Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 11
Reproducibility of nasal allergen challenge responses in adults with allergic rhinitis
Authors Pantin CT, Southworth T, Wetzel K, Singh D
Received 7 September 2018
Accepted for publication 30 January 2019
Published 13 May 2019 Volume 2019:11 Pages 67—76
DOI https://doi.org/10.2147/CPAA.S184404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Charles T Pantin,1 Thomas Southworth,1 Kristiane Wetzel,2 Dave Singh1
1Medicines Evaluation Unit, Manchester Academic Health Science Centre, Manchester University NHS Foundation Trust, University of Manchester, Manchester, UK; 2Translational Medicine and Clinical Pharmacology, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riss, Germany
Background: Allergic rhinitis is characterized by nasal inflammation in response to allergen exposure. Nasal allergen challenges are used in clinical trials evaluating drug effects. Reproducibility of nasal secretion cytokine responses and physiological measurements are needed to determine the optimum measurements and power calculations for future studies. We have investigated the reproducibility of nasal cytokine measurements, using ready-to-use polyvinyl acetate sponges to collect nasal secretions, and measurements of nasal physiological responses.
Methods: Twelve subjects with allergic rhinitis and no history of respiratory disease, and 12 subjects with asthma and allergic rhinitis underwent a nasal allergen challenge. This was repeated at 7–14 days later.
Results: There were increases in IL-5, CCL11, and CXCL8 responses post-challenge (all P<0.05). There was better reproducibility at later time points when higher cytokine levels were detected for IL-5 (ri =0.64 at 8 hours) and CXCL8 (ri =0.91 at 8 hours). Acoustic rhinometry provided good to excellent reproducibility (ri =0.66–0.89). Rhinomanometry had lower reproducibility with greater variation (ri =0.10–0.70), with some subjects unable to perform the measurement. Multiplex immunoassays provided greater sensitivity for CCL11 measurements. There were no differences between allergic rhinitis patients with and without asthma.
Conclusion: Polyvinyl acetate sponges are a practical and reproducible way to sample nasal secretions. Acoustic rhinometry is a practical and reproducible method for assessing physiological responses. There were no differences in nasal response due to the presence of concurrent asthma.
Keywords: acoustic rhinometry, total nasal symptom score, polyvinyl acetate sponges, IL-5, CXCL8, CCL11
Introduction
Allergic rhinitis is characterized by nasal inflammation that occurs in response to allergen exposure. T-helper type 2 (TH2) lymphocytes orchestrate this allergic inflammation through the production of cytokines including IL-4, IL-5, and IL-13, which cause a range of pathophysiological effects including TH2 differentiation, eosinophil recruitment and activation, and tissue remodeling.1,2 Many patients with allergic rhinitis also suffer with asthma, and allergen exposure in these individuals can trigger both nasal and lung inflammation.3–5
Nasal allergen challenges have been used to investigate the pathophysiology of allergic rhinitis and in clinical trials to evaluate the effects of drugs for the treatment of allergic rhinitis.6–8 Furthermore, nasal allergen challenge can be used as a surrogate model for allergic inflammation in the lungs, for the purpose of evaluating novel drugs for the treatment of asthma.9 There are no gold standard methods for measuring the nasal allergic response. Different methods have been used to sample nasal secretions including microsuction, nasal lavage, filter paper, cotton wool, and absorptive sponges for the purpose of measuring cytokine responses.10–14
There are different methods for measuring changes in nasal physiology after allergen challenge, including anterior and posterior rhinomanometry, and acoustic rhinometry.15,16 Rhinomanometry measures total nasal resistance, while acoustic rhinometry uses sound pulses to quantify the nostril lumen surface area.
It is important to determine the reproducibility of cytokine responses and physiological measurements after nasal allergen challenge, as this allows the optimum measurements to be selected and power calculations to be performed for future studies. For example, Scadding et al demonstrated that absorptive polyurethane sponges provided a superior nasal sampling method compared to synthetic filter paper, and used these polyurethane sponges to assess the reproducibility of cytokine responses to challenges performed on different days.17
We investigated nasal cytokine measurements after allergen challenge using polyvinyl acetate sponges to collect secretions.14 These polyvinyl acetate sponges are purpose built for sampling nasal secretions, as they are pre-sterilized and are designed for ease of manual placement in the nostrils. The main aims of this study were to 1) characterize the time course and reproducibility of cytokine responses measured using polyvinyl acetate sponges and 2) assess the reproducibility of rhinomanometry and acoustic rhinometry. We also compared the nasal allergen responses of two subsets of individuals with allergic rhinitis: individuals with asthma compared to individuals without asthma.
