Back to Journals » Cancer Management and Research » Volume 12
Research Progress Evaluating the Function and Mechanism of Anti-Tumor Peptides
Received 27 September 2019
Accepted for publication 8 December 2019
Published 16 January 2020 Volume 2020:12 Pages 397—409
DOI https://doi.org/10.2147/CMAR.S232708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Video abstract presented by Xuemei Jia.
Views: 1208
Xinxing Pan, Juan Xu, Xuemei Jia
Department of Gynecology, Women’s Hospital of Nanjing Medical University, Nanjing Maternity and Child Health Care Hospital, Nanjing, Jiangsu Province, People’s Republic of China
Correspondence: Xuemei Jia
Department of Gynecology, Women’s Hospital of Nanjing Medical University, Nanjing Maternity and Child Health Care Hospital, 123 Tianfeixiang, Mochou Road, Nanjing 210004, Jiangsu Province, People’s Republic of China
Tel/Fax +86-25-84460507
Email [email protected]
Abstract: Malignant tumors cause a high mortality rate worldwide, and they severely threaten human health and negatively affect the economy. Despite the advancements in tumor-related molecular genetics and effective new processes in anti-tumor drug development, the anti-tumor drugs currently used in clinical practice are inadequate due to their poor efficacy or severe side effects. Therefore, developing new safe and efficient drugs is a top priority for curing cancer. The peptide has become a suitable agent due to its exact molecular weight between whole protein and small molecule, and it has high targeting ability, high penetrability, low immunogenicity, and is convenient to synthesize and easy to modify. Because of these advantages, peptides have excellent prospect for application as anti-tumor agents. This article reviews the recent research progress evaluating anti-tumor peptides and their anti-tumor mechanisms, and may act as a reference for the future development and clinical application of anti-tumor peptides.
Keywords: anti-cancer, small biomolecule, medicine, drug development
Introduction
According to the latest cancer statistics of the International Agency for Research on Cancer, in 2018, there were an estimated 18.1 million new cancer cases and 9.6 million cancer deaths worldwide.1 Cancer severely threatens human health and decreases life expectancy, and it has become one of the most important public health problems in every country of the world in the 21st century.
Although the conventional anti-tumor drugs currently in use are multitudinous, their therapeutic effect remains unsatisfactory and could not meet clinical requirements. In addition, drug resistance, biological distribution change, biological transformation and drug clearance are currently common problems in cancer treatment. It has become clear that there is still an impending need for more effective anti-tumor candidate molecules with less side effects.
With an increasing number of protein receptors, peptide receptors, and protein-related signaling pathways that have been discovered, tumor-related peptides may lead to a surge of newly developed anti-tumor drugs. In addition, peptide-based anti-tumor drugs possess many exclusive advantages. Due to their small molecular weight, anti-cancer peptides can effectively spread through tissues or easily cross biological barriers that macromolecule-like mono-antibodies cannot cross. Because peptide therapeutics are not dependent on flow pumps, it is less likely that drug resistance will be encountered with their use compared with DNA alkylating agents. Peptides possess excellent clinical safety because their biocompatibility profile is superior to chemical compounds. Their degradation products are amino acids, which are natural entities that are used as nutrients or cellular building blocks. Peptides naturally participate in immune regulation and are more synergistic with the body’s immune system compared with common hormone agonists/antagonists. Theoretically, peptides can act precisely on any specified interaction site, and thus, are stronger targets than anti-metabolites. Additionally, there are rich sources of anti-tumor peptides, and they have the superiority of ease of acquisition, low preparation cost, and simple modification, which enables large-scale clinical applications.
Definition of Anti-Tumor Peptides
A peptide is a compound consisting of two or more α-amino acids joined together by a peptide bond. When 2 to 100 amino acids are joined, it is commonly called a polypeptide. Peptides participate in almost all cellular activities and are closely related to immune regulation,2,3 neurohormone transmitter regulation,4,5 and tumor lesions.6,7 From the point of view of clinical indications, different types of peptides are associated with different diseases, such as endocrine diseases, diabetes and tumors, among which peptides with specific anti-tumor activity are what we call “anti-tumor peptides”.8
Anti-tumor peptides are specifically applied in the field of cancer therapy. They are usually positively charged, with high hydrophobicity and strong penetrability. Compared with ordinary chemotherapy drugs, they are more specific to tumor cells and can affect host immunity through a variety of mechanisms to destroy various cancers.
Sources of Anti-Tumor Peptides
Peptides from various sources have been found to have anti-tumor effects (Table 1).
 |
Table 1 Diverse Sources of Anti-Tumor Peptides |
Naturally Existing Peptides
Peptides with anti-tumor activity are widely found in animals, plants, and microorganisms in nature. An example is the main composition of bee venom. The bee venom peptide (GIGAVLKVLTTGLPALISWIKRKRQQ) has a therapeutic effect on a variety of cancers, including breast cancer, ovarian cancer, prostate cancer, and melanoma.38 Two tripeptides extracted from pig spleen, Tyr-Ser-Leu (YSL) and Tyr-Ser-Val (YSV), play an important role in human liver cancer cells.39,40 The natural cyclic peptide RA-V extracted from the medicinal plant Ormosia yunnanensis Prain can induce mitochondrially mediated apoptosis by blocking pdk1-akt interaction, thus killing human breast cancer cells.41 A fungal metabolite, apicidin(cyclo(N-O-methyl-L-tryptophanyl-L-isoleucinyl-D-pipecolinyl-L-2-amino-8-oxodecanoyl)), can inhibit the proliferation of eight tumor cell lines, including the human cervical cancer cell line HeLa, human breast cancer cell line MCF-7, and human gastric adenocarcinoma cell line HBL-100.42
Peptides Obtained from a Combined Library
The combinatorial peptide libraries used to screen anti-tumor peptides can be divided into two categories: biological libraries and chemical libraries. The biological library contains DNA sequences encoding peptides, whose composition determines the abundance of the peptide sequences in the library. A typical example of this genotypic-phenotypic peptide library is the phage display library, which is also the most commonly used. Other types of biological libraries include bacteria, ribosomes, mRNAs, yeast, cDNA, retrovirus, baculovirus, and mammalian cell display libraries, but are not commonly used. Among the various types of chemical libraries, the one-bead-one-compound (OBOC) library and the positional scanning-synthetic peptide combinatorial library (PS-SPCL) are commonly used to screen anti-tumor peptides. The phage display library, OBOC library, and PS-SPCL are briefly described below.
