptoms and Treatment of Combined Pneumonitis |
In the treatment of pneumonia, in addition to cough and expectorant treatment, glucocorticoid treatment is key. It is recommended to use long-acting dexamethasone or prednisone, individualized treatment starting from a small dose and then adjusted according to the development of the disease and maintaining it for 3 to 4 weeks, with a slow reduction of the hormone to avoid recurrence. In cases where a definite diagnosis is not possible, oxygen support therapy and/or glucocorticoids may be prescribed, as appropriate, depending on the severity of the condition.108,109 At present, there is no clear conclusion on the specific principles of whether patients can resume radiotherapy and immunotherapy again after recovering from pneumonia during combination therapy. It is suggested clinicians should consider whether to continue to deliver an additional radiotherapy dose according to the clinical remission degree of radiation pneumonia after treatment and the recovery from inflammation in the irradiation field. It is recommended to evaluate whether to continue using immunotherapy drugs according to the remission degree of immune pneumonia after preclinical treatment: for patients who achieve a complete remission in the early stage, try to use drugs after observation; patients with early disease progression are no longer considered eligible to receive immunotherapy; and for those who have achieved a partial remission or disease stabilization, reintroducing immunotherapy may be considered. In addition to regular evaluation of efficacy, patients receiving immunotherapy should also be closely monitored for toxicity and side effects, including immune pneumonia and other irAEs. If immune pneumonia recurs again, immunotherapy needs to be permanently stopped.
Summary
Radiotherapy may improve the antigens presented by in situ vaccine release and help remove the inhibitory immune microenvironment, influence the tumour immune status, improve the immune system response to immune therapy, increase the efficacy of immune therapy, promote tumour cells to release tumour-specific antigens, improve the killing of tumour cells, and increase the immune response to treatment. Combination therapy can further promote the immune system’s antitumor processes and even increase the incidence of “distant effects”.110,111 However, when we use combination therapy to increase the distant effect, we should pay more attention to whether there will be a distant toxic reaction or metastasis. The future development of radiotherapy combined with ICIs in NSCLC is still worth looking forward. Most clinical studies showed that SBRT combined with immunotherapy did not significantly increase the incidence of pneumonia in early NSCLC, but a small number of studies showed that combined therapy increased the incidence of pneumonia in patients with advanced NSCLC. It is believed that with the innovation of radiotherapy technology, the continuous optimization of the timing of combination therapy and the exploration of more personalized dose parameters, the incidence of toxic reactions in the later stage of combination therapy can be reduced while improving the tumour control rate and the overall survival of patients.
The existing research results are mostly retrospective. Given the lack of a large amount of prospective data, future research should concentrate on exploring additional ICIs combined with radiotherapy technology for late adverse reaction mechanisms and how to accurately identify CIP, RP and combined treatment of pneumonia according to individual patient factors to achieve the ideal treatment plan. In addition, in view of the potentially high mortality of immune-mediated pneumonia, NSCLC patients receiving chest radiotherapy combined with ICIs should be more cautious in choosing the dose and timing of radiotherapy to complement the ICI treatment after the occurrence of relevant irAEs.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24. doi:10.1016/j.ccm.2019.10.001
