Back to Journals » International Journal of Nanomedicine » Volume 18
Research Progress of Design Drugs and Composite Biomaterials in Bone Tissue Engineering
Authors Guo X , Song P, Li F, Yan Q, Bai Y, He J, Che Q, Cao H, Guo J, Su Z
Received 4 April 2023
Accepted for publication 13 June 2023
Published 1 July 2023 Volume 2023:18 Pages 3595—3622
DOI https://doi.org/10.2147/IJN.S415666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Dongwoo Khang
Xinghua Guo,1,2,* Pan Song,1,2,* Feng Li,1,2,* Qihao Yan,1,2,* Yan Bai,3 Jincan He,3 Qishi Che,4 Hua Cao,5 Jiao Guo,2 Zhengquan Su1
1Guangdong Engineering Research Center of Natural Products and New Drugs, Guangdong Provincial University Engineering Technology Research Center of Natural Products and Drugs, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China; 2Guangdong Metabolic Disease Research Center of Integrated Chinese and Western Medicine, Key Laboratory of Glucolipid Metabolic Disorder, Ministry of Education of China, Guangdong TCM Key Laboratory for Metabolic Diseases, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China; 3School of Public Health, Guangdong Pharmaceutical University, Guangzhou, 510310, People’s Republic of China; 4Guangzhou Rainhome Pharm & Tech Co., Ltd, Science City, Guangzhou, 510663, People’s Republic of China; 5School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, 528458, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhengquan Su, Guangdong Engineering Research Center of Natural Products and New Drugs, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China, Tel +86-20-3935-2067, Fax +86-20-3935-2067, Email [email protected]
Abstract: Bone, like most organs, has the ability to heal naturally and can be repaired slowly when it is slightly injured. However, in the case of bone defects caused by diseases or large shocks, surgical intervention and treatment of bone substitutes are needed, and drugs are actively matched to promote osteogenesis or prevent infection. Oral administration or injection for systemic therapy is a common way of administration in clinic, although it is not suitable for the long treatment cycle of bone tissue, and the drugs cannot exert the greatest effect or even produce toxic and side effects. In order to solve this problem, the structure or carrier simulating natural bone tissue is constructed to control the loading or release of the preparation with osteogenic potential, thus accelerating the repair of bone defect. Bioactive materials provide potential advantages for bone tissue regeneration, such as physical support, cell coverage and growth factors. In this review, we discuss the application of bone scaffolds with different structural characteristics made of polymers, ceramics and other composite materials in bone regeneration engineering and drug release, and look forward to its prospect.
Keywords: bone scaffold, drug loading, slow release, osteogenesis, biomaterial
Graphical Abstract:
Introduction
Bones are a crucial organ in the human body and provide structural support to facilitate exercise and protect the body’s other organs. Bone acts as an important mineral warehouse in the human body that contains a large amount of calcium, phosphorus and other minerals, from which the internal electrolyte level of the human body can be regulated. Additionally, red bone marrow contained in bone is critical to the supply of red blood cells.1,2 Like most organs, bone tissue cells maintain homeostasis in constant proliferation and apoptosis. However, when encountering severe trauma, malignant tumor, infection and other reasons, the dynamic balance of bone repair will tilt to the bad direction. Every year there are many accidents involving bone caused by traffic accidents, sports, and large-scale machine work. Nearly 50% of people over 50 years old have a bone disease or related disease.3 Approximately 500,000 patients undergo bone transplants each year in the United States and many more globally.4 Allogenic, xenogenic and artificial bone transplantation are the most commonly used methods of treatment or repair in clinical practice. Autologous bone grafting is considered the “gold standard” because of the absence of immune response and infection issues during tissue growth. However, patients need to be further injured to obtain materials and must endure long-term postoperative pain.5 The use of allogeneic or xenogenic bone also needs careful consideration. Although this type of bone has strong bone conductivity and a similar structure, it is difficult to apply due to inflammation, immune response, infectious diseases and other reasons, which also makes the failure rate of allogeneic or xenogeneic bone transplantation higher than that of autologous bone transplantation.6,7 The main components of bone tissue include inorganic phase and organic phase. The inorganic phase is mainly calcium phosphate-based material, accounting for about 65% of bone tissue, while the organic component is type I collagen, accounting for 30%.8,9 Biological bone substitute materials are screened out according to the composition characteristics of natural bone, and are safe and non-toxic, rarely produce immune response to the human body, and their materials are relatively cheap and easy to obtain. Because of this, the use of biological bone substitute materials for treatment has become increasingly popular.
The repair of fractures caused by various unfavorable factors is usually divided into four stages: (1) Hematoma and inflammation; (2) Formation of cartilage callus; (3) The formation of hard bone callus; (4) Bone remodeling.10,11 Blood vessels in the bone and surrounding soft tissue rupture, bleed, form a hematoma, and are accompanied by an inflammatory response. The hematoma was slowly absorbed by macrophages and disappeared. Osteoclasts in the periosteum divide and proliferate and begin to clean up locally produced tissue fragments. While cleaning up the local tissue fragments of the fracture, osteoblasts, fibroblasts and capillaries on the periosteum gradually grow into the damaged bone tissue to form fibrous callus. Osteoblasts in fibrous callus secrete bone matrix to form bony callus. After the formation of bone callus, bone blood cavity gradually formed, bone callus began to calcification, bone mineral density began to recover. (Figure 1) Bone is a dynamic organ that requires living cells (osteoblasts, osteoclasts and osteocytes), sufficient blood supply, stable structure, and growth factors and growth matrix to maintain homeostasis. Therefore, ideal bone substitutes should be combined with key biological characteristics of bone, including conductivity, induction and integration. Bone substitutes have a 3D structure similar to real bone and are suitable for the ingrowth of cells and blood vessels. In addition, bone substitute materials should also have good biocompatibility and biodegradability.12,13 Notably, to improve bone formation and enhance the performance of bone substitutes, implants are typically combined with a variety of drugs with osteogenic potential, growth factors, metal ions or cells.4,11 However, there are still some challenges before this strategy is accepted for clinical treatment. For example, after adding drugs, the physical properties of the scaffold are affected, the activity of the encapsulated protein is degraded, and the drug loading and release do not reach the expected effect. The combination of different therapeutic factors and various types of scaffolds should provide a stable environment to promote the growth of bone tissue, which is the standard that each bone substitute needs to meet.
At present, due to the need of long-term clinical treatment and complications, people are actively looking for biological bone scaffold delivery schemes with excellent drug loading and sustained release properties. In recent years, most reviews have summarized the biological scaffolds for bone tissue engineering from the perspective of materials, focusing on their manufacturing technology and mechanical stability. However, there are few reviews on the structure and function based on the combination of bone regeneration drugs and drug-loaded preparations. Considering the wide application of bone implantation and the need for local drug release therapy, in this article, the therapeutic matrices and applications of various types of drugs are described, focusing on various types of drug delivery systems, including microspheres, liposomes, and mesoporous silica nanoparticles. (Figure 2) The scheme of combining a drug-loaded system with a bone scaffold is also discussed, and its mechanical properties, drug loading and release properties, 3D structure, osteogenic effect and biocompatibility are evaluated. Finally, the drug carrier materials currently used in bone tissue engineering are presented, and their future development is predicted. By understanding the uses of drug carrier materials in bone tissue engineering, they can be used to mimic the structure and function of natural bone in the human body, thereby promoting bone tissue regeneration.
 |
Figure 2 Application of different drug delivery systems in bone tissue engineering. |
Drugs Delivered in Bone Tissue Engineering Composite Scaffolds
In recent years, drugs, growth factors, natural pharmaceutical compounds and mesenchymal stem cells have been found to have osteogenic effects in vitro and in vivo, as shown in Table 1. Moreover, loading these osteogenic substances into bone scaffolds is widely considered to be a very effective strategy.
 |
Table 1 Drug Delivery in Composite Scaffolds for Bone Tissue Engineering |
Growth Factor: BMPs
Growth factors widely exist in organisms, and they are active protein or polypeptide substances that regulate the growth and development of organisms. Among them, BMPs is widely used to promote bone formation. Bone morphogenetic proteins (BMPs) is a group of highly conserved functional proteins with similar structures and belong to the transforming growth factor β (TGF-β) family. BMP can stimulate DNA synthesis and cell replication, thereby promoting the directional differentiation of mesenchymal cells into osteoblasts. BMPs help maintain healthy bones by stimulating the mineralization, differentiation and survival of osteoblasts. In recent years, a large number of in vivo and in vitro research have shown that BMPs seem to play an important role in regulating osteoblast-osteoclast communication.64,65 Therefore, in an increasing number of studies, BMPs are added to bone scaffolds with the goal of obtaining better osteogenic effects. Unfortunately, BMP is a protein drug that degrades rapidly in vivo. Studies have shown that when the bone scaffold is immersed in saline containing BMP and filled into the bone defect site, it is quickly cleared and cannot achieve the desired bone formation and healing.66 If the dose is increased to maintain osteogenesis in the body, it may lead to serious side effects, such as hematoma, inflammatory response, and ectopic bone formation.67 Thus, it is important to create a delivery system that can facilitate the release of a sufficient drug dose over time.
In 2018, Chen’s team67 surface-immobilized rhBMP-2 in three ways, physical adsorption, covalent grafting, and heparin binding, to investigate the effect of the surface immobilization mode on rhBMP-2-induced osteogenesis in vitro and in vivo. The results showed that the osteogenic effect of the modes was in the order of physical adsorption mode < covalent grafting mode < heparin binding mode. Heparin enhanced the recognition of rhBMP-2 by BMP receptors (BMPRs) and weakened its binding to Noggin. Overall, this selective binding ability holds promise as a growth factor enhancer.
Small Molecule Drugs
To speed up the regeneration and repair of bone injury, people are often committed to the effective combination of biomaterials and drug molecules. Some small molecule drugs, such as neovastatin, dexamethasone, deferoxamine, adenosine, and aspirin, have been shown to have great benefits in inducing bone regeneration, although they do not all target bone tissue. Although aspirin, as a non-steroidal anti-inflammatory drug, has also been shown to inhibit osteoclast formation and improve bone formation.34 Adenosine is a purine nucleoside that is ubiquitous inside and outside the cell. It is often used as an antiarrhythmic drug, and it is also actively involved in bone homeostasis and bone regulation.32 Biomaterial scaffolds act as a local drug delivery system, encapsulating drugs in scaffolds to maintain activity or releasing drugs as needed to regulate the activity of cells in tissues, including cell coordination, proliferation and differentiation. For example, alendronate, a representative drug for the treatment of osteoporosis, can prevent the loss of bone tissue and effectively inhibit bone resorption caused by bone cement particles such as calcium phosphate. However, alendronate can bind to the calcium in calcium phosphate, thereby affecting its curing. To make up for this defect, alendronate can be loaded into microspheres or liposomes.28,68,69
Antibiotics
Chronic osteomyelitis and infectious bone defects are great challenges in orthopedic treatment. This is a serious complication that can cause the lesion to be difficult to heal, threaten limb loss, prolong the treatment cycle and the patient ‘s pain.44 About two-thirds of bone infections are caused by Staphylococcus aureus, which can adhere to the surface of bone tissue to form a biofilm and is extremely difficult to remove after infection.43 Early use of antibiotics such as vancomycin, gentamicin, levofloxacin, ciprofloxacin, and cefalexin for prevention has been shown to significantly reduce the incidence of related bone infections. It is worth noting that tetracycline can react with hydroxyapatite, the main component of bone tissue, and then be absorbed by bone to achieve bone targeting.46 At present, the use of systemic antibiotic therapy is more popular in clinical practice. However, both oral and injection must be repeatedly administered at high doses to maintain the effective concentration in the blood. This can lead to increased bacterial resistance to antibiotics. In addition, systemic distribution patterns make it difficult to cross areas of impaired vascularization and biofilm formation to reach infected tissues.47 In current clinical practice, antibiotic-loaded bone cement has been developed to eliminate bacteria and promote bone regeneration.17,45,70 In this study, people are also keen to design bone scaffold strategies with large load and continuous release of antibiotics. These scaffolds are designed to reduce the large dead space, eliminate regional bacterial reproduction and bacterial biofilm formation, and achieve effective prevention and treatment of infection.
Natural Pharmaceutical Compounds
Natural pharmaceutical compounds are natural organic compounds extracted from herbaceous plants in nature, and their novel chemical structures give them wonderful functions. Because natural pharmaceutical compounds have a wide range of sources and are easy to extract and prepare, they are often more cost-effective. In addition, the active ingredients of natural drugs are safer and can replace synthetic drugs to reduce long-term side effects. Natural pharmaceutical compounds have great potential in bone tissue regeneration. Pharmacological studies have shown that natural pharmaceutical compounds contribute to bone regeneration. For example, the mechanism of action of icariin is to activate the ER-α-Wnt/β-catenin signaling pathway, thereby inducing bone formation.36 A previous study has shown that curcumin can inhibit the phosphorylation and degradation of nuclear factor-κβ inhibitor (Iκβ), leading to the formation of inactive conjugate NF-κβ-Iκβ. This pathway plays a role in the differentiation of osteoblasts and the inhibition of osteosarcoma growth.40 Cannabidiol (CBD) is a bioactive molecule extracted from cannabis. Kamali et al have shown that CBD enhances the migration of MSC by activating P42/44 MAPK, and it has been confirmed that CBD can induce MSC to differentiate into osteoblast lineage in vitro and in vivo.39 However, most natural drugs exhibit poor bioavailability and are rapidly metabolized and cleared after entering the body.38,41 Sarkar et al proposed using hydrophobic polyphenolic liposomes of curcumin to enhance absorption into cells and tissues and improve targeted distribution of drugs.40 Moreover, Lai et al studied polymer scaffolds incorporated with icariin to improve the mechanical properties of scaffolds.37 In summary, understanding the chemical and pharmacological mechanisms of natural medicines and the interaction of biological materials can aid in the design of more practical bone repair scaffolds.