(A) Phage display technology was first proposed by George Smith in 1985.43 He found that after cloning the DNA sequence encoding the unique peptide into the phage genome, the phage’s shell protein would display the peptide encoded by foreign DNA. According to the principle of molecular interaction, target ligand peptides can be screened from a phage library. This technology has been successfully used in ligand receptor interaction detection, affinity characterization, high affinity protein/peptide separation, epitope mapping, drug discovery, and other fields.
A variety of phage display peptide libraries have since been established, and many anti-tumor peptides have been separately identified. For example, a specific gastric cancer multi-drug resistant (MDR) cell-specific binding peptide, ETAPLSTMLSPY, was screened by a phage display. It can combine with MDR gastric cancer cells and reverse their MDR phenotype.44 As another example, Li isolated a synthetic peptide (CTPSPFSHC) with the ability to bind to colorectal cancer tissue that is 11–94 times higher than that of other tissues by using an in vivo phage library, and demonstrated the tumor homing ability of this peptide.45 Li identified an epidermal growth factor receptor (EGFR)-specific binding peptide GE11 (YHWYGYTPQNVI) from a phage display peptide library.46 In the H1299 xenograft mouse model, GE11 liposomes showed good tumor targeting and drug delivery ability.47
(B) The OBOC library is a compound peptide library constructed by solid phase synthesis on beads 80–100 μm, and its split-mix strategy was first described by Lam.48 Construction of easy linear α-amino acid libraries can be accomplished using standard solid-phase peptide chemical synthesis. Construction of complex peptide libraries such as cyclic peptides, branched-chain peptides, or peptides containing β or γ-amino acids requires adding specific tags to the bead structure.
APN is a cell membrane protein that plays a key role in tumor angiogenesis. Wang reported that AP-1 (YVEYHLC), a peptide with high affinity and specificity for APN, was screened by an OBOC combination peptide library.49 In vitro and in vivo optical imaging showed AP-1 accumulation in the tumor tissues of a xenograft mouse model of HepG2 liver cancer. Similarly, using OBOC library screening, Zhang identified a cyclic peptide of PLZ4 that specifically recognized and combined with human bladder cancer cells, and this specificity was demonstrated in mouse bladder cancer xenograft models.50 Xiao screened a cyclic peptide composed of nine amino acids named LXY30 and confirmed its tumor targeting by combining it with SKOV3 cells and clinical ovarian tumor tissues in vitro.51
(C) Positional scanning combinatorial synthesis of peptides (PS-SPCLs) has become a useful tool in recent years for cancer drug research, vaccine development, and protease research. In this research, many subset libraries are built, and each offshoot peptide set in the library maintains a designated site of amino acids that are changeless. The rest of the amino acids change, and then, the performance of the members of each subset is tested to identify the amino acid in designed sites with the highest activity. Bae identified a peptide anti-Flt1 (GNQWFI) from PS-SPCLs that blocks vascular endothelial growth factor (VEGF)-induced endothelial cell migration and angiogenesis in vitro and inhibits VEGF-secreted cancer cell growth and metastasis in vivo.52 Denholt identified a peptide H-FALGEA-NH with high EGFRvIII specificity, and it is expected to be used in the diagnosis and treatment of ovarian cancer, glioblastoma, and breast cancer with high EGFRvIII expression.53
Artificial Synthesis
Merrifield has made an outstanding contribution to the field of peptide synthesis by creating and developing a feasible method for the solid-phase synthesis of peptides.54 The principle of solid-phase synthesis is that the N-terminal of the first amino acid is linked to the resin, and then, amino acids are added to the peptide chain in a known sequence. In order to prevent side reactions, the amino acid side chains are protected throughout the reaction.
Anginex is an artificially designed peptide based on the sequence of several types of anti-angiogenesis proteins (including platelet factor 4 and interleukin-8). It is proven to block the growth of endothelial cells by combining with galactose clotting protein-1, thereby exerting strong anti-angiogenesis activity to inhibit tumor development.55 The expression of YAP is significantly upregulated in gastric cancer, and VGLL4 is a natural antagonistic protein of YAP by competitively binding TEAD4. Jiao found that VGLL4’s Tondu (TDU) domains are not only essential but also sufficient for its antagonism toward YAP. Thus they synthesized artificially a peptide called “Super-TDU” that can effectively hinder the progress of gastric cancer.56 Li determined that the gN spiral domains played an important role in FGF8b uniting its receptors by analyzing the FGF8b-FGFR structure.57 Therefore, a short gN spiral peptide 8b-13 (PNFTQHVREQSLV) was fabricated, and in vitro experiments confirmed that the peptide inhibited prostate cancer cell (such as PC-3, DU-145) proliferation by interfering with the function of FGF8b.