Materials and methods
Subjects
Twenty-four subjects participated: 12 subjects with allergic rhinitis and no history of respiratory disease, and 12 subjects with asthma and allergic rhinitis. All subjects were non-smokers with a <1 pack-year history and asthma patients were corticosteroid naïve. Inclusion criteria consisted of age between 18 and 70 years; a positive skin prick test to cat, house dust mite, or grass (ALK, Hø´rsholm, Denmark) at screening; and no use of medication for asthma in the last 4 weeks other than short-acting beta agonists. Exclusion criteria included uncontrolled asthma, cigarette smoking within the last 12 months, use of nasal medications, respiratory tract infection within 30 days of screening, ongoing symptoms of rhinitis or abnormal nasal examination, skin prick positive to cat with daily exposure to cats. Written informed consent was obtained and the study was approved by a local research ethics committee (Greater Manchester Central, 10/H1008/86).
Study design
The study was conducted outside the UK hay fever season. Demographic data were collected including FEV1 using a spirometer (Vitalograph, Buckinghamshire, UK) and related to reference values.18 Eligible subjects were administered a nasal allergen challenge using the allergen that had caused the greatest skin prick test reaction at screening. This was either cat, house dust mite, or grass. The nasal allergen challenge involved initial administration of 100 µL of normal saline allergen diluent into each nostril as previously described, and then 100 µL of the selected allergen (50,000 SQ-U/mL concentration) was administered into each nostril by Bidose Liquid nasal spray after 30 minutes (Aptar Pharma, Radolfzell, Germany).1,19,20 Nasal measurements were determined as follows: total nasal resistance using posterior and anterior rhinomanometry (rhinomanometer; GM Instruments Ltd., Kilwinning, Scotland, UK), and total nasal surface area using acoustic rhinometry (acoustic rhinometer; GM Instruments Ltd.). Rhinomanometry measures air flow and pressure through the nostrils to determine total nasal resistance (kPa/L/s). Acoustic rhinometry uses sound pulses emitted up a hollow, open ended tube sealed onto the nostril, and interprets the reflected sound waves to give a measurement of the nostril lumen surface area (cm2) at various distances into the nostril; we measured the surface area at 3 cm from the nostril opening, with the readings of both nostrils added together to give total surface area at 3 cm.21 For all three methods of nasal measurement, technically acceptable measurements were defined as subjects performing the measurement with reproducible results (three measurements within 20% of each other) at each time point. Nasal secretions were sampled using Ivalon® Post-op Sinus Pack K9 (Ivalon Inc., San Diego, USA) nasal sponges as detailed below. Nasal symptoms were quantified using the total nasal symptom score (TNSS) questionnaire, a 12-point score consisting of four categories rated 0–3: rhinorrhoea, sneezing, nasal itching, and nasal congestion. The nasal symptom score (NSS) questionnaire published by Riechelmann et al, consisting of a 6-point score assessing rhinorrhoea, sneezing, and extra-nasal symptoms, was also used.22 All measurements and nasal sampling were collected from post-diluent (10 minutes before allergen administration) and up to 8 hours after allergen administration (30, 60, 120, 240, 360, and 480 minutes). This nasal allergen challenge was repeated at 7–14 days later.
Nasal secretions
Nasal secretions were collected and processed as previously described.14 A pair of nasal sponges were weighed dry in a 15 mL tube, then inserted, one into each nostril, and left in situ for 5 minutes. On extraction, the sponges were reweighed. They were then irrigated with 3 mL of normal saline to flush out the cytokines and the sealed tube was placed in ice on a roller. After 2 hours, the sponges were inserted into a sterile 5 mL syringe barrel and placed back into the tube. This was then centrifuged at 1,500× g and 4°C for 15 minutes. The resulting supernatant was aliquoted and stored at –80°C for later analysis.