Stem Cells and microRNA
When the bone scaffold is implanted into the bone defect site, the lesion site produces an inflammatory response and recruits stem cells.71 The repair and functional reconstruction of bone injury ultimately depend on cells, and stem cells have been shown to play an important role in this process.71 Stem cells have the ability of self-renewal and multi-directional differentiation, and can differentiate into osteoblasts in bone tissue to promote bone tissue growth. Natural regeneration of bone tissue usually requires regenerative stem cells and scaffold-like extracellular matrix (ECM). The introduction of exogenous stem cells can regulate the microenvironment because they secrete a variety of factors, such as collagen, cytokines, and growth factors, thereby promoting osteoblast differentiation and mineralization.58 Mesenchymal stem cells (MSCs) have been widely used in bone regeneration engineering due to their excellent proliferation and differentiation ability and low immune rejection. Other stem cells such as induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) have also been shown to effectively promote bone regeneration.72 However, the migration, colonization, proliferation and differentiation of stem cells need to obtain information from the outside world. Its differentiation direction depends on the microenvironment. By receiving different signals, stem cells achieve satisfactory proliferation and differentiation to repair or form tissues. Therefore, it is necessary to design biomaterial scaffolds that mimic the ECM. Bone scaffolds fabricated by electrospinning and 3D printing are widely used to construct a suitable microenvironment for stem cell osteogenesis. They can provide irregular and rough surface, which is conducive to cell adhesion and proliferation, and is sought after.54,73
MicroRNAs (miRNA) are a kind of noncoding single-stranded RNA molecules with a length of about 22 nucleotides encoded by endogenous genes, which are involved in the regulation of post-transcriptional gene expression in bone tissues. In recent years, the use of microRNAs to induce osteogenesis and promote neovascularization of osteoblasts is expected to become an alternative approach for bone tissue engineering. An increasing number of miRNAs have been identified as regulators of factors specific to bone development, osteoblast differentiation and osteoporosis pathology.63 Some compounds, such as miRNA-26a, -135, and -148b, have been shown to induce new bone formation, as indicated by increased total alkaline phosphatase activity and increased expression of early specific biomarkers.74 They mainly regulate the osteogenic differentiation of MSCs from osteoblast precursor cells to mature osteoblasts through bone morphogenetic protein signaling pathway and wnt/β-catenin signaling pathway.75 In general, viruses, stem cells, or liposomes are commonly used delivery vectors for miRNAs in bone scaffolds. In the study of Liu et al, bone regeneration and newly formed bone volume were increased in the miRNA-26a-transfected BMSC group compared with the blank group, and the mRNA and protein expression levels of runt-related transcription factor 2 and osteocalcin were further increased.62
Drug-Loaded Bone Scaffolds
Various bone graft composite biomaterials have been clinically successful. The main biomaterials include polymers (natural polymers and synthetic polymers), inorganic calcium-containing compounds and degradable metals. Drugs, peptides, and stem cells with osteogenic potential are added to the implant to enhance osseointegration. The following focuses on commonly used methods of drug delivery and the interaction between medicinal chemistry and biomaterials. And using drug-loaded scaffolds is an innovative strategy proposed by researchers to repair bone defects.
Inorganic Drug-Loaded Bone Scaffolds
Bone is mainly composed of a composite of the inorganic minerals calcium phosphate and organic collagen, and the interconnected macroporous structure enables guest molecules and cells to pass through the inorganic network through pores and channels.10,76 In general, artificial bone scaffolds need to possess various properties similar to bone tissue to maintain bone strength in bone defect areas and promote bone regeneration; such properties include sufficient mechanical strength, excellent biocompatibility and degradability, and a porous network structure for cell growth.77–80 Hydroxyapatite (HAP),81 tricalcium phosphate (TCP),82,83 biphasic calcium phosphate (BCP),84 bioglass (BG),85 carbon nanotubes (CNTs)86,87 and other inorganic synthetic materials, because of their good biocompatibility, help the proliferation and adhesion of osteocytes and have become popular bone scaffold materials.88 Such inorganic bone implant materials, known as bioabsorbable materials, degrade over time and are replaced by bone tissue.89,90 In fact, the inorganic material HAP may still be integrated into the regenerated bone tissue after implantation, while TCP, etc., are completely resorbed.91,92
In order to construct a carrier-type slow-release bone scaffold, the underlying structure must be able to physically or chemically incorporate the drug, where its activity can be maintained. Then, as the scaffold gradually decomposes, the drug can be released over time.93 Calcium phosphates, as the most commonly used inorganic bone materials in bone tissue engineering, are generally divided into cement,94 nanoparticles,51,52 scaffolds25,76 and coatings95 for loading. During cement synthesis, drugs can be loaded from solution or added in the solid phase.94 In contrast, it is also possible to perform imprinting on scaffolds or nanoparticles by immersion, stirring and mixing in a solution containing the therapeutic factor.25,76,96 Although the treatments described in the literature are always effective, the loading of the drug cannot be determined, with pore size, porosity, and surface chemistry being the main influencing factors.90,97 Electrodeposition,95 biomimetic coating or mineralization,98 and plasma spraying99 are used to coat metal implants with a layer of drug-containing calcium phosphates to improve bioactivity and bone conductivity. Moreover, metal implants can cause infection during orthopedic surgery, posing a serious threat to long-term stability in the body.100,101 This challenge can be addressed by local delivery of antibiotic drugs from coated implants. The following mainly describes the design of inorganic bone tissue materials and the release kinetics of loaded antibiotics.
Since, in most cases, drugs or growth factors cannot specifically bind to the cement matrix, release is usually rapid, reaching >95% within a few days.96 However, some drugs may require a more gradual and sustained release strategy. For example, VEGF has a potential tumorigenic risk, so its uncontrolled release must be strictly avoided.102 For the treatment of infection, an initial burst of antibacterial and anti-inflammatory drugs is released at the implant site to effectively inhibit microbes, ideally in controlled amounts to avoid toxicity or bacterial resistance, and sustained release then continues at low concentrations.97,103,104 It is worth noting that in most calcium phosphate materials, there are two release concentration gradients, namely, the initial fast release period and the subsequent slow release period.76,105 Under actual experimental conditions, the drug release kinetics conformed to the Korsmeyer-Peppas equation and the port mode.94,96 Conversely, other factors may also affect drug release kinetics, such as diffusion, changes in cement matrix composition, different drug solubilities, and drug/matrix interactions.97,106
Results of the study by Lucas-Aparicio et al showed that the release rate of Si-β-TCP was faster than the initial release rate of silicon-free ceramic (β-TCP) (see Figure 3A).103 This result is consistent with a specific delivery mechanism that assumes a homogeneous distribution of pores, a combination of drug dissolution and diffusion processes in the matrix. The structural change of the Si-β-TCP matrix is caused by the influence of factors such as the microporosity, porosity, average pore size, and surface area of the TCP matrix.107,108 Although diffusion-controlled release is the main release mechanism of different types of cement, it can be seen from the graph that CPC and 40% Si-β-TCP showed an initial release. Additionally, the release rate changed with the drug concentration and followed first-order kinetics. However, the release rate of 80% Si-TCP showed linear behavior and was independent of the amount of dissolution. The Jatsue group also found that the release curve of bone cement prepared with β-TCP and 40% Mg-TCP followed the Fickian diffusion law (n<0.45), while the drug release rates of 26.67% Mg-TCP and 66.67% Mg-TCP were higher. Zero-order release and anomalous transfer mechanisms can account for 0.45<n<0.89 (Figure 3B).94 In summary, the pore size, pore distribution and surface area of the matrix are the main factors determining the release curve, and these properties are related to the pore connectivity, geometric shape and matrix stability. Porosity has a great influence on drug metabolism.
 |
Figure 3 (A) Pharmacokinetic analysis of vancomycin on β-TCP and Si-β-TCP. The halo images were suppressed by adsorbing 80% Si-β-TCP and 80% Si-CPC loaded with vancomycin. Pharmacokinetic analysis of vancomycin from CPC, 40% Si-CPC and 80% Si-CPC. (B) Release curves of cement prepared with three different concentrations of magnesium compared with unsubstituted cement. (C) The release spectra of four kinds of vancomycin mixed with HAP1 and HAP2 in different weight ratios and the time to complete the release of vancomycin in these four kinds of cement. (D) The release spectra of four kinds of ciprofloxacin mixed with HAP1 and HAP2 in different weight ratios and the time to complete the release of ciprofloxacin in these four kinds of cement. Notes: (A) Reprinted from Mater Sci Eng C-Mater Biol Appl, 106(8), Lucas-Aparicio J, Manchon A, Rueda C, et al. Silicon-calcium phosphate ceramics and silicon-calcium phosphate cements: Substrates to customize the release of antibiotics according to the idiosyncrasies of the patient. 110173. Copyright 2020, with permission from Elsevier.103 (B) Reprinted from Mater Sci Eng C Mater Biol Appl, 61, Cabrejos-Azama J, Alkhraisat MH, Rueda C, et al. Magnesium substitution in brushite cements: Efficacy of a new biomaterial loaded with vancomycin for the treatment of Staphylococcus aureus infections. 72–78. Copyright 2016, with permission from Elsevier.94 (C) and (D) Reprinted from Ghosh S, Wu V, Pernal S, Uskokovic V. Self-Setting Calcium Phosphate Cements with Tunable Antibiotic Release Rates for Advanced Antimicrobial Applications. ACS Appl Mater Interfaces. 2016;8(12):7691–7708. Copyright 2016, with permission from American Chemical Society.47 |
The research of Shreya team on calcium phosphate bone cement is also worth thinking about.47 HAP1 and HAP2, which are identical in chemistry, morphology and crystallography, are prepared, and the release kinetics of antibiotics is regulated by different proportions of them. As shown in the Figure 3D, the higher the content of HAP1 in the cement carrying ciprofloxacin, the slower the release rate; on the contrary, the greater the content of HAP2 in cement, the faster the release rate. The principle may be that HAP2 bypasses dicalcium phosphate as an intermediate product and directly transforms from amorphous to crystalline HAP. This process prolongs the time for HAP2 to remain amorphous. The entropy similarity between amorphous calcium phosphate and liquid phase makes it easier for drug molecules to transfer from the surface, which is conducive to drug loading and release. In addition, surface hydration weakens the binding of proteins and allows rapid drug release from HAP2.109,110 It is worth noting that the synergistic effect between the drug and the carrier is the ultimate factor determining the release characteristics. The release spectrum of vancomycin-loaded HAP was opposite to that of ciprofloxacin (Figure 3C and D).
Mesoporous Silica Drug-Loaded Bone Scaffolds
The proliferation, migration, adhesion and differentiation of bone cells contribute to bone regeneration.111,112 Ideally, bone scaffolds not only provide structural support for implanted cells but also provide osteogenic agents to regulate cellular responses and repair tissue repair.113–116 Therefore, the selection of scaffold materials and their surface properties are the key to bone tissue engineering. Inspired by some superior properties of mesoporous silica nanoparticles (MSNs), it was found that the integration of bone scaffolds and monodisperse silica may be a powerful strategy for the construction of bone regeneration control transfer systems.117
In recent years, MSNs have become an ideal drug carrier. Due to their low cytotoxicity,118,119 high porosity,120 high mechanical strength,121,122 high cost performance123 and good biocompatibility,124 MSNs have shown beneficial properties for bone tissue engineering. First, an appropriate amount of soluble orthosilicic acid can increase the expression of type I collagen in human osteoblasts, thereby accelerating the differentiation of osteoblasts.125,126 In particular, early studies have shown that silicon is one of the key elements in bone and cartilage formation and plays a crucial role in bone and cartilage formation.127,128 In addition, silicon nanomaterials have low toxicity and good biocompatibility; their large active surface area and large pore volume facilitate their use as carriers for drugs, growth factors, and essential minerals.17,112,129 Furthermore, mesoporous silica not only has good stability but is also easy to functionalize. Its surface chemistry can be changed to modify its drug release patterns, its specific targeting capabilities, and its role in stem cell or scaffold tissue engineering.126,130,131
Although most drugs are loaded in the pores of MSNs, drug release behavior is mainly controlled by diffusion.130,132 When macromolecular drugs containing antibiotic MSN were applied to composite stents, the drug loading and encapsulation efficiency were low, and the drugs are released rapidly after application.133–135 Moreover, the surface of MSNs lacks organic functional groups, and the infiltration of inorganic microcarriers on the surface of scaffolds is still difficult.116 To solve these problems, the best strategy is to modify MSNs with functional groups.