Mechanisms of Anti-Tumor Peptides
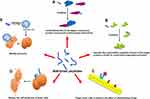
Peptides participate in all processes of life activities, and thus, they can assume anti-tumor roles in various aspects through a variety of ways, such as preventing the translation of genetic information, affecting energy metabolism, regulating immunity, and inhibiting tumor angiogenesis. The most important molecular mechanisms by which peptides exert their anti-tumor function are reviewed below (Figure 1).
Competitively Bind to the Targets of Precursor Proteins and Prevent Protein-Protein Interactions
The cell-penetrating peptide RT53 derived from AAC-11 can partially mimic the leucine repeat region of AAC-11, and then it reduces the generation of the endogenous AAC-11-Acinus complex and blocks the anti-apoptotic properties of AAC-11.58 Su found a new peptide, S7, that selectively binds to the interleukin (IL)-6 receptor gp80 and thus negatively regulates the IL-6 signaling pathway and reduces the IL-6 mediated occurrence and development of cervical cancer.59
The signaling pathway of β-catenin/LEF-1 plays an important role in tumor formation and evolution. The peptide BLBD synthesized by Hsieh can competitively bind to β-catenin with LEF-1 to interfere with the β-catenin/LEF-1 pathway, inhibiting growth, invasion, migration, and colony formation of breast cancer cells.60 The WASF3-CYFIP1 activated complex can promote actin cytoskeleton recombination, leading to the invasion and metastasis of cancer cells. The peptide WAHM synthesized by Yong has a high affinity for CYFIP1, which can prevent the formation of the WASF3-CYFIP1 complex.61
Simulate the Conformation Regulation Domain of the Target Protein to Inhibit Its Conformation-Dependent Activation
The activation of many proteins depends on conformational changes such as dimerization and isomerization. Therefore, peptides homologous to the conformational regulatory domain sequences of target proteins can be used as analogues to bind and aggregate with the target proteins and inactivate them. For example, previous studies have shown that the dimerization status of ErbB2, one of the ErbB family members, can trigger the MAPK and PI3K/Akt pathways, which are closely related to breast cancer progression, and this dimerization process is dependent on the transmembrane domain of normal ErbB2. Arpel subsequently designed a peptide that imitates the natural transmembrane domain of ErbB2 to block the dimerization reaction of ErbB2, and demonstrated the anti-cancer properties of this peptide when it was used in micromolar doses in a mouse tumor transplantation model.62
Soragni found that high-grade serous ovarian carcinoma (HGSOC) development was associated with p53 protein forming itself into an amyloid structure. Thus, he designed and synthesized a peptide named ReACp53, which can mask the 252–258 loci in the p53 protein’s adhesion region to restrain amyloid accumulation and preserve the function of p53.63 Moreover, intraperitoneal administration of ReACp53 was proved to shrink the peritoneal metastases in a mouse HGSOC xenotransplantation model.
NRP1 is a VEGF165 receptor, an important regulatory element of angiogenesis and tumor metastasis. Nasarre found that NRP1 has a GxxxG dimer motif that is necessary to trigger effective signal transmission in its transmembrane domain. On this basis, they designed a peptide to target the transmembrane sequence and inhibit NRP1 oligomerization. The experimental results showed that the synthetic peptide can prevent the subsequent biological function of NRP1 and act antagonistically in VEGF-dependent cell migration and cell proliferation.64
Target Tumor Cells to Enhance the Effect of Chemotherapy Drugs
The target polypeptide is a brand-new type of target carrier, like those such as nanoparticles and liposomes. The polypeptide carrier can directly position the loaded drug on the surface of the cell membrane receptor to exert pharmacological effect.
There is increased expression of membrane heat shock protein 70 (memHSP70) in breast cancer cells, which is significantly different from normal breast cells. Meng designed a memHSP70-targeted peptide called TKD according to the structural domain of memHSP70. The uptake of doxorubicin (DOX) modified with TKD was significantly increased in memHSP70-positive breast cancer cell lines in vitro.65 Similarly, the erbB2-targeted peptide LTVSPWY can enhance the pro-apoptotic effect of the vitamin E analogue α-TOS on erbB2-positive breast cancer cells.66 The α-enolase ligand peptide pHCT74 can target human colorectal cancer cells with high expression of α-enolase, and it can enhance the efficacy of anti-tumor drugs such as DOX and vinorelbine.67
Another exciting example is BT1718, which is a drug-binding peptide that has been initiated in Phase I/II clinical trials for advanced solid tumors. It specifically binds to overexpressed MMP14 in tumors, thereby enhancing drug targeting and penetration into tumors.68
Destroy the Cell Membrane of Tumor Cells
Some peptides are positively charged and can be folded or bent to form a special membrane structure, so as to dissolve the cancer cell membrane or form holes on the surface of the cell membrane, and then kill the tumor by lysing cells. A typical example is cell-penetrating peptide SVS-1, which is a small anti-cancer peptide. Due to the different lipid composition and electronegativity characteristics between tumor cells and normal cells on the cell membrane surface, SVS-1 only drives its own β-folding on the tumor cell surface, where it subsequently forms pores to destroy the cell membrane. Through this process, SVS-1 can kill A549 lung cancer, MCF-7 breast cancer, and other cell lines.69
Mediate Immunity
Polypeptides play an important role in the immune regulation of our body. Many polypeptides can kill tumor cells by activating the body’s own immune system and achieve the goal of curing cancer.