Measurement of nasal secretion supernatant biomarkers
The supernatants were assayed using the following ELISA kits according to manufacturer’s instructions: IL-5 (Human IL-5 DuoSet, range of quantification 23.4–2,000 pg/mL; R&D Systems, Abingdon, UK), CXCL8 (Human CXCL8/IL-8 DuoSet, range of quantification 31.2–2,000 pg/mL; R&D Systems), CCL11 (Human CCL11/Eotaxin Duoset, range of quantification 15.6–1,000 pg/mL; R&D Systems). Supernatants from the second challenge performed on the 12 patients with asthma and allergic rhinitis were also assayed using Mesoscale Sector® (from now on referred to as MS) Imager 6000 (Meso Scale Discovery, Rockville, MD, USA) according to manufacturer’s instructions for the following cytokines: CCL11, CCL26, CXCL10, CCL2, CCL13, CCL22, CCL4, CCL17, INF-γ, IL-1β, IL-2, IL-4, IL-5, CXCL8, IL-10, IL-12p70, IL-13, TNFα (Human TH1/TH2 10-Plex Ultra-Sensitive Kit; Meso Scale Discovery) and CCL11, CCL26, CXCL10, CCL2, CCL13, CCL22, CCL4, CCL17 (Human Chemokine 9-Plex Ultra-Sensitive Kit; Meso Scale Discovery).
Statistics
A sample size of 12 subjects per group was chosen for reproducibility experiments, as it allows sample size calculations using the within-subject SD to be performed for future studies with 90% power; these calculations still have 73% power if the true SD is 25% larger than observed.23 Results were assessed for normality using Kolmogorov–Smirnov test. CCL11 MS, CXCL8 MS, and CXCL10 MS data were normally distributed. Differences in these measurements pre- and post-allergen exposure were compared using one-way ANOVA with Dunnett’s multiple comparison post hoc test. All other measurements were not normally distributed, and differences between pre- and post-allergen measurements were compared using Friedman’s test with Dunn’s multiple comparison post hoc test. The pre-challenge value was the post-diluent time point. Comparisons between subjects with allergic rhinitis only and those with asthma and allergic rhinitis were performed using Mann–Whitney tests and estimating area under the curve. P-values <0.05 were considered significant. The reproducibility of nasal allergen challenges was assessed using intraclass correlation ri (ICC) using the Fleiss criteria (ri <0.40 poor reproducibility, 0.40–0.59 fair, 0.60–0.74 good, and >0.74 excellent reproducibility).24,25 Results below the lower limit of quantification were assigned a value of half of the lower limit of quantification. For ICC reproducibility analysis, only results above the lower limit of quantification were used. Cytokines measured by MS were assessed for correlation with ELISA data using Pearson’s correlation coefficient. Symptom scores were assessed for correlation with physiological measurements at the 30-minute time point using Spearman’s correlation coefficient. GraphPad PRISM® version 5.02 (GraphPad Software Inc., La Jolla, CA, USA) was used for all analysis except ICC, which was calculated using SPSS version 19 (IBM Corporation, Armonk, NY, USA).
Results
Figure 1 shows how the study population was recruited. For the five subjects who did not complete the study, two were withdrawn because of a coryzal illness (common cold), while three withdrew for personal reasons.
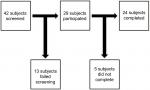  | Figure 1 Study population recruitment. |
The demographics of the participants are shown in Table 1. The measurements were performed on all 24 subjects unless stated otherwise.
Nasal symptom scores
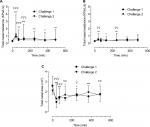
Both NSS (n=24) and TNSS (n=18) demonstrated significant increases in symptoms at 30 minutes after allergen challenge that steadily reduced thereafter (Figure 2A, B). The ICC for NSS on different days after nasal challenge varied from 0.52 to 0.69 at different time points (Table S1), with a mean of 0.60 indicating good reproducibility overall. The ICC for TNSS on different days after nasal challenge varied from 0.63 to 0.86 at different time points (Table S2), with a mean of 0.78 indicating excellent reproducibility overall. There was a correlation at 30 minutes post-allergen challenge between nasal symptoms and acoustic rhinometry measurements, eg, for TNSS the Spearman’s coefficients (r) were –0.59 (P=0.014) and –0.63 (P=0.005) for the first and second challenge, respectively. Associations between rhinomanometry and symptoms were mostly not significant (Tables S3 and S4).
Nasal secretion weight
There were significant increases in nasal secretion weights after allergen challenge (Figure 2C). Nasal secretion weights peaked 30 minutes after nasal challenge. The ICC for nasal challenge on different days varied from 0.63 to 0.87 at different time points (Table S5), with a mean of 0.74 indicating excellent reproducibility overall.