Dexamethasone (Dexa) is a synthetic glucocorticoid. As an excellent bone inducer, it can promote osteoblast differentiation and enhance alkaline phosphatase activity and bone mineralization.116,136 However, its induction of osteoblast differentiation has a typical dependence on time and dose.29 Daryasari et al29 loaded Dexa into MSNs (MSN-Dexa) and then coated a layer of CS (MSN-Dexa@CS) on the surface of the MSNs. Finally, MSN-Dexa@CS/PLLA composites were formed by electrospinning PLLA solution. As shown in Figure 4A, in this system, CS physically covered the MSN outlet to form a “gate-like” container to achieve pH-sensitive release of intracellularly loaded Dexa. After being internalized into the cells, the loaded Dexa was released into the cytoplasm with the decrease in pH and the change in CS dispersion. Thus, Dexa plays a role in osteogenic differentiation.
 |
Figure 4 (A) A schematic representation of a PLLA scaffold combined with MSN-Dexa@CS. (B) The fabrication process diagram of the Dexa@MSNs-NH2/PLLA/PCL composite scaffold. (C) A schematic of the effect of Dexa@MSNs-pep on osteogenic differentiation. (D) Difference of uptake of MSNs-pep and single nano-silicon in cells. TEM images show the absorption of MSN (a1 and a2) and MSNs-pep (b1 and b2) in BMSCs, and the comparison between them in measuring the silicon quality of BMSCs by ICP-OES (c). Compared with MSNs, the uptake of MSNs-pep nanoparticles increased significantly (**p<0.01). Notes: (A) Reprinted from Daryasari MP, Telgerd MD, Karami MH, et al. Poly-l-lactic acid scaffold incorporated chitosan-coated mesoporous silica nanoparticles as pH-sensitive composite for enhanced osteogenic differentiation of human adipose tissue stem cells by dexamethasone delivery. Artif Cell Nanomed Biotechnol. 2019;47(1):4020–4029. This is an open-access article distributed under the terms of the Creative Commons Attribution License. (http://creativecommons.org/licenses/by/4.0/).29 (B) Reprinted from Qiu KX, Chen B, Nie W, et al. Electrophoretic Deposition of Dexamethasone-Loaded Mesoporous Silica Nanoparticles onto Poly(L-Lactic Acid)/Poly(epsilon-Caprolactone) Composite Scaffold for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2016;8(6):4137–4148. Copyright 2016, with permission from American Chemical Society.116 (C) and (D) Reprinted from Zhou XJ, Feng W, Qiu KX, et al. BMP-2 Derived Peptide and Dexamethasone Incorporated Mesoporous Silica Nanoparticles for Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2015;7(29):15777–15789. Copyright 2015, with permission from American Chemical Society.137 |
Similar to the above example, Qiu’s team116 prepared aminated mesoporous silica particles (MSN-NH2) and loaded Dexa into it. Poly(l-lactic acid)/poly(ε-caprolactone) (PLLA/PCL) nanofiber scaffolds were prepared by thermally triggered segment separation and used as templates to deposit drug-loaded MSN-NH2 by electrophoresis (Figure 4B). Studies have shown that the surface of MSNs can be functionalized with appropriate organic molecules to better control the release of therapeutic factors. These molecules can also be incorporated into both MSN mesoporous channels and organic coatings.
Zhou et al137 concluded that BMP-2 and Dexa combined with MSNs can synergistically enhance the osteogenic differentiation of BMSCs, a technique which has potential application value in bone tissue engineering. They synthesized BMP-2 peptide-functionalized MSNs (MSNs-pep) by covalently grafting BMP-2 peptide onto the surface of nanoparticles through an aminosilane linker and loaded Dexa into the monodisperse two-nanotube channel to construct a nano-osteogenic delivery system (Dexa@MSNs-pep). As shown in Figure 4C and D, peptide grafting not only endows the nanoparticles with better dispersion performance and biocompatibility, but also improves their high affinity for BMP receptors. Electrostatic adsorption of non-uniform surface charges can also enhance cell uptake. In addition, the BMP-2 protein interacts with the BMP receptor on the cell surface, thereby generating a polypeptide/BMP receptor complex and activating the Smad signal transduction pathway. When absorbed by cells, Dexa enters the cytoplasm and binds to receptors.
Polymer Drug-Loaded Bone Scaffold
Generally, artificial bone scaffolds need to have various properties that are comparable to those of bone tissue to maintain bone strength in bone defect areas and promote bone regeneration, such as sufficient mechanical strength, excellent biocompatibility and degradability, and a porous network structure for cell growth.77–80 Inorganic synthetic materials such as calcium phosphate have become widely used bone scaffold materials because of their good biocompatibility, which promotes the growth and attachment of bone cells. However, they are too brittle to maintain bone strength and usually require appropriate additives to be improved.26,138 Some natural polymers, such as collagen, gelatin, alginate, chitosan, and hyaluronic acid, and synthetic polymers, such as polylactic acid-glycolic acid copolymer (PLGA), polyethylene glycol (PEG), polycaprolactone (PCL), polyhydroxyalkanoate (PHA) and polylactic acid (PLA), are broadly used as additives in bone tissue engineering.139,140
For the construction of a drug- or growth factor-loaded and sustained-release bone scaffold using a polymer as a carrier, the simplest way to load a drug in a ceramic scaffold is dip-coating.141 The method utilizes the porous structure of the ceramic scaffold142 by immersing it in a coating solution containing a specific amount of drug, and the process is repeated more than three times to achieve the desired drug layer thickness on the ceramic scaffold.44,143 It is worth mentioning that in this method, the drug is adsorbed on the ceramic surface by a weak binding force, which cannot guarantee the long-term release of the drug. Curcumin144 has anti-osteoclast activity and osteoblast-promoting activity, but its solubility and high metabolic rate often hinder the expected efficiency. Bose et al145 added PLGA-PEG and PCL-PEG polymers to a HAP matrix, and the results showed that the transferred curcumin expressed good bone conductivity (Figure 5A and B). In drug delivery applications, the most common method is to combine polymers containing drugs and then apply polymer coatings on the surface of bioceramics to control drug release behavior.44,146 Liang et al studied the application of polycarbonate with carboxylic acid structure in bone cement. Due to the covalent interaction with the antibiotic-gentamicin in commercial bone cement, the biocement has high processing capacity and adjustable release kinetics (Figure 5C).70 Polymers can be used to transport drugs with poor solubility.147,148 In addition, the addition of polymer can enhance the mechanical properties of bioceramic scaffolds and increase mechanical strength (Figure 5D).145
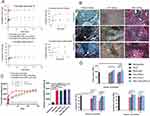 |
Figure 5 (A) The release profile of curcumin from HA in pH7.4 and pH5.0 showed that the release of curcumin in the first 24 hours increased by 39% and 64%, respectively, compared with that without polymer. (B) Optical microscopy images of extracellular matrix formation, angiogenesis and bone formation after 6 weeks of rat femur surgery. (C) In vitro release curve of gentamicin from each doped polymer (1%) cement. Compared with the control group (Gentamicin), the release of gentamicin mixed with cement increased significantly (***p<0.0001). (D) Elastic modulus, compressive yield and compressive strength of various polymer-doped cements with polymer content of 1% and 5%. Comparing the mechanical strength of each group, some of them have improved their mechanical properties in different degrees (*p<0.05, ***p<0.0001). Notes: (A) and (B) Reprinted from Mater Today Chem, 8, Bose S, Sarkar N, Banerjee D. Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. 110–120. Copyright 2018, with permission from Elsevier.145 (C) and (D) Reprinted from J Control Release, 329, Liang ZC, Yang C, Ding X, Hedrick JL, Wang W, Yang YY. Carboxylic acid-functionalized polycarbonates as bone cement additives for enhanced and sustained release of antibiotics. 871–881. Copyright 2021, with permission from Elsevier.70 |
In the past two decades, synthetic polymer-based drug-loaded scaffolds have been more popular than natural polymer-based drug-loaded scaffolds in bone tissue engineering due to their excellent porosity, degradation rate and mechanical properties.149 The lack of potential endotoxin substances and their inferior mechanical properties limits the use of natural polymers.150 In addition, to obtain better drug loading, mechanical properties, and biocompatibility, a series of nanofillers, such as nanohydroxyapatite (nHAP), mesoporous silica, and various nanoparticles, are incorporated into natural and/or synthetic polymer matrices and are widely used in bone tissue regeneration research platforms.
Microsphere Drug-Loaded Bone Scaffold
Prolonged drug release can also be achieved by creating absorbable microcarriers to release drugs within a designed time, and microsphere bone scaffolds are the more common type of drug-loaded bone scaffolds. Microspheres (1–1000 μm) are particle dispersion systems formed by dispersing drugs/bioactive molecules or being adsorbed in polymers or polymer matrices. Microspheres are ideal carrier materials due to their large surface and volume, which allow them to carry and release drugs.151,152 In general, any surgical procedure has a potential risk of inflammation and bone infection, which traditionally needs to be prevented by prolonged (2–6 weeks) antibiotic treatment,153,154 and the recovery of bone tissue is a long process. The sustained release of microsphere-loaded growth factors can promote osteogenesis.155–157 Chen et al prepared gentamicin sulfate/alendronate-double-loaded gelatin-modified PMMA bone cement (GAPBC) and found that the gel has multiple functions, fast and sustained antibiotic release, and can be effective in the treatment of osteoporosis.28
Moreover, the advantages of loading drugs, bioactive molecules, etc., on the microspheres also lie in their adsorption and encapsulation effects: (1) Some drugs have toxic effects on normal cells or tissues of the human body. The drugs encapsulated in the microspheres can maximize their efficacy and avoid adverse effects on other parts of the body.158 (2) Microspheres can easily adsorb aggregated drugs to achieve an effective therapeutic concentration.159,160 (3) When the bone cement is mixed, the drug and bioactive molecules are encapsulated in the particles to prevent the loss of efficacy or growth factor activity due to environmental changes (such as pH value and charge conditions).161,162 (4) Because of the chemical structure of some drugs, they easily react with bone matrix materials and affect the recovery process. For example, the high water solubility of alendronate prevents its incorporation into bone cement powder because it chelates calcium ions. Alendronate can dissolve quickly, which increases the content of calcium ions and affects bone adhesion.28 Tetracycline chelates Ca2+ ions, thereby interfering with the deposition of minerals, preventing mineralization, delaying the curing reaction, and prolonging the curing time.160,163
As mentioned in the previous section, many studies in bone tissue engineering directly load and release drugs or growth factors through ceramic scaffolds such as HAP, tricalcium phosphate, and bioglass.25,52,164 In general, the unmodified ceramic scaffold has an initial rapid release of drug, accompanied by relatively sustained release.143,165 Unstable release may be due to partial adsorption of the drug on the surface of the scaffold rather than being incorporated into the pores of the scaffold.166 Adding microspheres to ceramic scaffolds is an ideal solution to control drug release. Yu et al167 loaded naringin into gelatin microspheres and encapsulated these microspheres, which have good release performance, in silk cellulose/nHAP scaffolds. In addition, the large specific surface area of the microspheres can increase the surface roughness, thereby improving cell adhesion and proliferation, and is also conducive to mineral nucleation.168–170
Moreover, some studies have shown that the performance of microspheres (drug loading efficiency and encapsulation efficiency) is much higher than that of inorganic nanoparticles.152,171 In addition, microspheres can be designed as a stimuli-responsive drug-release system. Different media conditions lead to different degradability and different release behavior. The Bi team171 successfully prepared HAP/sodium alginate/chitosan (HAP/SA/CS) composite microspheres with good pH-sensitive drug release ability by utilizing the degradability of HAP and chitosan in acidic media (Figure 6A and B). It is worth noting that appropriate drug-loaded microspheres added to the scaffold can improve the porosity and mechanical properties of the composite scaffold and maintain structural stability. However, excessive addition may lead to a decrease in the compressive modulus.172,173 Therefore, the proportion of the introduced microspheres should be within a certain range, and maintaining an appropriate balance between overall porosity and mechanical properties is a key factor affecting the performance of microsphere scaffolds. Interestingly, microspheres were directly molded into scaffolds in one study. He’s team173 incorporated polylactic acid (PLLA), oleic acid-modified HAP (OA-HAP) and vancomycin hydrochloride into polytrimethyl carbonate microspheres. By adding the combination of PLLA and OA-HAP with polymer chains, the mechanical properties of the microsphere scaffolds were effectively improved, and the biodegradation of microspheres was slowed, showing acceptable biocompatibility for applications in bone tissue engineering (Figure 6C).