Alloferons, a group of peptides with anti-virus and anti-tumor activity, were primarily isolated from the hemolymph of insects after bacterial infection. Both alloferon-1 and alloferon-2 can stimulate cytotoxic activity of natural killer cells in mammals, including mice and humans. Alloferon-1 monotherapy showed moderate tumor suppressor and tumoricidal activity comparable to low dose chemotherapy in a mouse tumor transplantation model.70
In addition to naturally occurring immunoregulatory peptides, scientists are also trying to identify potential immunogenic peptides, for example, peptides containing MHCI mutations could be immunogenic, as they should be recognized as “non-self” neo-antigens by the adaptive immune system. On this basis, Yadav conducted an approach that combines whole-exome and transcriptome sequencing analysis with mass spectrometry, and two mutant peptides that can induce a T cell immune response in vivo were identified: AQL[P/A]NDVVL and ASMTN[R/M]ELM.71
In recent years, due to the development of biotechnology, a variety of tumor peptide vaccines also show a strong momentum of development. Thoman constructed a series of lipid ErbB2/HER2 epitope peptide vaccines against ErbB2 protein-expressing tumor cells.72 Granadillo designed a novel fusion protein vaccine by combining an immune-stimulating peptide LALF (32–51) with HPV 16 E7 antigen, which significantly improved the presentation of E7-derived peptides to T-cells in a preclinical model.73
Research Progress in the Processing and Modification of Anti-Tumor Peptides
Natural peptides are usually not suitable for direct application as drugs because they have inherent weaknesses, including poor chemical or physical stability, easy hydrolysis, and low concentration in tumors. Therefore, artificial modification of peptides is usually necessary to improve the physicochemical properties of peptide compounds. Chemical modification optimizes the original peptides with higher biological activity and lower immunogenicity and toxicity, and confers new desirable properties such as high targeting and excellent stability. The traditional modification methods are mainly based on the structure-activity relationship to change the main chain structure or side chain groups of the peptide to optimize the structural stability and physicochemical properties. Some common peptide modification methods are described below.
Polyethylene Glycol (PEG) Modification
PEG modification refers to covalent coupling of biomolecules with PEG, which is a non-toxic, non-immunogenic, non-ionic polymer that possesses excellent properties of biocompatibility, biodegradability, and hydrophilia. It is often applied in biopharmaceutical transformation to increase the size and molecular weight, improve the solubility, and reduce the immunogenicity. Moreover, PEG can increase the stability of the drug, reduce protein hydrolysis and renal excretion, and lengthen the retention time of the conjugate in the blood, thereby reducing the dose frequency.
Amino Acid Substitution Modification
Lycosin-I is an anti-cancer peptide extracted from spiders that can activate the mitochondrial death pathway to induce tumor cell apoptosis, but the peptide’s application was limited due to its low permeability in solid tumors. Zhang thus designed and synthesized arginine-modified lycosin-I (R-lycosin-I) by substituting arginine for lysine. Fluorescence analysis showed that the modified peptide significantly increased the uptake rate.74
The response of T cells to antigenic epitopes is influenced not only by their own affinity for the presented major histocompatibility complex (MHC) molecule but also by the affinity of the MHC-epitope complex for the T cell receptor (TCR). AH1 (SPSYVYHQF) is a specific tumor antigen peptide extracted from gp70, which is the main target of CD8 T cells in response to CT26 colorectal cancer, but its affinity for the TCR is weak. Slansky replaced the proline with alanine at position 5 of the AH1 peptide, which increased the affinity of the MHC peptide for TCR. Thus the modified peptide can more automatically activate the recognition by T cells, and thereby enhance systemic anti-tumor immunity.75
Kohno combined an EGFR-binding peptide with a newly designed cellular lytic peptide rich in cationic amino acids that can break down cell membranes and kill cancer cells, resulting in an EGFR-targeted hybrid peptide.76 In order to enhance the cytotoxic effect of this EGFR-lytic peptide on EGFR-positive tumor cells, based on analysis of biomolecular interaction and biophysical circular dichroism spectroscopy, they replaced histidine at the second site with arginine, and the cytotoxic activity of the modified hybrid peptide was 1.2–1.9 times that of the original.77
Cyclization Modification
Peptide drugs usually are degraded by enzymes in the digestive tract and have low bioavailability when taken orally. By cyclization, the cleavable N- and C-termini can be removed, the enzyme recognition site can be changed, and the degradation of peptides in the intestine, blood, and tissues can be reduced, thereby increasing the oral bioavailability degree.
The αvβ3 integrin receptor plays an important role in human tumor metastasis and tumor-induced angiogenesis, and the Arg-Gly-Asp (RGD) peptide can antagonize the function of the receptor. The FITC-conjugated cyclic RGD peptides are as effective as the fluorescence-labeled anti-integrin β3 antibody, and they can be used as fluorescent probes for staining integrin αvβ3/αvβ5 in Tumor Tissues.78 Moreover, Cyclic RGD peptide-modified liposomes may provide a targeted delivery system for apatinib in the treatment of colonic cancer.79
Gomesin is a dithiol-rich anti-microbial peptide derived from the Brazilian tarantula Acanthoscurria gomesiana and has been shown to have selective anti-cancer effects on melanoma cells. Henriques increased the anti-bacterial activity of gomesin 10 fold by designing circular gomesin analogues, which meanwhile increased the sensitivity and membrane binding affinity of the peptide to cancer cells.80
Fluorescence (FAM, FITC, TAMRA, CY) or Isotopic Labeling
The EPPT1 peptide can target the cell transmembrane underglycosylated MUC-1, which is overexpressed in colorectal cancer cells. After EPPT1 is fluorescently tagged, it can be used as a detection marker for malignant tissues in colorectal cancer after luminal instillation.81 CN-peptide and its analog-derived peptides (NS3)(CN-AVP) have strong specific recognition and ability to bind to the small cell lung cancer cell line H69. It is expected that labeling these peptides with 99m-Tc may be an ideal diagnostic radiopharmaceutical.82 Samit developed a peptide WL12 that specifically binds to PD-L1, and found that 64Cu-labeled WL12 can trace the expression of tumor PD-L1 to estimate tumor response to PD-1 or PD-L1 targeted therapy.83
Clinical Application of Anti-Tumor Peptides
Due to the many superior qualities of peptides mentioned above, there has been great activity in the development of peptides as anti-tumor drugs. In the past decade, an increasing number of peptide anti-tumor drugs have entered the market or have been tested in clinical trials. For example, gonadotropin-releasing hormone (GnRH) analog Lupron® (Abbott Laboratories, Chicago, Illinois, the USA), which is used to treat diseases such as prostate cancer, had global sales of more than $2 billion in 2011.84 A search of the US National Institutes of Health database ClinicalTrials.gov (https://clinicaltrials.gov/) using the phrase “anticancer peptides” performed in November 2019 found 1002 peptide-based clinical trials that target different types of cancer. As of Nov 2019, more than 20 anti-tumor peptides have been approved by the FDA and the EMA (Table 2).