4. Zhou Y, Zhang Y, Guo G, et al. Nivolumab plus ipilimumab versus pembrolizumab as chemotherapy-free, first-line treatment for PD-L1-positive non-small cell lung cancer. Clin Transl Med. 2020;10(1):107–115. doi:10.1002/ctm2.14
5. Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–195. doi:10.1016/j.lungcan.2019.07.004
6. Altorki NK, McGraw TE, Borczuk AC, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 2021;22(6):824–835. doi:10.1016/S1470-2045(21)00149-2
7. Lederman M. The early history of radiotherapy: 1895–1939. Int J Radiat Oncol Biol Phys. 1981;7(5):639–648. doi:10.1016/0360-3016(81)90379-5
8. Liu T, Mu Y, Dang J, Li G. The role of postoperative radiotherapy for completely resected pIIIA-N2 non-small cell lung cancer patients with different clinicopathological features: a systemic review and meta-analysis. J Cancer. 2019;10(17):3941–3949. doi:10.7150/jca.28680
9. Uchida Y, Tsugawa T, Tanaka-Mizuno S, et al. Exclusion of emphysematous lung from dose-volume estimates of risk improves prediction of radiation pneumonitis. Radiat Oncol. 2017;12(1):160. doi:10.1186/s13014-017-0891-z
10. Käsmann L, Dietrich A, Staab-Weijnitz CA, et al. Radiation-induced lung toxicity - cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol. 2020;15(1):214. doi:10.1186/s13014-020-01654-9
11. Keffer S, Guy CL, Weiss E. Fatal radiation pneumonitis: literature review and case series. Adv Radiat Oncol. 2020;5(2):238–249. doi:10.1016/j.adro.2019.08.010
12. Bledsoe TJ, Nath SK, Decker RH. Radiation Pneumonitis. Clin Chest Med. 2017;38(2):201–208. doi:10.1016/j.ccm.2016.12.004
13. Jain V, Berman AT. Radiation pneumonitis: old problem, new tricks. Cancers. 2018;10(7):222. doi:10.3390/cancers10070222
14. Simone CB. Thoracic radiation normal tissue injury. Semin Radiat Oncol. 2017;27(4):370–377. doi:10.1016/j.semradonc.2017.04.009
15. Wang C, Rimner A, Gelblum DY, et al. Analysis of pneumonitis and esophageal injury after stereotactic body radiation therapy for ultra-central lung tumors. Lung Cancer. 2020;147:45–48. doi:10.1016/j.lungcan.2020.07.009
16. Kasmann L, Dietrich A, Staab-Weijnitz CA, et al. Radiation-induced lung toxicity - cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol. 2020;15(1):214.
17. Giuranno L, Ient J, De Ruysscher D, Vooijs MA. Radiation-Induced Lung Injury (RILI). Front Oncol. 2019;9:877. doi:10.3389/fonc.2019.00877
18. Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, Muñoz-Montaño W, Nuñez-Baez M, Arrieta O. Radiation-induced lung injury: current evidence. BMC Pulm Med. 2021;21(1):9. doi:10.1186/s12890-020-01376-4
19. Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–162. doi:10.1016/j.chest.2019.03.033
20. Ullah T, Patel H, Pena GM, Shah R, Fein AM. A contemporary review of radiation pneumonitis. Curr Opin Pulm Med. 2020;26(4):321–325. doi:10.1097/MCP.0000000000000682
21. Takeda A, Ohashi T, Kunieda E, et al. Comparison of clinical, tumour-related and dosimetric factors in grade 0–1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br J Radiol. 2012;85(1013):636–642. doi:10.1259/bjr/71635286
22. Jin H, Tucker SL, Liu HH, et al. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol. 2009;91(3):427–432. doi:10.1016/j.radonc.2008.09.009
23. Faught AM, Yamamoto T, Castillo R, et al. Evaluating which dose-function metrics are most critical for functional-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):202–209. doi:10.1016/j.ijrobp.2017.03.051