 |
Figure 6 (A) Preparation process of hydroxyapatite/sodium alginate/chitosan (HA/SA/CS) composite microspheres. (B) In vitro drug release curves of HA/SA/CS composite microspheres loaded with DOX at different pH values. (C) Scaffold characteristics: SEM images (a1–a4); compression modulus and compressive strength (b); porosity and pore size (c); water absorption of microspheres (d) and hydrolysis (e) and enzymatic hydrolysis (f) in the degradation cycle (Compared with the control value, *p<0.05, **p<0.01, ***p<0.0001). Notes: (A) and (B) Reprinted from Mater Sci Eng C-Mater Biol Appl, 100, Bi YG, Lin ZT, Deng ST. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. 576–583. Copyright 2019, with permission from Elsevier.171 (C) Reprinted from Carbohydr Polym, 253 (9), He J, Hu XL, Cao JF, et al. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. 117198. Copyright 2021, with permission from Elsevier.173. |
Liposome Drug-Loaded Bone Scaffold
Liposomes are closed vesicles with a two-layer structure that are formed by dispersing amphiphilic molecules such as phospholipids and cholesterol into water.174–176 Due to the hydrophobicity of the tail and the hydrophilicity of the head, the tails are easily aggregated to form an inner layer to avoid contact with water, and the heads form an outer layer where they are exposed to water.177,178 This special self-assembled vesicle structure enables these carriers to load hydrophilic substances in the internal aqueous phase and hydrophobic substances in the bilayer.31,174,179 Liposomes were discovered by Benham in 1964; since then, an increasing number of scholars have studied them. Liposome nanotechnology has been developed significantly.180 The advantages of good biocompatibility, satisfactory drug loading and stability, low cytotoxicity and immunogenicity, strong passive targeting ability and easy surface modification make liposomes excellent carriers.34,35,181,182 When liposomes are fixed/bound to biomaterial scaffolds, they have special advantages in controlling the transmission of growth factors, genes, proteins, etc. Their structures and surface properties can be optimized for specific needs.174,183,184
In a composite scaffold delivery system, liposomes can also play the same role as microspheres. In general, carrier devices for the delivery of proteins and other active biomolecules should be able to maintain the stability of the delivered proteins.185 An ideal drug delivery system can prevent the denaturation of drug molecules and achieve efficient delivery.186,187 Core-shell nanofibers can prevent denaturation and facilitate the injection of sensitive molecules into liposomes.188 In addition, a mixture of cationic liposomes and neutral lipids can be used to promote nucleic acid transmission.189 Viruses and nonviruses (ie, liposomes, cationic lipids, polymers, proteins) can act as vectors of transfected cells.24,190 The use of viral vectors increases gene expression and transduction efficiency, but also brings immunological risks and has limited applicability and limitations in gene insertion.191 Fortunately, cationic lipids have become the choice for use in gene transfer, and some lipids with cationic head groups have been synthesized and evaluated for cell transfection.192 Unfortunately, their transfection efficiency is relatively low.193,194
Drugs are coupled with biomaterial scaffolds to provide ideal therapeutic performance and mechanical support. However, the use of this technique alone may lead to problems, such as the abrupt release of drugs and potent side effects in unintended locations.195
Lee and her collaborators created apatite-PLGA 3D scaffolds that mimic trabecular bone and were combined with bone-induced oxidation liposomes.184 Sequential functionalization of polydopamine (PD) and bone-induced oxysterol (Oxy) liposomes altered the chemical composition of the scaffold surface, which indicated that Oxy liposomes were successfully added to the scaffold. By coordinating with hydroxyl-rich catechol groups and cations like calcium, the PD substrate was easily immobilized on the surface of apatite-PLGA to create PD-coated apatite-PLGA scaffolds under weakly alkaline conditions (Figure 7). Additionally, a Schiff base reaction and Michael addition reaction between catechol on PD-PLGA and amine on Oxy liposomes anchored Oxy liposomes on the surface of PD scaffolds.196 Interestingly, Oxy liposomes were immobilized to protect the scaffold from the potential toxicity of many positive charges and ensure its biocompatibility. Further PD coating significantly reduced the toxicity.
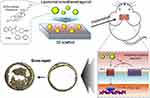 |
Figure 7 Synthesis of smooth agonist (SAG)-loaded oxidized liposome-coated PLGA scaffolds for bone transplantation to treat skull defects in mice. Notes: Reprinted from Lee CS, Hsu GCY, Sono T, Lee M, James AW. Development of a Biomaterial Scaffold Integrated with Osteoinductive Oxysterol Liposomes to Enhance Hedgehog Signaling and Bone Repair. Mol Pharm. 2021;18(4):1677–1689. Copyright 2021, with permission from American Chemical Society.184 |
The traditional drug delivery system is passive release based on polymer materials. The rate of drug release depends on the rate of diffusion of the carrier and/or the rate of degradation of the carrier.197 Lee et al used sterol oxide to construct nonphospholipid liposomes, which are a potential self-assembly drug transport carrier for applications in bone tissue engineering.184,198,199 Nonphospholipid vesicles have large sterol levels compared to phospholipid liposomes, which creates a durable double molecular layer with the lowest permeability, allowing for the controlled and sustained distribution of drugs (Figure 7).200
Hydrogel Drug-Loaded Scaffold
Hydrogel is a kind of extremely hydrophilic three-dimensional network structure gel. It swells rapidly in water and can maintain a large volume of water without dissolution. Because of its good biocompatibility, high water content, mechanical elasticity and good permeability to nutrients, it constructs a microenvironment suitable for cell growth and is widely used in drug delivery of bone tissue engineering, especially stem cell delivery.201,202 They can not only produce porous structures to capture cells or proteins, but also form chemical bonds with drug genes, leading to drug release.203,204 Hou et al14 prepared degradable polyvinyl alcohol (PVA) microgels by high-throughput microfluidic technology, so that hMSCs loaded in them maintained high activity. In addition, regulating the crosslinking density of PVA microgels can affect cell proliferation and migration, and co-loaded BMP-2 promotes osteogenic differentiation of hMSCs (Figure 8A and B). It is worth noting that hydrogels can be designed to deliver encapsulated therapeutic factors, proteins and cells to specific sites corresponding to physiological stimuli such as pH, enzyme and temperature.205,206 Kim’s team207 synthesized a bioconjugate by postmodification by combining temperature-responsive PCLA and O-phosphorylated functional groups. Free flow at low temperature (37°C) can be transformed into a stable viscoelastic gel. BMP-2-loaded bioconjugates formed stable gels in vivo and showed sustained release (Figure 8C and D).
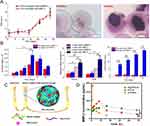 |
Figure 8 (A) During the incubation period of 4 weeks, the proliferation rate of hMSCs in polymer hydrogel and the alizarin red staining of its cells were observed. (B) The ALP activity and relative Runx2 and OPN gene expression of hMSCs cultured in BMP-2-treated or untreated hydrogels (mean ± SD, n = 3, *p<0.05, **p<0.01). (C) Extracellular simulation of in situ formation of injectable bio-coupled hydrogel loaded with BMP-2 and its sustained release to repair bone defects. (D) The concentration of BMP-2 in serum of SD rats at each time point. Notes: (A) and (B) Reprinted from Acta Biomater, 77, Hou Y, Xie W, Achazi K, et al. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. 28–37. Copyright 2018, with permission from Elsevier.14 (C) and (D) Reprinted from Carbohydr Polym, 233(11), Kim SH, Thambi T, Phan VHG, Lee DS. Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. 115832. Copyright 2020, with permission from Elsevier.207 |
Auxiliary Drug-Loaded Bone Scaffold Technology: 3D Printing Technology and Electrospinning Technology
3D printing technology is expected to promote the development of customized and integrated engineered bone tissue. 3D printing technology is a combination of software design and materials to accurately produce personalized bone tissue engineering scaffolds. Due to the imaging observation and analysis of bone defects and lesions, the 3D printed bone scaffold is perfectly bridged with the desired site. The purpose of biomaterials is to promote the growth of bone tissue at the defect site and eventually degrade in situ and be replaced by new bone tissue. Through 3D printing technology, a variety of polymer materials, such as artificial materials, natural materials, or a mixture of the two, can be used to prepare diversified bone tissue scaffolds with nanostructures.208 Due to its ability to create structures with high specific surface area, high porosity, bionic ECM structure and good biocompatibility, 3D printing technology has broad application prospects.209–212 In addition, functional nanofibers can carry many biologically active proteins, peptides, and small molecule drugs, thereby enhancing cell adhesion, proliferation, and differentiation.213–215 Therefore, 3D-printed scaffolds have become important in the biomedical field due to their ECM-like structure and bioactive material loading.216,217
The disadvantage is that neither drugs, peptides, proteins, targeted substances and cell cultures cannot be added by 3D printing because elevated temperature will lose the biological activity of these therapeutic molecules.218,219 In addition, porous scaffolds with large specific surface areas based on 3D printing are generally loaded by impregnation.11 However, in this physical mode of adsorption, the drug release rate is faster mainly due to weak force interactions such as electrostatic adsorption, hydrogen bonding, and hydrophobic interactions.220 Dang et al215 used PCL and fused deposition modeling (FDM) to prepare a dual microporous-macroporous scaffold and chose doxorubicin (DOX), paclitaxel (PTX) and cefazolin (CEF) as model drugs with properties and solubilities conducive to scaffold immersion loading. It was found that the microscale porosity significantly reduced the burst release, permitting the microporous scaffold to release DOX, PTX and CEF for up to 200, 500 and 150 hours, respectively. In addition to changing the microporous structure, the use of surface modification or coating growth factors are also commonly used strategies.67 Lai et al221 prepared porous PLGA/β-TCP/Mg (PTM) scaffolds by low-temperature rapid prototyping. The results showed that the early release of Mg2+ showed burst release through diffusion, which was regulated by Mg and TCP content. After 4 weeks, Mg2+ was released slowly and continuously with the degradation of PLGA (Figure 9A). During these two release processes, the concentration of Mg2+ was within the normal reference range, and in vivo omics analysis also showed the osteogenic and angiogenic ability of PTM scaffolds (Figure 9B).
 |
Figure 9 (A) Release of surface particles and accumulated magnesium ions and calcium ions during degradation by scaffold analysis. SEM images showed the morphology and particle distribution of the scaffold (a). Proportion ratio of each element in PTM stent (b) and cumulative release of Mg2+ (c) and Ca2+ (d). (B) Histological analysis of bone formation after stent implantation. Histological analysis of decalcified sections, in which the blue arrow shows new bone (a). Fluorescence microscope image of decalcified section, in which white arrow indicates bone formation (b). Quantitative analysis of new bone formation (c1–c4). n=3 in each group; compared with the control group, ***p<0.0001; compared with PT group, ##p<0.01, ###p<0.0001. Notes: (A) and (B) Reprinted from Biomaterials, 197, Lai Y, Li Y, Cao H, et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. 207–219. Copyright 2019, with permission from Elsevier.221 |
Similarly, fibrous bone scaffolds prepared by electrospinning technology are also a research hotspot in bone tissue engineering. Electrospinning technology is to eject the molten polymer through a syringe, form a fiber through the collector, and then stack the fibers in a specific program or irregularly to form a porous nano-scaffold. Because of the irregular arrangement of the scaffold, its total surface area increases, so that cells can better adhere and proliferate on the rough surface. In general electrospinning and 3D printing are quite different techniques, however, in some cases even electrospun scaffolds are referred to as 3D scaffolds. Different electrospinning techniques form hollow, core-shell, beaded nanofibers with different structures, making them high-load drug delivery materials. Because of the degradation performance and high porosity of drug-loaded electrospinning fibers, the 3D structure effectively controls drug release and has high affinity for local tissue.222,223 To achieve long-term release of drugs from electrospun materials, coaxial electrospinning is generally used to prepare drug-loaded fiber scaffolds. As a classic sustained-release structure, coaxial electrospinning can produce core-shell fibers, which can merge and release bioactive agents such as drugs, growth factors, and cells in a controlled manner.223–225 Carvalho et al226 used electrospinning to fabricate cell-derived ECM mesh scaffolds with high porosity and interconnectivity, simulating the structure and composition of natural bone ECM (Figure 10A and B). In addition, adenosine is incorporated into the internal layer of coaxial nanofibers to achieve controlled release of adenosine while avoiding excessive adenosine accumulation that may lead to skeletal system disorders.32 In an in vitro study, the core-shell nanofiber structure protected platelet-rich fibrin in the core layer from solvent and microenvironment changes. Compared with uniaxial nanofibers, nanofibers have lower platelet-rich fibrin release.21
 |
Figure 10 (A) Schematic of the fabrication of cell-derived ECM microfibrous scaffolds. (B) SEM observations at lower magnification (a) and at a higher magnification (b), and fiber diameter distributions (c) of ECM PCL electrospun scaffolds from different cell sources. Scale bar 5 μm. Notes: (A) and (B) Reprinted from C-Mater Biol Appl, 99, Carvalho MS, Silva JC, Udangawa RN, et al. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. 479–490. Copyright 2019, with permission from Elsevier.226 |
Conclusion and Future Prospects
As an increasing number of drugs are investigated for osteogenic properties, it has become clear that various types of substances can be used to treat orthopedic diseases and promote osteogenic differentiation. Oral administration or injection may be used for systemic administration of some drugs, but this method is not ideal for targeting a bone defect site, and some serious side effects may occur. Local administration by utilizing the implant method can concentrate the effect and avoid possible adverse reactions. However, these drugs with osteogenic potential have their own unique structures, which will be disturbed by the in vivo environment. For example, polypeptide proteins such as BMP and VEGF may be difficult to maintain their stable activity during loading into bone scaffolds and storage, resulting in drug waste and even potential toxicity. In addition, the problem of hydrophobicity of some natural pharmaceutical compounds also needs to be solved. The long period (weeks to months) of orthopedic diseases forces drug-loaded bone scaffolds to be designed for high drug loading and sustained release. Or load different drugs and release them at different release gradients to meet the osteogenic needs of each stage. These are undoubtedly a huge challenge. This review discusses drug-loaded bone scaffolds, hydrogels, cements, etc., formed from natural/synthetic polymers and inorganic substances such as calcium phosphate, calcium sulfate, and mesoporous silica. These materials all have suitable mechanical properties, degradability and biocompatibility. They mimic the natural bone environment with a porous structure, which facilitates angiogenesis and cell adhesion to the growth matrix. Therapeutic drugs are bound to different biomaterials according to their properties, such as the strong charge interaction between the scaffold and the drug and the immobilization of the drug on the surface of the scaffold by covalent bonds. Drug delivery systems may also be constructed with nanoparticles such as microspheres. From a broader perspective, fields such as medicine, chemistry, materials science, and pathology are involved in the development of effective strategies to repair critical-sized bone defects, which is a unique characteristic of bone tissue engineering.