 |
Table 2 Currently Marketed Anti-Tumor Peptides |
Peptide drugs have become an emerging weapon for the treatment of tumors, showing not only good clinical efficacy, but also considerable economic benefits. However, the clinical application and marketization of peptide drugs still face many challenges. One of the main technical challenges is the large and complex procedures in preclinical and clinical trials, such as the need for continuous functional verification and modification of candidate peptides. These procedures also ensure higher therapeutic performance and safety for peptides in clinical trials than similar drugs in existing markets.
Anti-tumor peptides, as a new class of anti-cancer drugs, are expected be a prominent target for designing innovative drugs. It is expected that they will have a huge development space and broad prospects in future clinical applications.
Conclusion and Perspectives
The development of anti-tumor drugs has been focused on DNA alkylating agents, hormone agonists/antagonists, and anti-metabolites. Anti-tumor polypeptides currently show a strong development momentum in the field of cancer drug therapy due to their small molecular weight, weak antigenicity, good targeting, convenient synthesis, and many other natural advantages. Anti-tumor peptides inhibit tumorigenesis and development by preventing protein interaction, regulating the conformation of biomolecules, competing for binding receptors, and destroying cell membranes.
It is highly likely that the future development of anti-tumor peptides will continue to build on the advantages of natural peptides, and use new polypeptide modification methods to create peptides with longer half-life, increased resistance to enzymatic hydrolysis, and compensation for other deficiencies, so as to obtain relatively perfect pharmacokinetic properties. Perhaps, in the near future, a gratifying breakthrough in the area of tumor therapy will emerge, and the efficacious agent will be an anti-tumor peptide.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 81602285& 81872126) and Nanjing Medical Science and technique Development Foundation (No. JQX17009).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
2. Mohan JF, Unanue ER. Unconventional recognition of peptides by T cells and the implications for autoimmunity. Nat Rev Immunol. 2012;12(10):721–728. doi:10.1038/nri3294
3. Hilchie AL, Wuerth K, Hancock REW. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–768. doi:10.1038/nchembio.1393
4. Garcia-Ratés S, Morrill P, Tu H, et al. (I) Pharmacological profiling of a novel modulator of the α7 nicotinic receptor: blockade of a toxic acetylcholinesterase-derived peptide increased in Alzheimer brains. Neuropharmacology. 2016;105:487–499. doi:10.1016/j.neuropharm.2016.02.006
5. Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85(1):1–74. doi:10.1016/j.pneurobio.2008.01.004
6. Nakagawa H, Mizukoshi E, Kobayashi E, et al. Association between high-avidity T-cell receptors, induced by α-fetoprotein−derived peptides, and anti-tumor effects in patients with hepatocellular carcinoma. Gastroenterology. 2017;152(6):1395–1406. doi:10.1053/j.gastro.2017.02.001
7. Arnoldini S, Moscaroli A, Chabria M, et al. Novel peptide probes to assess the tensional state of fibronectin fibers in cancer. Nat Commun. 2017;8(1):1793. doi:10.1038/s41467-017-01846-0
8. Qin Y, Qin ZD, Chen J, et al. From antimicrobial to anticancer peptides: the transformation of peptides. Recent Pat Anticancer Drug Discov. 2019;14(1):70–84. doi:10.2174/1574892814666190119165157
9. Yu A, Wang Y, Bian Y, et al. IL-1β promotes the nuclear translocaiton of S100A4 protein in gastric cancer cells MGC803 and the cell’s stem-like properties through PI3K pathway. J Cell Biochem. 2018;119(10):8163–8173. doi:10.1002/jcb.26813
10. Hung CC, Yang YH, Kuo PF, Hsu KC. Protein hydrolysates from tuna cooking juice inhibit cell growth and induce apoptosis of human breast cancer cell line MCF-7. J Funct Foods. 2014;11:563–570. doi:10.1016/j.jff.2014.08.015
11. Wang Z, Zhang X. Isolation and identification of anti-proliferative peptides from Spirulina platensis using three-step hydrolysis. J Sci Food Agric. 2017;97(3):713–1047.