24. Zhou Y, Yan T, Zhou X, et al. Acute severe radiation pneumonitis among non-small cell lung cancer (NSCLC) patients with moderate pulmonary dysfunction receiving definitive concurrent chemoradiotherapy: impact of pre-treatment pulmonary function parameters. Strahlenther Onkol. 2020;196(6):505–514. doi:10.1007/s00066-019-01552-4
25. Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol. 2015;10(1):116–125. doi:10.1097/JTO.0000000000000359
26. Takeda A, Kunieda E, Ohashi T, et al. Severe COPD is correlated with mild radiation pneumonitis following stereotactic body radiotherapy. Chest. 2012;141(4):858–866. doi:10.1378/chest.11-1193
27. Kimura T, Togami T, Takashima H, Nishiyama Y, Ohkawa M, Nagata Y. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema. Br J Radiol. 2012;85(1010):135–141. doi:10.1259/bjr/32629867
28. Zhao J, Yorke ED, Li L, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys. 2016;95(5):1357–1366. doi:10.1016/j.ijrobp.2016.03.024
29. Dang J, Li G, Zang S, Zhang S, Yao L. Risk and predictors for early radiation pneumonitis in patients with stage III non-small cell lung cancer treated with concurrent or sequential chemoradiotherapy. Radiat Oncol. 2014;9(1):172. doi:10.1186/1748-717X-9-172
30. Tucker SL, Liao ZX, Travis EL. Estimation of the spatial distribution of target cells for radiation pneumonitis in mouse lung. Int J Radiat Oncol Biol Phys. 1997;38(5):1055–1066. doi:10.1016/S0360-3016(97)00131-4
31. Bradley JD, Hope A, El Naqa I, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69(4):985–992. doi:10.1016/j.ijrobp.2007.04.077
32. Hope AJ, Lindsay PE, El Naqa I, et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys. 2006;65(1):112–124. doi:10.1016/j.ijrobp.2005.11.046
33. Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63(1):5–24. doi:10.1016/j.ijrobp.2005.03.047
34. Katsui K, Ogata T, Watanabe K, et al. Dose-volume parameters predict radiation pneumonitis after induction chemoradiotherapy followed by surgery for non-small cell lung cancer: a retrospective analysis. BMC Cancer. 2019;19(1):1144. doi:10.1186/s12885-019-6359-9
35. Ryckman JM, Baine M, Carmicheal J, et al. Correlation of dosimetric factors with the development of symptomatic radiation pneumonitis in stereotactic body radiotherapy. Radiat Oncol. 2020;15(1):33. doi:10.1186/s13014-020-1479-6
36. Boonyawan K, Gomez DR, Komaki R, et al. Clinical and Dosimetric Factors Predicting Grade ≥2 Radiation Pneumonitis After Postoperative Radiotherapy For Patients With Non-Small Cell Lung Carcinoma. Int J Radiat Oncol Biol Phys. 2018;101(4):919–926. doi:10.1016/j.ijrobp.2018.04.012
37. Yu JH, Wang CL, Liu Y, et al. Study of the predictors for radiation pneumonitis in patient with non-small cell lung cancer received radiotherapy after pneumonectomy. Cancer Radiother. 2021;25(4):323–329. doi:10.1016/j.canrad.2020.11.001
38. Tomita N, Okuda K, Ogawa Y, et al. Relationship between radiation doses to heart substructures and radiation pneumonitis in patients with thymic epithelial tumors. Sci Rep. 2020;10(1):11191. doi:10.1038/s41598-020-68168-y
39. Banfill K, Giuliani M, Aznar M, et al. Cardiac toxicity of thoracic radiotherapy: existing evidence and future directions. J Thorac Oncol. 2021;16(2):216–227. doi:10.1016/j.jtho.2020.11.002
40. Qiu F, Liang CL, Liu H, et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. 2017;8(1):268–284. doi:10.18632/oncotarget.13613
41. Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol. 2012;51(8):975–983. doi:10.3109/0284186X.2012.718093
42. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56–62. doi:10.1200/JCO.2016.69.1378
43. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–1353.
44. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. doi:10.1038/s41572-020-0160-6
45. Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi:10.1016/j.ctrv.2020.102134
46. Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19(1):558. doi:10.1186/s12885-019-5701-6
47. Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018;9:1430. doi:10.3389/fphar.2018.01430
48. Postow MA, Sidlow R, Hellmann MD, Longo DL. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481
49. Toi Y, Sugawara S, Sugisaka J, et al. Profiling preexisting antibodies in patients treated with Anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019;5(3):376–383. doi:10.1001/jamaoncol.2018.5860
50. Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving Anti-PD-1-based immunotherapy. Clin Cancer Res. 2019;25(5):1557–1563. doi:10.1158/1078-0432.CCR-18-2795
51. Wang PF, Chen Y, Song SY, et al. Immune-related adverse events associated with Anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. doi:10.3389/fphar.2017.00730
52. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–717. doi:10.1200/JCO.2016.68.2005
53. Sun Y, Shao C, Li S, et al. Programmed cell death 1 (PD-1)/PD-ligand 1(PD-L1) inhibitors-related pneumonitis in patients with advanced non-small cell lung cancer. Asia Pac J Clin Oncol. 2020;16(6):299–304. doi:10.1111/ajco.13380
54. Reuss JE, Suresh K, Naidoo J. Checkpoint inhibitor pneumonitis: mechanisms, characteristics, management strategies, and beyond. Curr Oncol Rep. 2020;22(6):56. doi:10.1007/s11912-020-00920-z
55. Naidoo J, Nishino M, Patel SP, et al. Immune-related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectable stage iii non-small-cell lung cancer. Clin Lung Cancer. 2020;21(5):e435–e444. doi:10.1016/j.cllc.2020.02.025
56. Shannon VR. Pneumonitis associated with immune checkpoint inhibitors among patients with non-small cell lung cancer. Curr Opin Pulm Med. 2020;26(4):326–340. doi:10.1097/MCP.0000000000000689
57. Zhai X, Zhang J, Tian Y, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med. 2020;17(3):599–611. doi:10.20892/j.issn.2095-3941.2020.0102
58. Ferguson EC, Berkowitz EA, Lung CT. Part 2, The interstitial pneumonias–clinical, histologic, and CT manifestations. AJR Am J Roentgenol. 2012;199(4):W464–476. doi:10.2214/AJR.10.7309
59. Baba T, Sakai F, Kato T, et al. Radiologic features of pneumonitis associated with nivolumab in non-small-cell lung cancer and malignant melanoma. Future Oncol. 2019;15(16):1911–1920. doi:10.2217/fon-2019-0102
60. Nishino M, Hatabu H, Hodi FS, Ramaiya NH. Drug-related pneumonitis in the era of precision cancer therapy. JCO Precis Oncol. 2017;1. doi:10.1200/PO.17.00026
61. Miller TP, Fisher BT, Getz KD, et al. Unintended consequences of evolution of the common terminology criteria for adverse events. Pediatr Blood Cancer. 2019;66(7):e27747. doi:10.1002/pbc.27747
62. Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50(2):1700050. doi:10.1183/13993003.00050-2017
63. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–1939. doi:10.1016/j.jtho.2018.08.2035
64. Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–156. doi:10.1016/j.lungcan.2018.09.015
65. Galant-Swafford J, Troesch A, Tran L, Weaver A, Doherty TA, Patel SP. Landscape of immune-related pneumonitis in cancer patients with asthma being treated with immune checkpoint blockade. Oncology. 2020;98(2):123–130. doi:10.1159/000503566
66. Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother. 2020;69(1):15–22. doi:10.1007/s00262-019-02431-8
67. Filho MM, Aguiar PN, de Mello RA. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Ann Transl Med. 2019;7(Suppl 1):S42. doi:10.21037/atm.2019.02.27
68. Pozzessere C, Bouchaab H, Jumeau R, et al. Relationship between pneumonitis induced by immune checkpoint inhibitors and the underlying parenchymal status: a retrospective study. ERJ Open Res. 2020;6(1):00165–2019. doi:10.1183/23120541.00165-2019
69. Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271–281. doi:10.1016/j.chest.2017.04.177
70. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi:10.1056/NEJMoa1810865
71. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385