However, most recent studies only involve in vitro or animal models and have not been clinically proven to be effective for patients. In addition, due to the complex operation process of some strategies, the reproducibility and stability of the entire drug-loaded bone scaffold system need to be carefully considered. Difficulties in large-scale production and testing also hinder commercialization. It is worth mentioning that 3D printing technology provides unprecedented opportunities for bone tissue engineering. It can flexibly construct implants that adapt to each individual patient and can act as a scaffold to control drug delivery. This undoubtedly demonstrates the great potential of 3D printing in drug delivery and tissue engineering for orthopedic applications. Although 3D printing still has some shortcomings, such as difficult to control the fine structure and the hardness of the material is not suitable for cell growth. In the future, with the development of modern bone tissue engineering technology, innovative bone transplantation programs and treatment strategies will emerge, opening up a new era of dynamic research.
Acknowledgments
This work was financially supported by the Science and Technology Program of Guangzhou, China (NO.202103000089) the Guangdong Demonstration Base for Joint Cultivation of Postgraduates (2019), the Science Foundation for Distinguished Young Scholars of Guangdong (2020B1515020026).
Disclosure
Qishi Che is affiliated with Guangzhou Rainhome Pharm & Tech Co., Ltd. The authors report no other conflicts of interest in this work.
References
1. Chande S, Bergwitz CJNr C. Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol. 2018;14(11):637–655. doi:10.1038/s41574-018-0076-3
2. Farokhi M, Mottaghitalab F, Shokrgozar M, Ou K, Mao C, Hosseinkhani HJ. Importance of dual delivery systems for bone tissue engineering. J Control Release. 2016;225:152–169. doi:10.1016/j.jconrel.2016.01.033
3. Qi JQ, Yu TQ, Hu BY, Wu HW, Ouyang HW. Current biomaterial-based bone tissue engineering and translational medicine. Review. Int J Mol Sci. 2021;22(19):
4. Bose S, Sarkar NJ. Natural medicinal compounds in bone tissue engineering. Trends Biotechnol. 2020;38(4):404–417. doi:10.1016/j.tibtech.2019.11.005
5. Kashirina A, Yao Y, Liu Y, Leng JJ. Biopolymers as bone substitutes: a review. Biomater Sci. 2019;7(10):3961–3983. doi:10.1039/c9bm00664h
6. Amini Z, Lari R. A systematic review of decellularized allograft and xenograft-derived scaffolds in bone tissue regeneration. Review. Tissue Cell. 2021;69:101494. doi:10.1016/j.tice.2021.101494
7. Nikolaou VS, Giannoudis PV. History of osteochondral allograft transplantation. Article. Inj Int J Care Inj. 2017;48(7):1283–1286. doi:10.1016/j.injury.2017.05.005
8. Guo L, Liang Z, Yang L, et al. The role of natural polymers in bone tissue engineering. J J Control Release. 2021;338:571–582. doi:10.1016/j.jconrel.2021.08.055
9. Li Z, Du T, Ruan C, Niu X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact Mater. 2021;6(5):1491–1511. doi:10.1016/j.bioactmat.2020.11.004
10. Qin D, Wang N, You X, Zhang A, Chen X, Liu Y. Collagen-based biocomposites inspired by bone hierarchical structures for advanced bone regeneration: ongoing research and perspectives. Biomater Sci. 2022;10(2):318–353. doi:10.1039/d1bm01294k
11. Wang Z, Wang Y, Yan J, et al. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504–534. doi:10.1016/j.addr.2021.05.007
12. Bow A, Anderson D, Dhar M. Commercially available bone graft substitutes: the impact of origin and processing on graft functionality. Drug Metab Rev. 2019;51(4):533–544. doi:10.1080/03602532.2019.1671860
13. Sohn H, JJBr O. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res. 2019;23:9. doi:10.1186/s40824-019-0157-y
14. Hou Y, Xie W, Achazi K, et al. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018;77:28–37. doi:10.1016/j.actbio.2018.07.003
15. Suliman S, Sun Y, Pedersen TO, et al. In vivo host response and degradation of copolymer scaffolds functionalized with nanodiamonds and bone morphogenetic protein 2. Adv Healthc Mater. 2016;5(6):730–742. doi:10.1002/adhm.201500723
16. Yao Q, Liu Y, Selvaratnam B, Koodali RT, Sun H. Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering. J Control Release. 2018;279:69–78. doi:10.1016/j.jconrel.2018.04.011
17. Paris J, Lafuente-Gómez N, Cabañas M, Román J, Peña J, Vallet-Regí M. Fabrication of a nanoparticle-containing 3D porous bone scaffold with proangiogenic and antibacterial properties. Acta Biomater. 2019;86:441–449. doi:10.1016/j.actbio.2019.01.013
18. Chen S, Shi Y, Zhang X, Ma J. Evaluation of BMP-2 and VEGF loaded 3D printed hydroxyapatite composite scaffolds with enhanced osteogenic capacity in vitro and in vivo. Mater Sci Eng C Mater Biol Appl. 2020;112:110893. doi:10.1016/j.msec.2020.110893
19. De-Paula MMM, Afewerki S, Viana BC, Webster TJ, Lobo AO, Marciano FR. Dual effective core-shell electrospun scaffolds: promoting osteoblast maturation and reducing bacteria activity. Mater Sci Eng C Mater Biol Appl. 2019;103:109778. doi:10.1016/j.msec.2019.109778
20. Chu YS, Wong PC, Jang JS, Chen CH, Wu SH. Combining Mg-Zn-Ca bulk metallic glass with a mesoporous silica nanocomposite for bone tissue engineering. Pharmaceutics. 2022;14(5). doi:10.3390/pharmaceutics14051078
21. Rastegar A, Mahmoodi M, Mirjalili M, Nasirizadeh N. Platelet-rich fibrin-loaded PCL/chitosan core-shell fibers scaffold for enhanced osteogenic differentiation of mesenchymal stem cells. Article. Carbohydr Polym. 2021;269:
22. Filova E, Rampichova M, Litvinec A, et al. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int J Pharm. 2013;447(1–2):139–149. doi:10.1016/j.ijpharm.2013.02.056
23. Chen Y, Wu T, Huang S, et al. Sustained release SDF-1alpha/TGF-beta1-loaded silk fibroin-porous gelatin scaffold promotes cartilage repair. ACS Appl Mater Interfaces. 2019;11(16):14608–14618. doi:10.1021/acsami.9b01532
24. Monteiro N, Ribeiro D, Martins A, et al. Instructive nanofibrous scaffold comprising runt-related transcription factor 2 gene delivery for bone tissue engineering. Article. ACS Nano. 2014;8(8):8082–8094. doi:10.1021/nn5021049
25. Li T, Peng MZ, Yang ZZ, et al. 3D-printed IFN-gamma-loading calcium silicate-beta-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Article. Acta Biomater. 2018;71:96–107. doi:10.1016/j.actbio.2018.03.012
26. Wang CZ, Wang YH, Lin CW, et al. Combination of a bioceramic scaffold and simvastatin nanoparticles as a synthetic alternative to autologous bone grafting. Article. Int J Mol Sci. 2018;19(12):
27. Venkatesan N, Liyanage ADT, Castro-Nunez J, et al. Biodegradable polymerized simvastatin stimulates bone formation. Acta Biomater. 2019;93:192–199. doi:10.1016/j.actbio.2019.04.059
28. Chen L, Tang Y, Zhao K, et al. Sequential release of double drug (graded distribution) loaded gelatin microspheres/PMMA bone cement. J Mater Chem B. 2021;9(2):508–522. doi:10.1039/d0tb01452d
29. Daryasari MP, Telgerd MD, Karami MH, et al. Poly-l-lactic acid scaffold incorporated chitosan-coated mesoporous silica nanoparticles as pH-sensitive composite for enhanced osteogenic differentiation of human adipose tissue stem cells by dexamethasone delivery. Article. Artif Cells Nanomed Biotechnol. 2019;47(1):4020–4029. doi:10.1080/21691401.2019.1658594
30. Zhou X, Liu P, Nie W, et al. Incorporation of dexamethasone-loaded mesoporous silica nanoparticles into mineralized porous biocomposite scaffolds for improving osteogenic activity. Int J Biol Macromol. 2020;149:116–126. doi:10.1016/j.ijbiomac.2020.01.237
31. Han XY, Sun MJ, Chen B, et al. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Article. Bioact Mater. 2021;6(6):1639–1652. doi:10.1016/j.bioactmat.2020.11.019
32. Cheng X, Cheng G, Xing X, et al. Controlled release of adenosine from core-shell nanofibers to promote bone regeneration through STAT3 signaling pathway. J Controlled Release. 2020;319:234–245. doi:10.1016/j.jconrel.2019.12.048
33. Sumathra M, Munusamy MA, Alarfaj AA, Rajan M. Osteoblast response to vitamin D3 loaded cellulose enriched hydroxyapatite Mesoporous silica nanoparticles composite. Biomed Pharmacother. 2018;103:858–868. doi:10.1016/j.biopha.2018.04.078
34. Li Y, Li Q, Li H, et al. An effective dual-factor modified 3D-printed PCL scaffold for bone defect repair. J Biomed Mater Res B Appl Biomater. 2020;108(5):2167–2179. doi:10.1002/jbm.b.34555
35. Li Y, Bai YJ, Pan JJ, et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. Article. J Mat Chem B. 2019;7(4):619–629. doi:10.1039/c8tb02756k
36. Monavari M, Homaeigohar S, Fuentes-Chandía M, et al. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO-CaO nanoparticles for bone tissue engineering. Mater Sci Eng. 2021;131:112470. doi:10.1016/j.msec.2021.112470
37. Lai Y, Cao H, Wang X, et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials. 2018;153:1–13. doi:10.1016/j.biomaterials.2017.10.025
38. Jin S, Gao J, Yang R, et al. A baicalin-loaded coaxial nanofiber scaffold regulated inflammation and osteoclast differentiation for vascularized bone regeneration. Bioact Mater. 2022;8:559–572. doi:10.1016/j.bioactmat.2021.06.028
39. Kamali A, Oryan A, Hosseini S, et al. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater Sci Eng C Mater Biol Appl. 2019;101:64–75. doi:10.1016/j.msec.2019.03.070
40. Sarkar N, Bose S. Liposome-encapsulated curcumin-loaded 3D printed scaffold for bone tissue engineering. ACS Appl Mat Interfaces. 2019;11(19):17184–17192. doi:10.1021/acsami.9b01218
41. Ji C, Bi L, Li J, Fan J. Salvianolic acid B-loaded chitosan/hydroxyapatite scaffolds promotes the repair of segmental bone defect by angiogenesis and osteogenesis. Int J Nanomed. 2019;14:8271–8284. doi:10.2147/ijn.S219105
42. Liu Y, Wang R, Chen S, et al. Heparan sulfate loaded polycaprolactone-hydroxyapatite scaffolds with 3D printing for bone defect repair. Int J Biol Macromol. 2020;148:153–162. doi:10.1016/j.ijbiomac.2020.01.109
43. Rumian Ł, Tiainen H, Cibor U, et al. Ceramic scaffolds enriched with gentamicin loaded poly(lactide-co-glycolide) microparticles for prevention and treatment of bone tissue infections. Mater Sci Eng C Mater Biol Appl. 2016;69:856–864. doi:10.1016/j.msec.2016.07.065
44. Cheng T, Qu H, Zhang G. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif Cells Nanomed Biotechnol. 2018;46(8):1935–1947. doi:10.1080/21691401.2017.1396997
45. Ferreira M, Rzhepishevska O, Grenho L, et al. Levofloxacin-loaded bone cement delivery system: highly effective against intracellular bacteria and Staphylococcus aureus biofilms. Int J Pharm. 2017;532(1):241–248. doi:10.1016/j.ijpharm.2017.08.089
46. Wang H, Liu J, Tao S, et al. Tetracycline-grafted PLGA nanoparticles as bone-targeting drug delivery system. Int J Nanomed. 2015;10:5671–5685. doi:10.2147/ijn.S88798
47. Ghosh S, Wu V, Pernal S, Uskoković V. Self-setting calcium phosphate cements with tunable antibiotic release rates for advanced antimicrobial applications. ACS Appl Mater Interfaces. 2016;8(12):7691–7708. doi:10.1021/acsami.6b01160
48. Bigham A, Aghajanian AH, Behzadzadeh S, et al. Nanostructured magnetic Mg2SiO4-CoFe2O4 composite scaffold with multiple capabilities for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2019;99:83–95. doi:10.1016/j.msec.2019.01.096
49. Liang W, Gao M, Lou J, et al. Integrating silicon/zinc dual elements with PLGA microspheres in calcium phosphate cement scaffolds synergistically enhances bone regeneration. J Mater Chem B. 2020;8(15):3038–3049. doi:10.1039/c9tb02901j
50. Rohnke M, Pfitzenreuter S, Mogwitz B, et al. Strontium release from Sr(2+)-loaded bone cements and dispersion in healthy and osteoporotic rat bone. J Control Release. 2017;262:159–169. doi:10.1016/j.jconrel.2017.07.036
51. Liao F, Peng XY, Yang F, Ke QF, Zhu ZH, Guo YP. Gadolinium-doped mesoporous calcium silicate/chitosan scaffolds enhanced bone regeneration ability. Article. Mater Sci Eng C Mater Biol Appl. 2019;104:
52. Peng XY, Hu M, Liao F, et al. La-Doped mesoporous calcium silicate/chitosan scaffolds for bone tissue engineering. Article. Biomater Sci. 2019;7(4):1565–1573. doi:10.1039/c8bm01498a