12. Pan X, Zhao YQ, Hu FY, Chi CF, Wang B. Anticancer activity of a hexapeptide from skate (Raja porosa) cartilage protein hydrolysate in HeLa cells. Mar Drugs. 2016;14(8):153. doi:10.3390/md14080153
13. Luna Vital DA, González De Mejía E, Dia VP, Loarca-Piña G. Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chem. 2014;157:347–355. doi:10.1016/j.foodchem.2014.02.050
14. Rong WK, Zhi ZB, Zhang W, Yan JX, Li J, Wang R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29(6):963–968. doi:10.1016/j.peptides.2008.01.015
15. Miguel-Lillo B, Valenzuela B, Peris-Ribera JE, Soto-Matos A, Pérez-Ruixo JJ. Population pharmacokinetics of kahalalide F in advanced cancer patients. Cancer Chemother Pharmacol. 2015;76(2):365–374. doi:10.1007/s00280-015-2800-1
16. Chernysh S, Kozuharova I. Anti-tumor activity of a peptide combining patterns of insect alloferons and mammalian immunoglobulins in naïve and tumor antigen vaccinated mice. Int Immunopharmacol. 2013;17(4):1090–1093. doi:10.1016/j.intimp.2013.10.014
17. Guo X, Ma C, Du Q, et al. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: evaluation of their antimicrobial and anticancer activities. Biochimie. 2013;95(9):1784–1794. doi:10.1016/j.biochi.2013.06.003
18. Oller-Salvia B, Teixidó M, Giralt E. From venoms to BBB shuttles: synthesis and blood-brain barrier transport assessment of apamin and a nontoxic analog. Biopolymers. 2013;100(6):675–686. doi:10.1002/bip.v100.6
19. Gajski G, Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ Toxicol Pharmacol. 2013;36(2):697–705. doi:10.1016/j.etap.2013.06.009
20. Han YY, Liu HY, Han DJ, Zong XC, Zhang SQ, Chen YQ. Role of glycosylation in the anticancer activity of antibacterial peptides against breast cancer cells. Biochem Pharmacol. 2013;86(9):1254–1262. doi:10.1016/j.bcp.2013.08.008
21. Lin MC, Pan CY, Hui CF, Chen JY, Wu JL. Shrimp anti-lipopolysaccharide factor (SALF), an antimicrobial peptide, inhibits proinflammatory cytokine expressions through the MAPK and NF-κB pathways in LPS-induced HeLa cells. Peptides. 2013;40:42–48. doi:10.1016/j.peptides.2012.11.010
22. Wang K, Yan J, Dang W, et al. Membrane active antimicrobial activity and molecular dynamics study of a novel cationic antimicrobial peptide polybia-MPI, from the venom of Polybia paulista. Peptides. 2013;39:80–88. doi:10.1016/j.peptides.2012.11.002
23. Jaśkiewicz M, Neubauer D, Kazor K, Bartoszewska S, Kamysz W. Antimicrobial activity of selected antimicrobial peptides against planktonic culture and biofilm of acinetobacter baumannii. Probiotics Antimicrob Proteins. 2019;11:317–324. doi:10.1007/s12602-018-9444-5
24. Hashem L, Swedrowska M, Vllasaliu D. Intestinal uptake and transport of albumin nanoparticles: potential for oral delivery. Nanomedicine. 2018;13(11):1255–1265. doi:10.2217/nnm-2018-0029
25. Liu C, Chen C, Yang F, Li X, Cheng L, Song Y. Phytic acid improves intestinal mucosal barrier damage and reduces serum levels of proinflammatory cytokines in a 1,2-dimethylhydrazine-induced rat colorectal cancer model. Br J Nutr. 2018;120(2):121–130. doi:10.1017/S0007114518001290
26. Zheng L, Xu Y, Lin X, et al. Recent progress of marine polypeptides as anticancer agents. Recent Pat Anticancer Drug Discov. 2018;13(4):445–454. doi:10.2174/1574892813666180430110033
27. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi:10.1056/NEJMoa1716948
28. Guo Z, Li D, Peng H, et al. Specific hepatic stellate cell-penetrating peptide targeted delivery of a KLA peptide reduces collagen accumulation by inducing apoptosis. J Drug Target. 2017;25(8):715–723. doi:10.1080/1061186X.2017.1322598
29. Higa M, Katagiri C, Shimizu-Okabe C, et al. Identification of a novel cell-penetrating peptide targeting human glioblastoma cell lines as a cancer-homing transporter. Biochem Biophys Res Commun. 2015;457(2):206–212. doi:10.1016/j.bbrc.2014.12.089
30. Soler M, González-Bártulos M, Soriano-Castell D, et al. Identification of BP16 as a non-toxic cell-penetrating peptide with highly efficient drug delivery properties. Org Biomol Chem. 2014;12:1652–1663. doi:10.1039/C3OB42422G
31. Tagliabue A, Rappuoli R. Changing priorities in vaccinology: antibiotic resistance moving to the top. Front Immunol. 2018;9:1068. doi:10.3389/fimmu.2018.01068
32. Ratain MJ, Geary D, Undevia SD, et al. First-in-human, phase I study of elisidepsin (PM02734) administered as a 30-min or as a 3-hour intravenous infusion every three weeks in patients with advanced solid tumors. Invest New Drugs. 2015;33(4):901–910. doi:10.1007/s10637-015-0247-1
33. Huang CY, Huang HY, Forrest MD, Pan YR, Wu WJ, Chen HM. Inhibition effect of a custom peptide on lung tumors. PLoS One. 2014;9(10):109–174.