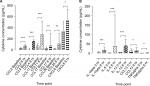
Cytokines
No subjects produced detectable IL-5 concentrations before allergen challenge. IL-5 concentrations increased significantly after both nasal allergen challenges (Figure 3A), with 12 and 16 subjects producing detectable IL-5 concentrations after the first and second nasal allergen challenges, respectively, at 480 minutes. The ICC for IL-5 measurements after nasal challenge on different days varied from 0.17 to 0.64 at different time points (Table S6), with good reproducibility (ICC =0.64) at 480 minutes when the highest IL-5 levels were observed.
CXCL8 was detected in all subjects before and after allergen challenge. CXCL8 concentrations increased significantly after both nasal allergen challenges (Figure 3B). The ICC ranged from 0.51 to 0.91 at different time points (Table S7), with excellent reproducibility at the later time points (360 and 480 minutes) when the highest CXCL8 levels were observed.
CCL11 concentrations increased significantly after both nasal allergen challenges (Figure 3C). CCL11 was detectable in eight subjects before the first challenge, and six of these subjects before the second challenge. Eighteen and 19 subjects produced detectable concentrations of CCL11 after the first and second nasal allergen challenges, respectively. The ICC at different time points varied from 0.45 to 0.94 (Table S8), with better reproducibility at the earlier time points (30–240 minutes) when low CCL11 levels were observed.
Analysis of MS data for samples collected during the second allergen challenge in 12 asthma patients with allergic rhinitis showed statistically significant increases in CCL2, CCL4, CCL11, CCL17, CCL26, IL-1β, IL-5, IL-13, CCL13, TNFα, and CXCL8 (Figure 4 and Figure S1). Other cytokines showed no statistically significant increases. The Pearson’s coefficients (r) of the MS and ELISA cytokine data for IL-5 (r=0.97, P<0.0001) and CXCL8 (r=0.58, P<0.0001) demonstrated good correlation between methods, while for CCL11 (r=0.46, P=0.021) the correlation was lower (Figure S1). The MS CCL11 results were higher compared to the ELISA data at every time point.
Posterior and anterior rhinomanometry
Out of 23 subjects, 19 produced technically acceptable posterior rhinomanometry measurements. There was an increase in total nasal resistance 30 minutes after nasal allergen administration (Figure 5A and Table S9). The range of ICC for posterior rhinomanometry at different time points varied from 0.32 to 0.56 (Table S9) with a mean of 0.43 indicating fair reproducibility overall.
Out of 22 subjects, 12 produced technically acceptable anterior rhinomanometry data. There was a significant increase in total nasal resistance 30 minutes after nasal allergen administration (Figure 5B and Table S10). The range of ICC for anterior rhinomanometry at different time points varied from 0.10 to 0.70 (Table S10) with a mean of 0.36 indicating poor reproducibility overall.
Acoustic rhinometry
All subjects who performed acoustic rhinometry produced technically acceptable data (n=21). There were significant decreases in total nasal area 30 minutes after allergen challenge (Figure 5C and Table S11). The range of ICC at different time points after nasal challenge varied from 0.66 to 0.89 (Table S11) with a mean of 0.81 indicating excellent reproducibility overall.
Allergic rhinitis versus asthma and allergic rhinitis subjects
There was no statistical difference found between the 12 allergic rhinitis subjects with asthma and the 12 allergic rhinitis subjects without asthma on any measurement (Figures S2–S5, which also show area under the curve analysis).
Discussion
The main novel aspects of this study were to document the reproducibility of nasal cytokine measurements after allergen challenge using polyvinyl acetate sponges, and to compare the practicality and reproducibility of nasal physiological measurements during challenges. We observed good reproducibility for IL-5, CXCL8, and CCL11 levels after allergen challenge, but this reproducibility varied according to the time point. For IL-5 and CXCL8, there was better reproducibility at later time points during the late allergic response when the cytokine response was higher. This highlights the importance of selecting the optimum time points to perform these measurements. Furthermore, we demonstrated that acoustic rhinometry is the method that all subjects were able to perform and provided good to excellent reproducibility. In contrast, rhinomanometry was less practical and had lower reproducibility. Acoustic rhinometry was also more closely associated with nasal symptoms compared to rhinomanometry.