53. Kurtuldu F, Mutlu N, Michalek M, et al. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: bioactivity, biocompatibility and antibacterial activity. Mater Sci Eng C Mater Biol Appl. 2021;124:112050. doi:10.1016/j.msec.2021.112050
54. Singh B, Pramanik K. Generation of bioactive nano-composite scaffold of nanobioglass/silk fibroin/carboxymethyl cellulose for bone tissue engineering. J Biomater Sci Polym Ed. 2018;29(16):2011–2034. doi:10.1080/09205063.2018.1523525
55. Lin CY, Chang YH, Li KC, et al. The use of ASCs engineered to express BMP2 or TGF-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials. 2013;34(37):9401–9412. doi:10.1016/j.biomaterials.2013.08.051
56. Tevlin R, desJardins-Park H, Huber J, DiIorio SE, Longaker MT, Wan DC. Musculoskeletal tissue engineering: adipose derived stromal cell implementation for the treatment of osteoarthritis. Biomaterials. 2022;286:121544. doi:10.1016/j.biomaterials.2022.121544
57. Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836–849. doi:10.7150/ijbs.14809
58. Xie J, Peng C, Zhao Q, et al. Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater. 2016;29:365–379. doi:10.1016/j.actbio.2015.10.007
59. Zhang M, Shi J, Xie M, et al. Recapitulation of cartilage/bone formation using iPSCs via biomimetic 3D rotary culture approach for developmental engineering. Biomaterials. 2020;260:120334. doi:10.1016/j.biomaterials.2020.120334
60. Evans ND, Gentleman E, Chen X, Roberts CJ, Polak JM, Stevens MM. Extracellular matrix-mediated osteogenic differentiation of murine embryonic stem cells. Biomaterials. 2010;31(12):3244–3252. doi:10.1016/j.biomaterials.2010.01.039
61. Gamie Z, Tran GT, Vyzas G, et al. Stem cells combined with bone graft substitutes in skeletal tissue engineering. Review. Expert Opin Biol Ther. 2012;12(6):713–729. doi:10.1517/14712598.2012.679652
62. Liu Z, Chang H, Hou Y, et al. Lentivirus-mediated microRNA-26a overexpression in bone mesenchymal stem cells facilitates bone regeneration in bone defects of calvaria in mice. Mol Med Rep. 2018;18(6):5317–5326. doi:10.3892/mmr.2018.9596
63. Deng Y, Zhou H, Gu P, Fan X. Repair of canine medial orbital bone defects with miR-31-modified bone marrow mesenchymal stem cells. Invest Ophthalmol Vis Sci. 2014;55(9):6016–6023. doi:10.1167/iovs.14-14977
64. Bordukalo-Nikšić T, Kufner V, Vukičević S. The role of BMPs in the regulation of osteoclasts resorption and bone remodeling: from experimental models to clinical applications. Front Immunol. 2022;13:869422. doi:10.3389/fimmu.2022.869422
65. Shi C, Zhang H, Louie K, Mishina Y, Sun HJ. BMP signaling mediated by BMPR1A in osteoclasts negatively regulates osteoblast mineralization through suppression of Cx43. J Cell Biochem. 2017;118(3):605–614. doi:10.1002/jcb.25746
66. Noori A, Ashrafi S, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomed. 2017;12:4937–4961. doi:10.2147/ijn.S124671
67. Chen R, Yu Y, Zhang W, et al. Tuning the bioactivity of bone morphogenetic protein-2 with surface immobilization strategies. Acta Biomater. 2018;80:108–120. doi:10.1016/j.actbio.2018.09.011
68. Dolci L, Panzavolta S, Torricelli P, et al. Modulation of Alendronate release from a calcium phosphate bone cement: an in vitro osteoblast-osteoclast co-culture study. Int J Pharm. 2019;554:245–255. doi:10.1016/j.ijpharm.2018.11.023
69. Dolci L, Panzavolta S, Albertini B, et al. Spray-congealed solid lipid microparticles as a new tool for the controlled release of bisphosphonates from a calcium phosphate bone cement. Eur J Pharm Biopharm. 2018;122:6–16. doi:10.1016/j.ejpb.2017.10.002
70. Liang ZC, Yang C, Ding X, Hedrick JL, Wang W, Yang YJ. Carboxylic acid-functionalized polycarbonates as bone cement additives for enhanced and sustained release of antibiotics. J Controlled Release. 2021;329:871–881. doi:10.1016/j.jconrel.2020.10.018
71. Xia B, Deng Y, Lv Y, Chen G. Stem cell recruitment based on scaffold features for bone tissue engineering. Biomater Sci. 2021;9(4):1189–1203. doi:10.1039/d0bm01591a
72. Hao Z, Song Z, Huang J, et al. The scaffold microenvironment for stem cell based bone tissue engineering. Biomater Sci. 2017;5(8):1382–1392. doi:10.1039/c7bm00146k
73. Noreikaitė A, Antanavičiūtė I, Mikalayeva V, et al. Scaffold design for artificial tissue with bone marrow stem cells. Medicina. 2017;53(3):203–210. doi:10.1016/j.medici.2017.07.001
74. Qureshi A, Doyle A, Chen C, et al. Photoactivated miR-148b-nanoparticle conjugates improve closure of critical size mouse calvarial defects. Acta Biomater. 2015;12:166–173. doi:10.1016/j.actbio.2014.10.010
75. Arriaga M, Ding M, Gutierrez A, Chew S. The application of microRNAs in biomaterial scaffold-based therapies for bone tissue engineering. Biotechnol J. 2019;14(10):e1900084. doi:10.1002/biot.201900084
76. Li N, Jiang C, Zhang XD, et al. Preparation of an rhBMP-2 loaded mesoporous bioactive glass/calcium phosphate cement porous composite scaffold for rapid bone tissue regeneration. Article. J Mat Chem B. 2015;3(43):8558–8566. doi:10.1039/c5tb01423a
77. Babaie E, Bhaduri SB. Fabrication aspects of porous biomaterials in orthopedic applications: a review. Review. ACS Biomater Sci Eng. 2018;4(1):1–39. doi:10.1021/acsbiomaterials.7b00615
78. Chen XN, Fan HY, Deng XW, et al. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Review. Nanomaterials. 2018;8(11):
79. Zhang J, Wang L, Zhang W, Zhang M, Luo ZP. Synchronization of calcium sulphate cement degradation and new bone formation is improved by external mechanical regulation. Article. J Orthop Res. 2015;33(5):685–691. doi:10.1002/jor.22839
80. Zhao DY, Zhu TT, Li J, et al. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Review. Bioact Mater. 2021;6(2):346–360. doi:10.1016/j.bioactmat.2020.08.016
81. Venkatesan J, Kim SK. Nano-hydroxyapatite composite biomaterials for bone tissue engineering-a review. Review. J Biomed Nanotechnol. 2014;10(10):3124–3140. doi:10.1166/jbn.2014.1893
82. Yuan B, Wang ZW, Zhao Y, et al. In vitro and in vivo study of a novel nanoscale demineralized bone matrix coated PCL/beta-TCP scaffold for bone regeneration. Article. Macromol Biosci. 2021;21(3):
83. Bagherifard A, Yekta FJ, Aghdam HA, et al. Improvement in osseointegration of tricalcium phosphate-zircon for orthopedic applications: an in vitro and in vivo evaluation. Med Biol Eng Comput. 2020;58(8):1681–1693. doi:10.1007/s11517-020-02157-1
84. Ballouze R, Marahat MH, Mohamad S, Saidin NA, Kasim SR, Ooi JP. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. Review. J Biomed Mater Res Part B. 2021;109(10):1426–1435. doi:10.1002/jbm.b.34802
85. El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Review. Acta Biomater. 2017;62:1–28. doi:10.1016/j.actbio.2017.08.030
86. Pei BQ, Wang W, Dunne N, Li XM. Applications of carbon nanotubes in bone tissue regeneration and engineering: superiority, concerns, current advancements, and prospects. Review. Nanomaterials. 2019;9(10):1501. doi:10.3390/nano9101501
87. Qian WM, Vahid MH, Sun YL, et al. Investigation on the effect of functionalization of single-walled carbon nanotubes on the mechanical properties of epoxy glass composites: experimental and molecular dynamics simulation. J Mater Res Technol. 2021;12:1931–1945. doi:10.1016/j.jmrt.2021.03.104
88. Sahmani S, Saber-Samandari S, Khandan A, Aghdam MM. Nonlinear resonance investigation of nanoclay based bio-nanocomposite scaffolds with enhanced properties for bone substitute applications. J Alloys Compd. 2019;773:636–653. doi:10.1016/j.jallcom.2018.09.211
89. Hertz A, Bruce IJ. Inorganic materials for bone repair or replacement applications. Review. Nanomedicine. 2007;2(6):899–918. doi:10.2217/17435889.2.6.899
90. Xiao DQ, Zhang JW, Zhang CD, et al. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Review. Acta Biomater. 2020;106:22–33. doi:10.1016/j.actbio.2019.12.034
91. Takahashi Y, Yamamoto M, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Article. Biomaterials. 2005;26(17):3587–3596. doi:10.1016/j.biomaterials.2004.09.046
92. Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. Review. J Control Release. 2006;113(2):102–110. doi:10.1016/j.jconrel.2006.04.007
93. Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Review. Acta Biomater. 2012;8(4):1401–1421. doi:10.1016/j.actbio.2011.11.017
94. Cabrejos-Azama J, Alkhraisat MH, Rueda C, et al. Magnesium substitution in brushite cements: efficacy of a new biomaterial loaded with vancomycin for the treatment of Staphylococcus aureus infections. Article. Mater Sci Eng C Mater Biol Appl. 2016;61:72–78. doi:10.1016/j.msec.2015.10.092
95. Roy M, Bandyopadhyay A, Bose S. In vitro antimicrobial and biological properties of laser assisted tricalcium phosphate coating on titanium for load bearing implant. Article. Mater Sci Eng C Mater Biol Appl. 2009;29(6):1965–1968. doi:10.1016/j.msec.2009.03.009
96. Schumacher M, Reither L, Thomas J, et al. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Article. Biomater Sci. 2017;5(3):578–588. doi:10.1039/c6bm00903d
97. Mistry S, Roy S, Maitra NJ, et al. A novel, multi-barrier, drug eluting calcium sulfate/biphasic calcium phosphate biodegradable composite bone cement for treatment of experimental MRSA osteomyelitis in rabbit model. Article. J Control Release. 2016;239:169–181. doi:10.1016/j.jconrel.2016.08.014
98. Garcia-Gareta E, Hua J, Knowles JC, Blunn GW. Comparison of mesenchymal stem cell proliferation and differentiation between biomimetic and electrochemical coatings on different topographic surfaces. Article. J Mater Sci Mater Med. 2013;24(1):199–210. doi:10.1007/s10856-012-4789-x
99. Surmenev RA. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Review. Surf Coat Technol. 2012;206(8–9):2035–2056. doi:10.1016/j.surfcoat.2011.11.002
100. Sia IG, Berbari EF. Osteomyelitis. Article. Best Pract Res Clin Rheumatol. 2006;20(6):1065–1081. doi:10.1016/j.berh.2006.08.014
101. Schafer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Article. Clin Infect Dis. 2008;47(11):1403–1409. doi:10.1086/592973
102. Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium - deleterious effects of unregulated expression. Article. Circulation. 2000;102(8):898–901. doi:10.1161/01.CIR.102.8.898
103. Lucas-Aparicio J, Manchon A, Rueda C, et al. Silicon-calcium phosphate ceramics and silicon-calcium phosphate cements: substrates to customize the release of antibiotics according to the idiosyncrasies of the patient. Article. Mater Sci Eng C Mater Biol Appl. 2020;106:
104. Pastorino D, Canal C, Ginebra MP. Drug delivery from injectable calcium phosphate foams by tailoring the macroporosity-drug interaction. Article. Acta Biomater. 2015;12:250–259. doi:10.1016/j.actbio.2014.10.031
105. Murphy CM, Schindeler A, Gleeson JP, et al. A collagen-hydroxyapatite scaffold allows for binding and co-delivery of recombinant bone morphogenetic proteins and bisphosphonates. Article. Acta Biomater. 2014;10(5):2250–2258. doi:10.1016/j.actbio.2014.01.016
106. Bohner M, Lemaitre J, Merkle HP, Gander B. Control of gentamicin release from a calcium phosphate cement by admixed poly(acrylic acid). Article. J Pharm Sci. 2000;89(10):1262–1270. doi:10.1002/1520-6017(200010)89:10<1262::Aid-jps4>3.0.Co;2-7
107. Manchon A, Alkhraisat M, Rueda-Rodriguez C, et al. Silicon calcium phosphate ceramic as novel biomaterial to simulate the bone regenerative properties of autologous bone. Article. J Biomed Mater Res A. 2015;103(2):479–488. doi:10.1002/jbm.a.35196
108. Aparicio JL, Rueda C, Manchon A, et al. Effect of physicochemical properties of a cement based on silicocarnotite/calcium silicate on in vitro cell adhesion and in vivo cement degradation. Article. Biomed Mater. 2016;11(4):
109. Hanein D, Geiger B, Addadi L. Fibronectin adsorption to surfaces of hydrated crystals - an analysis of the importance of bound water in protein substrate interactions. Article. Langmuir. 1993;9(4):1058–1065. doi:10.1021/la00028a030
110. Flade K, Lau C, Mertig M, Pompe W. Osteocalcin-controlled dissolution-reprecipitation of calcium phosphate under biomimetic conditions. Article. Chem Mat. 2001;13(10):3596–3602. doi:10.1021/cm011063z