34. Zhou Z, Qutaish M, Han Z, et al. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat Commun. 2015;6:7984. doi:10.1038/ncomms8984
35. Tisseyre C, Bahembera E, Dardevet L, Sabatier JM, Ronjat M, De Waard M. Cell penetration properties of a highly efficient mini maurocalcine peptide. Pharmaceuticals. 2013;6(3):320–339. doi:10.3390/ph6030320
36. Messina CS, Weiher H, Schmidt-Wolf IGH. Targeting prostate cancer with a combination of WNT inhibitors and a bi-functional peptide. Anticancer Res. 2017;37(2):555–560. doi:10.21873/anticanres.11348
37. Kim JJY, Han JH, Park G, et al. Necrosis-inducing peptide has the beneficial effect on killing tumor cells through neuropilin (NRP-1) targeting. Oncotarget. 2016;7(22):32449–32461. doi:10.18632/oncotarget.8719
38. Rady I, Siddiqui IA, Rady M, Mukhtar H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017;402(608):16–31. doi:10.1016/j.canlet.2017.05.010
39. Yao Z, Lu R, Jia J, et al. The effect of tripeptide tyroserleutide (YSL) on animal models of hepatocarcinoma. Peptides. 2006;27(6):1167–1172. doi:10.1016/j.peptides.2005.02.026
40. Jia J, Lu R, Qiu S, et al. Preliminary investigation of the inhibitory effects of the tyroservaltide (YSV) tripeptide on human hepatocarcinoma BEL-7402. Cancer Biol Ther. 2005;4(9):993–997. doi:10.4161/cbt.4.9.1968
41. Fang XY, Chen W, Fan JT, et al. Plant cyclopeptide RA-V kills human breast cancer cells by inducing mitochondria-mediated apoptosis through blocking PDK1-AKT interaction. Toxicol Appl Pharmacol. 2013;267(1):95–103. doi:10.1016/j.taap.2012.12.010
42. Han JW, Ahn SH, Park SH, et al. Apicidin, a histone deacetylase inhibitor, inhibits proliferation of tumor cells via induction of p21(WAF1/Cip1) and gelsolin. Cancer Res. 2000;60(21):6068–6074.
43. Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315–1317. doi:10.1126/science.4001944
44. Kang J, Zhao G, Lin T, et al. A peptide derived from phage display library exhibits anti-tumor activity by targeting GRP78 in gastric cancer multidrug resistance cells. Cancer Lett. 2013;339(2):247–259. doi:10.1016/j.canlet.2013.06.016
45. Li ZJ, Wu WKK, Ng SSM, et al. A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J Control Release. 2010;148(3):292–302. doi:10.1016/j.jconrel.2010.09.015
46. Li Z, Zhao R, Wu X, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J. 2005;19(14):1978–1985. doi:10.1096/fj.05-4058com
47. Song S, Liu D, Peng J, et al. Peptide ligand-mediated liposome distribution and targeting to EGFR expressing tumor in vivo. Int J Pharm. 2008;363(1–2):155–161. doi:10.1016/j.ijpharm.2008.07.012
48. Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierskit WM, Knappt RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354(6348):82–84. doi:10.1038/354082a0
49. Wang W, Wei Z, Zhang D, et al. Rapid screening of peptide probes through in situ single-bead sequencing microarray. Anal Chem. 2014;86(23):11854–11859. doi:10.1021/ac503454z
50. Zhang H, Aina OH, Lam KS, et al. Identification of a bladder cancer-specific ligand using a combinatorial chemistry approach. Urol Oncol Semin Orig Investig. 2012;30(5):635–645. doi:10.1016/j.urolonc.2010.06.011
51. Xiao W, Li T, Bononi FC, et al. Discovery and characterization of a high-affinity and high-specificity peptide ligand LXY30 for in vivo targeting of α3 integrin-expressing human tumors. EJNMMI Res. 2016;6(1):18. doi:10.1186/s13550-016-0165-z
52. Bae DG, Kim TD, Li G, Yoon WH, Chae CB. Anti-Flt1 peptide, a vascular endothelial growth factor receptor 1 - specific hexapeptide, inhibits tumor growth and metastasis. Clin Cancer Res. 2005;11(7):2651–2661. doi:10.1158/1078-0432.CCR-04-1564
53. Denholt CL, Hansen PR, Pedersen N, Poulsen HS, Gillings N, Kjær A. Identification of novel peptide ligands for the cancer-specific receptor mutation EFGRvIII using a mixture-based synthetic combinatorial library. Biopolymers. 2009;91(3):201–206. doi:10.1002/bip.v91:3
54. Merrifield RB. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85(14):2149–2154. doi:10.1021/ja00897a025
55. van der Schaft DWJ, Dings RPM, de Lussanet QG, et al. The designer anti-angiogenic peptide anginex targets tumor endothelial cells and inhibits tumor growth in animal models. FASEB J. 2002;16(14):1991–1993. doi:10.1096/fj.02-0509fje
56. Jiao S, Wang H, Shi Z, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25(2):166–180. doi:10.1016/j.ccr.2014.01.010
57. Li T, Luo W, He D, et al. A short peptide derived from the gN helix domain of FGF8b suppresses the growth of human prostate cancer cells. Cancer Lett. 2013;339(2):226–236. doi:10.1016/j.canlet.2013.06.001
58. Jagot-Lacoussiere L, Kotula E, Villoutreix BO, Bruzzoni-Giovanelli H, Poyet JL. A cell-penetrating peptide targeting AAC-11 specifically induces cancer cells death. Cancer Res. 2016;76(18):5479–5490. doi:10.1158/0008-5472.CAN-16-0302
59. Su JL, Lai KP, Chen CA, et al. A novel peptide specifically binding to interleukin-6 receptor (gp80) inhibits angiogenesis and tumor growth. Cancer Res. 2005;65(11):4827–4835. doi:10.1158/0008-5472.CAN-05-0188