72. Fujii T, Colen RR, Bilen MA, et al. Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs. 2018;36(4):638–646. doi:10.1007/s10637-017-0534-0
73. Sprung CN, Forrester HB, Siva S, Martin OA. Immunological markers that predict radiation toxicity. Cancer Lett. 2015;368(2):191–197. doi:10.1016/j.canlet.2015.01.045
74. Li B, Jiang C, Pang L, et al. Toxicity profile of combining PD-1/PD-L1 inhibitors and thoracic radiotherapy in non-small cell lung cancer: a systematic review. Front Immunol. 2021;12:627197. doi:10.3389/fimmu.2021.627197
75. Zhang Z, Zhou J, Verma V, et al. Crossed pathways for radiation-induced and immunotherapy-related lung injury. Front Immunol. 2021;12:774807. doi:10.3389/fimmu.2021.774807
76. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi:10.1172/JCI67313
77. Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR. Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: evidence from a case report. Acta Oncol. 2018;57(5):705–708. doi:10.1080/0284186X.2017.1406668
78. Voong KR, Hazell SZ, Fu W, et al. Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2019;20(4):e470–e479. doi:10.1016/j.cllc.2019.02.018
79. Shaverdian N, Beattie J, Thor M, et al. Safety of thoracic radiotherapy in patients with prior immune-related adverse events from immune checkpoint inhibitors. Ann Oncol. 2020;31(12):1719–1724. doi:10.1016/j.annonc.2020.09.016
80. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi:10.1016/S1470-2045(17)30380-7
81. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937
82. Schoenfeld JD, Nishino M, Severgnini M, Manos M, Mak RH, Hodi FS. Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circulating biomarker features. J Immunother Cancer. 2019;7(1):112. doi:10.1186/s40425-019-0583-3
83. Zeng J, Rengan R, Santana-Davila R, et al. Early assessment of liquid biomarkers to predict pneumonitis after chemoradiation in patients with locally advanced non-small cell lung cancer (LA-NSCLC). Cancer Res. 2020;80(16):6497. doi:10.1158/1538-7445.AM2020-6497
84. Chen Y, Huang Z, Xing L, Meng X, Yu J. Radiation recall pneumonitis induced by Anti-PD-1 blockade: a case report and review of the literature. Front Oncol. 2020;10:561. doi:10.3389/fonc.2020.00561
85. McGovern K, Ghaly M, Esposito M, Barnaby K, Seetharamu N. Radiation recall pneumonitis in the setting of immunotherapy and radiation: a focused review. Future Sci OA. 2019;5(5):Fso378. doi:10.2144/fsoa-2018-0123
86. Teng F, Li M, Yu J. Radiation recall pneumonitis induced by PD-1/PD-L1 blockades: mechanisms and therapeutic implications. BMC Med. 2020;18(1):275. doi:10.1186/s12916-020-01718-3
87. Willsmore ZN, Coumbe BGT, Crescioli S, et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: treatment of melanoma and immune mechanisms of action. Eur J Immunol. 2021;51(3):544–556. doi:10.1002/eji.202048747
88. Witt K, Evans-Axelsson S, Lundqvist A, Johansson M, Bjartell A, Hellsten R. Inhibition of STAT3 augments antitumor efficacy of anti-CTLA-4 treatment against prostate cancer. Cancer Immunol Immunother. 2021;70(11):3155–3166. doi:10.1007/s00262-021-02915-6
89. Chen D, Menon H, Verma V, et al. Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: retrospective analysis of two single-institution prospective trials. J Immunother Cancer. 2020;8(1):e000492. doi:10.1136/jitc-2019-000492
90. Chhabra N, Kennedy J. A review of cancer immunotherapy toxicity: immune checkpoint inhibitors. J Med Toxicol. 2021;17(4):411–424. doi:10.1007/s13181-021-00833-8
91. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. doi:10.3322/caac.21596
92. Chhabra N, Kennedy J. A review of cancer immunotherapy toxicity II: adoptive cellular therapies, kinase inhibitors, monoclonal antibodies, and oncolytic viruses. J Med Toxicol. 2022;18(1):43–55. doi:10.1007/s13181-021-00835-6
93. Johnson CB, Win SY. Combination therapy with PD-1/PD-L1 blockade: an overview of ongoing clinical trials. Oncoimmunology. 2018;7(4):e1408744. doi:10.1080/2162402X.2017.1408744
94. Gray JE, Villegas A, Daniel D, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15(2):288–293. doi:10.1016/j.jtho.2019.10.002