111. Thomas A, Jones F. Biological surface science. Editorial material. J Phys Condes Matter. 2004;16(26):1.
112. Eivazzadeh-Keihan R, Chenab KK, Taheri-Ledari R, et al. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Review. Mater Sci Eng C Mater Biol Appl. 2020;107:
113. He CL, Nie W, Feng W. Engineering of biomimetic nanofibrous matrices for drug delivery and tissue engineering. Article. J Mat Chem B. 2014;2(45):7828–7848. doi:10.1039/c4tb01464b
114. Liao JF, Qu Y, Chu BY, Zhang XN, Qian ZY. Biodegradable CSMA/PECA/graphene porous hybrid scaffold for cartilage tissue engineering. Article. Sci Rep. 2015;5:
115. Liao JF, Shi K, Ding QX, Qu Y, Luo F, Qian ZY. Recent developments in scaffold-guided cartilage tissue regeneration. Article. J Biomed Nanotechnol. 2014;10(10):3085–3104. doi:10.1166/jbn.2014.1934
116. Qiu KX, Chen B, Nie W, et al. Electrophoretic deposition of dexamethasone-loaded mesoporous silica nanoparticles onto Poly(L-Lactic Acid)/Poly(epsilon-Caprolactone) composite scaffold for bone tissue engineering. Article. ACS Appl Mater Interfaces. 2016;8(6):4137–4148. doi:10.1021/acsami.5b11879
117. Zhou XJ, Weng WZ, Chen B, et al. Mesoporous silica nanoparticles/gelatin porous composite scaffolds with localized and sustained release of vancomycin for treatment of infected bone defects. Article. J Mat Chem B. 2018;6(5):740–752. doi:10.1039/c7tb01246b
118. Gu WY, Wu CT, Chen JZ, Xiao Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Review. Int J Nanomed. 2013;8:13. doi:10.2147/ijn.S44393
119. Tavakol S, Nikpour MR, Hoveizi E, et al. Investigating the effects of particle size and chemical structure on cytotoxicity and bacteriostatic potential of nano hydroxyapatite/chitosan/silica and nano hydroxyapatite/chitosan/silver; as antibacterial bone substitutes. Article. J Nanopart Res. 2014;16(10):
120. Ding YP, Yao QQ, Li W, Schubert DW, Boccaccini AR, Roether JA. The evaluation of physical properties and in vitro cell behavior of PHB/PCL/sol-gel derived silica hybrid scaffolds and PHB/PCL/fumed silica composite scaffolds. Article. Colloids Surf B Biointerfaces. 2015;136:93–98. doi:10.1016/j.colsurfb.2015.08.023
121. Zhou J, Lin H, Fang TL, et al. The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Article. Biomaterials. 2010;31(6):1171–1179. doi:10.1016/j.biomaterials.2009.10.043
122. Nguyen LH, Annabi N, Nikkhah M, et al. Vascularized bone tissue engineering: approaches for potential improvement. Article. Tissue Eng Part B Rev. 2012;18(5):363–382. doi:10.1089/ten.teb.2012.0012
123. Shabafrooz V, Mozafari M, Vashaee D, Tayebi L. Electrospun nanofibers: from filtration membranes to highly specialized tissue engineering scaffolds. Review. J Nanosci Nanotechnol. 2014;14(1):522–534. doi:10.1166/jnn.2014.9195
124. Fernandes JS, Gentile P, Pires RA, Reis RL, Hatton PV. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Review. Acta Biomater. 2017;59:2–11. doi:10.1016/j.actbio.2017.06.046
125. Reffitt DM, Ogston N, Jugdaohsingh R, et al. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Article. Bone. 2003;32(2):127–135. doi:10.1016/s8756-3282(02)00950-x
126. Chen L, Zhou XJ, He CL. Mesoporous silica nanoparticles for tissue-engineering applications. Review. Wiley Interdiscip Rev Nanomed. 2019;11(6):
127. Carlisle EM. Silicon: a possible factor in bone calcification. Science. 1970;167(3916):279. doi:10.1126/science.167.3916.279
128. Carlisle EM. Silicon: an essential element for the chick. Nutr Rev. 2009;40(7):210–213. doi:10.1111/j.1753-4887.1982.tb05313.x
129. Zhang QQ, Qin M, Zhou XJ, et al. Porous nanofibrous scaffold incorporated with S1P loaded mesoporous silica nanoparticles and BMP-2 encapsulated PLGA microspheres for enhancing angiogenesis and osteogenesis. Article. J Mat Chem B. 2018;6(42):6731–6743. doi:10.1039/c8tb02138d
130. Li HW, Gu JS, Shah LA, et al. Bone cement based on vancomycin loaded mesoporous silica nanoparticle and calcium sulfate composites. Article. Mater Sci Eng C Mater Biol Appl. 2015;49:210–216. doi:10.1016/j.msec.2014.12.082
131. Cui W, Liu QQ, Yang L, et al. Sustained delivery of BMP-2-related peptide from the true bone ceramics/hollow mesoporous silica nanoparticles scaffold for bone tissue regeneration. Article. ACS Biomater Sci Eng. 2018;4(1):211–221. doi:10.1021/acsbiomaterials.7b00506
132. Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Review. Adv Drug Deliv Rev. 2008;60(11):1278–1288. doi:10.1016/j.addr.2008.03.012
133. Cauda V, Onida B, Platschek B, Muhlstein L, Bein T. Large antibiotic molecule diffusion in confined mesoporous silica with controlled morphology. Article. J Mater Chem. 2008;18(48):5888–5899. doi:10.1039/b805395b
134. Lai CY, Trewyn BG, Jeftinija DM, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. Article. J Am Chem Soc. 2003;125(15):4451–4459. doi:10.1021/ja028650l
135. Trewyn BG, Slowing II, S G, Chen HT, Lin VSY. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Review. Acc Chem Res. 2007;40(9):846–853. doi:10.1021/ar600032u
136. de Matos MBC, Piedade AP, Alvarez-Lorenzo C, Concheiro A, Braga MEM, de Sousa HC. Dexamethasone-loaded poly(epsilon-caprolactone)/silica nanoparticles composites prepared by supercritical CO2 foaming/mixing and deposition. Article. Int J Pharm. 2013;456(2):269–281. doi:10.1016/j.ijpharm.2013.08.042
137. Zhou XJ, Feng W, Qiu KX, et al. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. Article. ACS Appl Mater Interfaces. 2015;7(29):15777–15789. doi:10.1021/acsami.5b02636
138. Wang X, Zhang GL, Qi F, et al. Enhanced bone regeneration using an insulin-loaded nano-hydroxyapatite/collagen/PLGA composite scaffold. Article. Int J Nanomed. 2018;13:117–127. doi:10.2147/ijn.S150818
139. Bharadwaz A, Jayasuriya AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Review. Mater Sci Eng C Mater Biol Appl. 2020;110:
140. Pryadko A, Surmeneva M, Surmenev RJP. Review of hybrid materials based on polyhydroxyalkanoates for tissue engineering applications. Polymers. 2021;13(11). doi:10.3390/polym13111738
141. Li Y, Liu YZ, Long T, et al. Mesoporous bioactive glass as a drug delivery system: fabrication, bactericidal properties and biocompatibility. Article. J Mater Sci Mater Med. 2013;24(8):1951–1961. doi:10.1007/s10856-013-4960-z
142. Zhu M, Zhang LX, He QJ, Zhao JJ, Guo LM, Shi JL. Mesoporous bioactive glass-coated poly(L-lactic acid) scaffolds: a sustained antibiotic drug release system for bone repairing. Article. J Mater Chem. 2011;21(4):1064–1072. doi:10.1039/c0jm02179b
143. Farzin A, Etesami SA, Goodarzi A, Ai J. A facile way for development of three-dimensional localized drug delivery system for bone tissue engineering. Article. Mater Sci Eng C Mater Biol Appl. 2019;105:
144. Wang JZ, You ML, Ding ZQ, Ye WB. A review of emerging bone tissue engineering via PEG conjugated biodegradable amphiphilic copolymers. Review. Mater Sci Eng C Mater Biol Appl. 2019;97:1021–1035. doi:10.1016/j.msec.2019.01.057
145. Bose S, Sarkar N, Banerjee D. Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. Article. Mater Today Chem. 2018;8:110–120. doi:10.1016/j.mtchem.2018.03.005
146. Kim SS, Park MS, Jeon O, Choi CY, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Article. Biomaterials. 2006;27(8):1399–1409. doi:10.1016/j.biomaterials.2005.08.016
147. Subhapradha N, Abudhahir M, Aathira A, Srinivasan N, Moorthi A. Polymer coated mesoporous ceramic for drug delivery in bone tissue engineering. Review. Int J Biol Macromol. 2018;110:65–73. doi:10.1016/j.ijbiomac.2017.11.146
148. Ke XY, Ng VWL, Ono RJ, et al. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. Article. J Control Release. 2014;193:9–26. doi:10.1016/j.jconrel.2014.06.061
149. Oh SH, Kang SG, Kim ES, Cho SH, Lee JH. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Article. Biomaterials. 2003;24(22):4011–4021. doi:10.1016/s0142-9612(03)00284-9
150. Zhang L, Yang GJ, Johnson BN, Jia XF. Three-dimensional (3D) printed scaffold and material selection for bone repair. Review. Acta Biomater. 2019;84:16–33. doi:10.1016/j.actbio.2018.11.039
151. Gupta V, Khan Y, Berkland CJ, Laurencin CT, Detamore MS. Microsphere-based scaffolds in regenerative engineering. In: Yarmush ML, editor. Annual Review of Biomedical Engineering. Vol. 19. Annual Reviews; 2017:135–161.
152. He J, Lin ZD, Hu XL, et al. Biocompatible and biodegradable scaffold based on polytrimethylene carbonate-tricalcium phosphate microspheres for tissue engineering. Article. Colloids Surf B Biointerfaces. 2021;204:
153. Xie ZP, Liu X, Jia WT, Zhang CQ, Huang WH, Wang JQ. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. Article. J Control Release. 2009;139(2):118–126. doi:10.1016/j.jconrel.2009.06.012
154. Loca D, Sokolova M, Locs J, Smirnova A, Irbe Z. Calcium phosphate bone cements for local vancomycin delivery. Article. Mater Sci Eng C Mater Biol Appl. 2015;49:106–113. doi:10.1016/j.msec.2014.12.075
155. Calasans-Maia MD, Barboza CA, Soriano-Souza CA, et al. Microspheres of alginate encapsulated minocycline-loaded nanocrystalline carbonated hydroxyapatite: therapeutic potential and effects on bone regeneration. Article. Int J Nanomed. 2019;14:4559–4571. doi:10.2147/ijn.S201631
156. Cai B, Zou Q, Zuo Y, et al. Injectable gel constructs with regenerative and anti-infective dual effects based on assembled chitosan microspheres. Article. ACS Appl Mater Interfaces. 2018;10(30):25099–25112. doi:10.1021/acsami.8b06648
157. Shen XF, Zhang YX, Gu Y, et al. Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Article. Biomaterials. 2016;106:205–216. doi:10.1016/j.biomaterials.2016.08.023
158. Akkineni AR, Luo YX, Schumacher M, Nies B, Lode A, Gelinsky M. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Article. Acta Biomater. 2015;27:264–274. doi:10.1016/j.actbio.2015.08.036
159. Soriano-Souza C, Valiense H, Mavropoulos E, et al. Doxycycline containing hydroxyapatite ceramic microspheres as a bone-targeting drug delivery system. Article. J Biomed Mater Res Part B. 2020;108(4):1351–1362. doi:10.1002/jbm.b.34484
160. Ginebra MP, Canal C, Espanol M, Pastorino D, Montufar EB. Calcium phosphate cements as drug delivery materials. Review. Adv Drug Deliv Rev. 2012;64(12):1090–1110. doi:10.1016/j.addr.2012.01.008
161. Ruhe PQ, Boerman OC, Russel RGM, Spauwen PHM, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded Poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. In: Nakamura T, Yamashita K, Neo M, editors. Bioceramics 18, Pts 1 and 2. Trans Tech Publications Ltd; 2006:973–976.