The physiological response to allergen challenge has been measured using a variety of methods, but there is no accepted gold standard.26–30 We investigated three methods of monitoring nasal physiological responses after allergen challenge: posterior rhinomanometry, anterior rhinomanometry, and acoustic rhinometry. In terms of reproducibility and practicality, we found that acoustic rhinometry was the superior technique. Posterior rhinomanometry required subject participation in holding their throat and tongue still, which some participants were unable to do. Anterior rhinomanometry does not require subject participation, but suffered from missing data at time points when rhinorrhea occurred which prevented sticking of the tape required to occlude the nostril. Additionally, in some cases, complete nasal congestion occurred, so the anterior rhinomanometer could not register a nasal pressure gradient. In contrast, acoustic rhinometry was an easy procedure for participants to learn and had excellent reproducibility. It also was the only physiological method with significant correlation with TNSS at 30 minutes in both challenges. An association between physiological changes and nasal symptoms has previously been reported.15
We chose to measure IL-5, CXCL8, and CCL11 based on consistent previous literature showing an elevation in the levels of these mediators after nasal allergen challenge.6,7,17,31,32 We used standard immunoassay techniques for measuring cytokines published in the literature.6,7,14,32 Based on previous studies, we used a washout period of 7–21 days, which is also a common washout period in crossover clinical trials investigating drug effects.6,7,32
We did not set out to characterize the early-phase response in detail, but focused on sampling during the late-phase response. For both IL-5 and CXCL8, there were clear late-phase responses after both challenges, as shown in previous studies.6,13,17,31,32 IL-5 was not measurable before allergen challenge in any individual, making it easier to observe the late-phase response. At early time points, only small concentrations were being secreted or were not quantifiable as in the case of IL-5.6,32 This produces a relatively wide range of concentrations compared to the mean at early time points, reducing the ICC. At later time points, the concentrations secreted increase, thereby reducing the between-patient variability and improving ICC.
For CCL11, the ELISA measurements were able to demonstrate the late-phase response, but the reproducibility was lower at later time points after allergen challenge. Some previous studies have shown no significant increase after allergen challenge for this eosinophil chemoattractant, although some studies have shown a positive response.6,13,33–35 The wide variation in the late-phase CCL11 concentrations appears to be due to some individuals producing CCL11 and those that are “non-producers”. The low CCL11 concentrations at early time points combined with the use of half the lower limit of quantification for data points of subjects not producing CCL11 means that the ICC values at early time points are driven by low/absent values. ICC decreased as the mean concentration increased at later time points. These differences in the literature and in reproducibility may be related to the sensitivity of the immunoassays to detect CCL11; we found that MS measurements provided greater sensitivity for CCL11 measurements and were able to demonstrate a significant increase during the late phase. This highlights the importance of using sensitive immunoassays to measure cytokine responses after nasal allergen challenge.
The MS measurements demonstrated significant increases in TH2 cytokines (IL-5, IL-13, CCL11, and CCL26) during the late-phase response. This is in agreement with previous publications.6,12,13 IL-1β, CCL2, CCL4, CCL13, CCL17, CXCL8, and TNFα were also found to significantly increase post-allergen challenge, although for CCL13 and TNFα, these increases were numerically small. Due to budget constraints, further MS measurements were not performed.
There is limited evidence in the literature concerning reproducibility of nasal allergen challenges. Proud et al investigated the reproducibility of nasal allergen challenges following intranasal corticosteroid treatment.20 There was excellent reproducibility for sneezing and lysozyme secretion, good reproducibility for symptom scores and albumin release, but only poor reproducibility for kinin release. Scadding et al investigated the nasal responses of 20 grass pollen allergic subjects, comparing three mediums for collecting nasal samples: Accuwick Ultra (fibrous hydroxylated polyester), 111 (100% cellulose fibers), and synthetic polyurethane sponge. The different mediums were tested in pairs in each subject and inserted into different nostrils using forceps. The polyurethane sponge was reported to be the best medium for collection of nasal secretions.17 All of the methods used required insertion with forceps, which risks trauma to the nasal mucosa. We used nasal sponges made of stiff, sterilized polyvinyl acetate, and were able to efficiently absorb nasal secretions without the need for forceps. We found these sponges easy to use, and they were able to collect sufficient material for immunoassay measurements. A potential limitation is the cost; these sponges are purchased already pre-packaged in sterilized foil packets in pairs.
We have presented the within-subject SD for the measurements performed (see Supplement). These can be used to perform power calculations for future studies, including clinical trials of drugs designed to modulate the nasal allergen challenge response.
There was no clear difference in the nasal allergen challenge responses of asthma patients with allergic rhinitis compared to subjects with allergic rhinitis only. This indicates that subjects with and without asthma could be recruited into studies using the nasal allergen challenge model, and little differences are expected due to the presence of asthma. Area under the curve analysis (see Supplement) highlighted that there are some individuals with very high cytokine responses relative to the rest of the group, and the limited sample size of this sub-analysis means that some differences in allergen response due to the presence of asthma could not be completely ruled out.