95. Chang JY, Lin SH, Yao LY, et al. I-SABR phase II randomized study of nivolumab immunotherapy and stereotactic ablative radiotherapy in early stage NSCLC: interim analysis adverse effects. J Clin Oncol. 2020;38(15):9035. doi:10.1200/JCO.2020.38.15_suppl.9035
96. Saha A, Beasley M, Hatton N, et al. Clinical and dosimetric predictors of radiation pneumonitis in early-stage lung cancer treated with Stereotactic Ablative radiotherapy (SABR) - An analysis of UK’s largest cohort of lung SABR patients. Radiother Oncol. 2021;156:153–159. doi:10.1016/j.radonc.2020.12.015
97. Moore R, Lau S, Bezjak A, et al. The clinical relevance and management of grade 2 pneumonitis in stage III non-small cell lung cancer patients on adjuvant durvalumab. Int J Radiat Oncol Biol Phys. 2020;108(3):E100–E100. doi:10.1016/j.ijrobp.2020.07.1211
98. Barrón F, Sánchez R, Arroyo-Hernández M, et al. Risk of developing checkpoint immune pneumonitis and its effect on overall survival in non-small cell lung cancer patients previously treated with radiotherapy. Front Oncol. 2020;10:570233. doi:10.3389/fonc.2020.570233
99. Botticella A, Ibrahim T, Mezquita L, et al. Immune-related pneumonitis in NSCLC patients treated with ICI: impact of previous thoracic RT. Radiother Oncol. 2019;133:S109–S109. doi:10.1016/S0167-8140(19)30628-0
100. Jabbour SK, Berman AT, Decker RH, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: a nonrandomized controlled trial. JAMA Oncol. 2020;6(6):848–855. doi:10.1001/jamaoncol.2019.6731
101. Lin SH, Lin Y, Yao L, et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J Thorac Oncol. 2020;15(2):248–257. doi:10.1016/j.jtho.2019.10.024
102. Tian S, Switchenko JM, Buchwald ZS, et al. Lung stereotactic body radiation therapy and concurrent immunotherapy: a multicenter safety and toxicity analysis. Int J Radiat Oncol Biol Phys. 2020;108(1):304–313. doi:10.1016/j.ijrobp.2019.12.030
103. Miyamoto S, Nomura R, Sato K, et al. Nivolumab and stereotactic radiation therapy for the treatment of patients with Stage IV non-small-cell lung cancer. Jpn J Clin Oncol. 2019;49(2):160–164. doi:10.1093/jjco/hyy171
104. Harary M, Reardon DA, Iorgulescu JB. Efficacy and safety of immune checkpoint blockade for brain metastases. CNS Oncol. 2019;8(2):Cns33. doi:10.2217/cns-2018-0018
105. Qin A, Rengan R, Lee S, et al. A pilot study of atezolizumab plus hypofractionated image guided radiation therapy for the treatment of advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2020;108(1):170–177. doi:10.1016/j.ijrobp.2019.10.047
106. Yu H, Liu N, Wang H, Shang Q, Jiang P, Zhang Y. Different responses of tumor and normal cells to low-dose radiation. Contemp Oncol. 2013;17(4):356–362.
107. Su S, Zou Z, Chen F, et al. CRISPR-Cas9-mediated disruption of PD-1 on human T cells for adoptive cellular therapies of EBV positive gastric cancer. Oncoimmunology. 2017;6(1):e1249558. doi:10.1080/2162402X.2016.1249558
108. Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131(5). doi:10.1172/JCI145186
109. Yan Y, Fu J, Kowalchuk RO, et al. Exploration of radiation-induced lung injury, from mechanism to treatment: a narrative review. Transl Lung Cancer Res. 2022;11(2):307–322. doi:10.21037/tlcr-22-108
110. Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi:10.1056/NEJMoa1112824
111. Wang H, Lin X, Luo Y, et al. α-PD-L1 mAb enhances the abscopal effect of hypo-fractionated radiation by attenuating PD-L1 expression and inducing CD8(+) T-cell infiltration. Immunotherapy. 2019;11(2):101–118. doi:10.2217/imt-2018-0049
112. Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–448. doi:10.1097/SLA.0000000000003471
113. Fathi Z, Syn NL, Zhou J-G, Roudi R. Molecular epidemiology of lung cancer in Iran: implications for drug development and cancer prevention. J Hum Genet. 2018;63(7):783–794. doi:10.1038/s10038-018-0450-y
114. Tartarone A, Lerose R, Aieta M. Focus on lung cancer screening. J Thorac Dis. 2020;12(7):3815–3820. doi:10.21037/jtd.2020.02.17
115. Raoufi E, Hemmati M, Eftekhari S, et al. Epitope prediction by novel immunoinformatics approach: a state-of-the-art review. Int J Pept Res Ther. 2020;26(2):1155–1163. doi:10.1007/s10989-019-09918-z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.