162. Lanao RPF, Bosco R, Leeuwenburgh SCG, et al. RANKL delivery from calcium phosphate containing PLGA microspheres. Article. J Biomed Mater Res A. 2013;101(11):3123–3130. doi:10.1002/jbm.a.34623
163. Alkhraisat MH, Rueda C, Cabrejos-Azama J, et al. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Article. Acta Biomater. 2010;6(4):1522–1528. doi:10.1016/j.actbio.2009.10.043
164. Farzin A, Fathi M, Emadi R. Multifunctional magnetic nanostructured hardystonite scaffold for hyperthermia, drug delivery and tissue engineering applications. Article. Mater Sci Eng C Mater Biol Appl. 2017;70:21–31. doi:10.1016/j.msec.2016.08.060
165. Habraken W, Wolke JGC, Jansen JA. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Review. Adv Drug Deliv Rev. 2007;59(4–5):234–248. doi:10.1016/j.addr.2007.03.011
166. Kong DY, Shi YB, Gao Y, Fu MG, Kong SL, Lin GM. Preparation of BMP-2 loaded MPEG-PCL microspheres and evaluation of their bone repair properties. Article. Biomed Pharmacother. 2020;130:
167. Yu X, Shen GY, Shang Q, et al. A naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat. Article. Int J Biol Macromol. 2021;193:510–518. doi:10.1016/j.ijbiomac.2021.10.036
168. Kawashita M. Development and evaluation of the properties of functional ceramic microspheres for biomedical applications. Review. J Ceram Soc Jpn. 2018;126(1):1–7. doi:10.2109/jcersj2.17192
169. Chen MQ, Wang X, Ye ZY, Zhang Y, Zhou Y, Tan WS. A modular approach to the engineering of a centimeter-sized bone tissue construct with human amniotic mesenchymal stem cells-laden microcarriers. Article. Biomaterials. 2011;32(30):7532–7542. doi:10.1016/j.biomaterials.2011.06.054
170. Yuan ZY, Wei PF, Huang YQ, et al. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Article. Acta Biomater. 2019;85:294–309. doi:10.1016/j.actbio.2018.12.017
171. Bi YG, Lin ZT, Deng ST. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Article. Mater Sci Eng C Mater Biol Appl. 2019;100:576–583. doi:10.1016/j.msec.2019.03.040
172. Fan M, Ma Y, Tan HP, et al. Covalent and injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Article. Mater Sci Eng C Mater Biol Appl. 2017;71:67–74. doi:10.1016/j.msec.2016.09.068
173. He J, Hu XL, Cao JF, et al. Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Article. Carbohydr Polym. 2021;253:
174. Cheng RY, Liu LL, Xiang Y, et al. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Review. Biomaterials. 2020;232:
175. Elizondo E, Moreno E, Cabrera I, et al. Liposomes and other vesicular systems: structural characteristics, methods of preparation, and use in nanomedicine. In: Villaverde A, editor. Nanoparticles in Translational Science and Medicine. Elsevier Academic Press Inc; 2011:1–52.
176. Dubnika A, Egle K, Skrinda-Melne M, Skadins I, Rajadas J, Salma I. Development of vancomycin delivery systems based on autologous 3D platelet-rich fibrin matrices for bone tissue engineering. Article. Biomedicines. 2021;9(7):
177. Guimaraes D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Review. Int J Pharm. 2021;601:
178. Li J, Wang XL, Zhang T, et al. A review on phospholipids and their main applications in drug delivery systems. Review. Asian J Pharm Sci. 2015;10(2):81–98. doi:10.1016/j.ajps.2014.09.004
179. Liu LL, Xiang Y, Wang Z, et al. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. Article. NPG Asia Mater. 2019;11(1):
180. Bangham AD, Horne RW. Negative staining of phospholipids + their structural modification by-surface active agents as observed in electron microscope. Article. J Mol Biol. 1964;8(5):660. doi:10.1016/s0022-2836(64)80115-7
181. Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Review. Trends Biotechnol. 2014;32(1):32–45. doi:10.1016/j.tibtech.2013.09.007
182. Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Review. Nanomedicine. 2013;8(9):1509–1528. doi:10.2217/nnm.13.118
183. Monteiro N, Martins A, Pires R, et al. Immobilization of bioactive factor-loaded liposomes on the surface of electrospun nanofibers targeting tissue engineering. Article. Biomater Sci. 2014;2(9):1195–1209. doi:10.1039/c4bm00069b
184. Lee CS, Hsu GCY, Sono T, Lee M, James AW. Development of a biomaterial scaffold integrated with osteoinductive oxysterol liposomes to enhance hedgehog signaling and bone repair. Article. Mol Pharm. 2021;18(4):1677–1689. doi:10.1021/acs.molpharmaceut.0c01136
185. Amler E, Filova E, Buzgo M, et al. Functionalized nanofibers as drug-delivery systems for osteochondral regeneration. Review. Nanomedicine. 2014;9(7):1083–1094. doi:10.2217/nnm.14.57
186. Wang D, Miller SC, Kopeckova P, Kopecek J. Bone-targeting macromolecular therapeutics. Review. Adv Drug Deliv Rev. 2005;57(7):1049–1076. doi:10.1016/j.addr.2004.12.011
187. Wang GL, Mostafa NZ, Incani V, Kucharski C, Uludag H. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. Article. J Biomed Mater Res A. 2012;100A(3):684–693. doi:10.1002/jbm.a.34002
188. Mickova A, Buzgo M, Benada O, et al. Core/shell nanofibers with embedded liposomes as a drug delivery system. Meeting abstract. J Tissue Eng Regen Med. 2012;6:35.
189. Monteiro N, Martins A, Ribeiro D, et al. On the use of dexamethasone-loaded liposomes to induce the osteogenic differentiation of human mesenchymal stem cells. Article. J Tissue Eng Regen Med. 2015;9(9):1056–1066. doi:10.1002/term.1817
190. Pornpattananangkul D, Olson S, Aryal S, et al. Stimuli-responsive liposome fusion mediated by gold nanoparticles. Article. ACS Nano. 2010;4(4):1935–1942. doi:10.1021/nn9018587
191. Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Review. Adv Drug Deliv Rev. 2012;64(12):1292–1309. doi:10.1016/j.addr.2012.01.016
192. Woodle MC, Scaria P. Cationic liposomes and nucleic acids. Review. Curr Opin Colloid Interface Sci. 2001;6(1):78–84. doi:10.1016/s1359-0294(00)00091-1
193. Storrie H, Mooney DJ. Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Review. Adv Drug Deliv Rev. 2006;58(4):500–514. doi:10.1016/j.addr.2006.03.004
194. Kulkarni M, Greiser U, O’Brien T, Pandit A. Liposomal gene delivery mediated by tissue-engineered scaffolds. Review. Trends Biotechnol. 2010;28(1):28–36. doi:10.1016/j.tibtech.2009.10.003
195. Clare K, Hardwick SJ, Carpenter KLH, Weeratunge N, Mitchinson MJ. Toxicity of oxysterols to human monocyte-macrophages. Article. Atherosclerosis. 1995;118(1):67–75. doi:10.1016/0021-9150(95)05594-m
196. Park J, Brust TF, Lee HJ, Lee SC, Watts VJ, Yeo Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. Article. ACS Nano. 2014;8(4):3347–3356. doi:10.1021/nn405809c
197. Sahoo S, Ang LT, Goh JCH, Toh SL. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. Article. J Biomed Mater Res A. 2010;93A(4):1539–1550. doi:10.1002/jbm.a.32645
198. Cui ZK, Kim S, Baljon JJ, et al. Design and characterization of a therapeutic non-phospholipid liposomal nanocarrier with osteoinductive characteristics to promote bone formation. Article. ACS Nano. 2017;11(8):8055–8063. doi:10.1021/acsnano.7b02702
199. Lee CS, Kim S, Fan JB, Hwang HS, Aghaloo T, Lee M. Smoothened agonist sterosome immobilized hybrid scaffold for bone regeneration. Article. Sci Adv. 2020;6(17):
200. Cui ZK, Fan JB, Kim S, et al. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. Article. J Control Release. 2015;217:42–52. doi:10.1016/j.jconrel.2015.08.031
201. Kang M, Kang J, Le Thi P, et al. Three-dimensional printable gelatin hydrogels incorporating graphene oxide to enable spontaneous myogenic differentiation. ACS Macro Lett. 2021;10(4):426–432. doi:10.1021/acsmacrolett.0c00845
202. Bendtsen S, Quinnell S, Wei MJJobmr PA. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J Biomed Mater Res A. 2017;105(5):1457–1468. doi:10.1002/jbm.a.36036
203. Bai X, Gao MZ, Syed S, Zhuang J, Xu XY, Zhang XQ. Bioactive hydrogels for bone regeneration. Review. Bioact Mater. 2018;3(4):401–417. doi:10.1016/j.bioactmat.2018.05.006
204. Li JY, Mooney DJ. Designing hydrogels for controlled drug delivery. Review. Nat Rev Mater. 2016;1(12):
205. Du XW, Zhou J, Shi JF, Xu B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Review. Chem Rev. 2015;115(24):13165–13307. doi:10.1021/acs.chemrev.5b00299
206. Kim YM, Park MR, Song SC. Injectable polyplex hydrogel for localized and long-term delivery of siRNA. Article. ACS Nano. 2012;6(7):5757–5766. doi:10.1021/nn300842a
207. Kim SH, Thambi T, Phan VHG, Lee DS. Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Article. Carbohydr Polym. 2020;233:
208. Lee S, Kang M, Jeon S, et al. 3D bioprinting of human mesenchymal stem cells-laden hydrogels incorporating MXene for spontaneous osteodifferentiation. Heliyon. 2023;9(3):e14490. doi:10.1016/j.heliyon.2023.e14490
209. Mitragotri S, Anderson DG, Chen XY, et al. Accelerating the translation of nanomaterials in biomedicine. Article. ACS Nano. 2015;9(7):6644–6654. doi:10.1021/acsnano.5b03569
210. Jiang L, Jiang YC, Stiadle J, et al. Electrospun nanofibrous thermoplastic polyurethane/poly(glycerol sebacate) hybrid scaffolds for vocal fold tissue engineering applications. Article. Mater Sci Eng C Mater Biol Appl. 2019;94:740–749. doi:10.1016/j.msec.2018.10.027
211. Ye KQ, Kuang HZ, You ZW, Morsi Y, Mo XM. Electrospun nanofibers for tissue engineering with drug loading and release. Review. Pharmaceutics. 2019;11(4):
212. Moarrefzadeh A, Morovvati M, Angili S, Smaisim G, Khandan A, Toghraie DJ. Fabrication and finite element simulation of 3D printed poly L-lactic acid scaffolds coated with alginate/carbon nanotubes for bone engineering applications. Int J Biol Macromol. 2023;224:1496–1508. doi:10.1016/j.ijbiomac.2022.10.238
213. Sadeghi A, Moztarzadeh F, Mohandesi JA. Investigating the effect of chitosan on hydrophilicity and bioactivity of conductive electrospun composite scaffold for neural tissue engineering. Article. Int J Biol Macromol. 2019;121:625–632. doi:10.1016/j.ijbiomac.2018.10.022
214. Venugopal E, Rajeswaran N, Sahanand KS, Bhattacharyya A, Rajendran S. In vitro evaluation of phytochemical loaded electrospun gelatin nanofibers for application in bone and cartilage tissue engineering. Article. Biomed Mater. 2019;14(1):
215. Dang HP, Shabab T, Shafiee A, et al. 3D printed dual macro-, microscale porous network as a tissue engineering scaffold with drug delivering function. Article. Biofabrication. 2019;11(3):
216. Ding YP, Li W, Zhang F, et al. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Review. Adv Funct Mater. 2019;29(2):
217. Iranmanesh P, Ehsani A, Khademi A, et al. Application of 3D bioprinters for dental pulp regeneration and tissue engineering (porous architecture). Transp Porous Media. 2022;142(1–2):265–293. doi:10.1007/s11242-021-01618-x
218. Do AV, Khorsand B, Geary SM, Salem AK. 3D printing of scaffolds for tissue regeneration applications. Review. Adv Healthc Mater. 2015;4(12):1742–1762. doi:10.1002/adhm.201500168
219. Qu MY, Wang CR, Zhou XW, et al. Multi-dimensional printing for bone tissue engineering. Review. Adv Healthc Mater. 2021;10(11):
220. Ye KQ, Liu DH, Kuang HZ, et al. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. Article. J Colloid Interface Sci. 2019;534:625–636. doi:10.1016/j.jcis.2018.09.071
221. Lai Y, Li Y, Cao H, et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials. 2019;197:207–219. doi:10.1016/j.biomaterials.2019.01.013
222. Wroblewska-Krepsztul J, Rydzkowski T, Michalska-Pozoga I, Thakur VK. Biopolymers for biomedical and pharmaceutical applications: recent advances and overview of alginate electrospinning. Review. Nanomaterials. 2019;9(3):
223. Mao YJ, Zhao YP, Guan JJ, et al. Electrospun fibers: an innovative delivery method for the treatment of bone diseases. Review. Expert Opin Drug Deliv. 2020;17(7):993–1005. doi:10.1080/17425247.2020.1767583
224. Wang J, Windbergs M. Influence of polymer composition and drug loading procedure on dual drug release from PLGA:PEG electrospun fibers. Article. Eur J Pharm Sci. 2018;124:71–79. doi:10.1016/j.ejps.2018.08.028
225. Silva JC, Udangawa RN, Chen JL, et al. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Article. Mater Sci Eng C Mater Biol Appl. 2020;107:
226. Carvalho MS, Silva JC, Udangawa RN, et al. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Article. Mater Sci Eng C Mater Biol Appl. 2019;99:479–490. doi:10.1016/j.msec.2019.01.127
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