60. Hsieh TH, Hsu CY, Tsai CF, et al. A novel cell-penetrating peptide suppresses breast tumorigenesis by inhibiting β-catenin/LEF-1 signaling. Sci Rep. 2016;6:1–12. doi:10.1038/srep19156
61. Teng Y, Bahassan A, Dong D, et al. Targeting the WASF3-CYFIP1 complex using stapled peptides suppresses cancer cell invasion. Cancer Res. 2016;76(4):965–973. doi:10.1158/0008-5472.CAN-15-1680
62. Arpel A, Sawma P, Spenlé C, et al. Transmembrane domain targeting peptide antagonizing ErbB2/Neu inhibits breast tumor growth and metastasis. Cell Rep. 2014;8(6):1714–1721. doi:10.1016/j.celrep.2014.07.044
63. Soragni A, Janzen DM, Johnson LM, et al. A designed inhibitor of p53 aggregation rescues p53 tumor suppression in ovarian carcinomas. Cancer Cell. 2016;29(1):90–103. doi:10.1016/j.ccell.2015.12.002
64. Nasarre C, Roth M, Jacob L, et al. Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene. 2010;29(16):2381–2392. doi:10.1038/onc.2010.9
65. Meng Y, Wang S, Li C, et al. TKD peptide as a ligand targeting drug delivery systems to memHsp70-positive breast cancer. Int J Pharm. 2016;498(1–2):40–48. doi:10.1016/j.ijpharm.2015.12.013
66. Wang XF, Birringer M, Dong LF, et al. A peptide conjugate of vitamin E succinate targets breast cancer cells with high ErbB2 expression. Cancer Res. 2007;67(7):3337–3344. doi:10.1158/0008-5472.CAN-06-2480
67. Wu CH, Kuo YH, Hong RL, Wu HC. α-enolase-binding peptide enhances drug delivery efficiency and therapeutic efficacy against colorectal cancer. Sci Transl Med. 2015;7(290):290–291. doi:10.1126/scitranslmed.aaa9391
68. Bennett G, Harrison H, Campbell S, et al. Development of BT1718, a Bicycle Drug Conjugate® (BDC) targeting MT1-MMP for treatment of solid tumours. Eur J Cancer. 2016;69(1):21. doi:10.1016/S0959-8049(16)32642-9
69. Sinthuvanich C, Veiga AS, Gupta K, Gaspar D, Blumenthal R, Schneider JP. Anticancer β-hairpin peptides: membrane-induced folding triggers activityc. J Am Chem Soc. 2012;134(14):6210–6217. doi:10.1021/ja210569f
70. Chernysh S, Irina K, Irina A. Anti-tumor activity of immunomodulatory peptide alloferon-1 in mouse tumor transplantation model. Int Immunopharmacol. 2012;12(1):312–314. doi:10.1016/j.intimp.2011.10.016
71. Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. doi:10.1038/nature14001
72. Thomann JS, Heurtault B, Weidner S, et al. Antitumor activity of liposomal ErbB2/HER2 epitope peptide-based vaccine constructs incorporating TLR agonists and mannose receptor targeting. Biomaterials. 2011;32(20):4574–4583. doi:10.1016/j.biomaterials.2011.03.015
73. Granadillo M, Vallespi MG, Batte A, et al. A novel fusion protein-based vaccine comprising a cell penetrating and immunostimulatory peptide linked to human papillomavirus (HPV) type 16 E7 antigen generates potent immunologic and anti-tumor responses in mice. Vaccine. 2011;29(5):920–930. doi:10.1016/j.vaccine.2010.11.083
74. Zhang P, Ma J, Yan Y, et al. Arginine modification of lycosin-I to improve inhibitory activity against cancer cells. Org Biomol Chem. 2017;15(44):9379–9388. doi:10.1039/C7OB02233F
75. Slansky JE, Rattis FM, Boyd LF, et al. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13(4):529–538. doi:10.1016/S1074-7613(00)00052-2
76. Kohno M, Horibe T, Haramoto M, et al. A novel hybrid peptide targeting EGFR-expressing cancers. Eur J Cancer. 2011;47(5):773–783. doi:10.1016/j.ejca.2010.10.021
77. Tada N, Horibe T, Haramoto M, Ohara K, Kohno M, Kawakami K. A single replacement of histidine to arginine in EGFR-lytic hybrid peptide demonstrates the improved anticancer activity. Biochem Biophys Res Commun. 2011;407(2):383–388. doi:10.1016/j.bbrc.2011.03.030
78. Zheng Y, Ji S, Czerwinski A, Valenzuela F, Pennington M, Liu S. FITC-conjugated cyclic RGD peptides as fluorescent probes for staining integrin αvβ3/αvβ5 in tumor tissues. Bioconjug Chem. 2014;25(11):1925–1941. doi:10.1021/bc500452y
79. Song Z, Lin Y, Zhang X, et al. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: enhanced cellular uptake and improved therapeutic effects. Int J Nanomedicine. 2017;12:1941–1958. doi:10.2147/IJN
80. Henriques ST, Lawrence N, Chaousis S, et al. Redesigned spider peptide with improved antimicrobial and anticancer properties. ACS Chem Biol. 2017;12(9):2324–2334. doi:10.1021/acschembio.7b00459
81. Bloch M, Kam Y, Yavin E, et al. The relative roles of charge and a recognition peptide in luminal targeting of colorectal cancer by fluorescent polyacrylamide. Eur J Pharm Sci. 2012;47(5):904–913. doi:10.1016/j.ejps.2012.09.003
82. Gniazdowska E, Koźmiński P, Bańkowski K, Ochman P. 99mTc-labeled vasopressin peptide as a radiopharmaceutical for small-cell lung cancer (SCLC) diagnosis. J Med Chem. 2014;57(14):5986–5994. doi:10.1021/jm500272r
83. Chatterjee S, Lesniak WG, Miller MS, et al. Rapid PD-L1 detection in tumors with PET using a highly specific peptide. Biochem Biophys Res Commun. 2017;483(1):258–263. doi:10.1016/j.bbrc.2016.12.156
84. Kaspar AA, Reichert JM. Future directions for peptide therapeutics development. Drug Discov Today. 2013;18(17–18):807–817. doi:10.1016/j.drudis.2013.05.011
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.