Possible future improvements to the nasal supernatant analysis after allergen challenge include normalization of protein levels to secreted substances that are less affected by nasal allergen such as urea. Also, we were not able to investigate other relevant mediators due to limitations of sample volume for analysis, such as histamine, immunoglobulin E, and leukotriene C4. These could be investigated in future work along with more detailed study of the early-phase response to nasal allergen.
Conclusion
In summary, we show that polyvinyl acetate sponges can be used to collect nasal samples after allergen challenge, with reproducible cytokine responses. A panel of TH2 cytokines can be detected using this method. We also show acoustic rhinometry to be a practical and reproducible measurement after nasal allergen challenge.
Ethics approval and consent to participate
This study was approved by a local research ethics committee (Greater Manchester Central, 10/H1008/86) and all subjects provided written informed consent. This research was performed in accordance with the Declaration of Helsinki.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This report is independent research supported by National Institute for Health Research South Manchester Respiratory and Allergy Clinical Research Facility at University Hospital of South Manchester NHS Foundation Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. We would also like acknowledge Boehringer Ingelheim for providing the analysis of nasal secretion samples using the Mesoscale SECTOR® Imager 6000. This work was partially funded by the Medicines Evaluation Unit and the North West Lung Research Charity, Manchester, UK.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
DS is supported by the National Institute for Health Research Manchester Biomedical Research Centre and has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards, and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Skypharma, Takeda, Teva, Therevance, and Verona. KW is an employee of Boehringer Ingelheim. The authors report no other conflicts of interest in this work.
References
Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28(1):445–489. | ||
Williams CMM, Rahman S, Hubeau C, Ma H-L. Cytokine pathways in allergic disease. Toxicol Pathol. 2012;40(2):205–215. | ||
Chakir J, Laviolette M, Turcotte H, Boutet M, Boulet LP. Cytokine expression in the lower airways of nonasthmatic subjects with allergic rhinitis: influence of natural allergen exposure. J Allergy Clin Immunol. 2000;106(5):904–910. | ||
Beeh KM, Beier J, Kornmann O, et al. A single nasal allergen challenge increases induced sputum inflammatory markers in non-asthmatic subjects with seasonal allergic rhinitis: correlation with plasma interleukin-5. Clin Exp Allergy. 2003;33(4):475–482. | ||
Braunstahl GJ, Fokkens WJ, Overbeek SE, et al. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airways. Clin Exp Allergy. 2003;33(5):579–587. | ||
Erin EM, Zacharasiewicz AS, Nicholson GC, et al. Topical corticosteroid inhibits interleukin-4, -5 and -13 in nasal secretions following allergen challenge. Clin Exp Allergy. 2005;35(12):1608–1614. | ||
Nicholson GC, Kariyawasam HH, Tan AJ, et al. The effects of an anti-IL-13 mAb on cytokine levels and nasal symptoms following nasal allergen challenge. J Allergy Clin Immunol. 2011;128(4):800–807. | ||
Stokes JR, Romero FA, Allan RJ, et al. The effects of an H3 receptor antagonist (PF-03654746) with fexofenadine on reducing allergic rhinitis symptoms. J Allergy Clin Immunol. 2012;129(2):409–412. | ||
Scadding GW, Eifan A, Penagos M, et al. Local and systemic effects of cat allergen nasal provocation. Clin Exp Allergy. 2015;45(3):613–623. | ||
Clement P, Wang D. Nasal allergen challenge: changes in nasal patency and in biochemical markers. Allergy. 1997;52(suppl 1):24–27. | ||
Linden M, Greiff L, Andersson M, et al. Nasal cytokines in common cold and allergic rhinitis. Clin Exp Allergy. 1995;25(2)):166–172. | ||
McDermott RA, Nelson HS, Dreskin SC. Mediator measurements after daily instillation of allergen: increased IL-5 and decreased IFN-gamma. Allergy Asthma Proc. 2008;29(2):146–151. | ||
Kramer MF, Jordan TR, Klemens C, et al. Factors contributing to nasal allergic late phase eosinophilia. Am J Otolaryngol. 2006;27(3):190–199. | ||
Watelet J-B, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Collection of nasal secretions for immunological analysis. Eur Arch Otorhinolaryngol. 2004;261(5):242–246. | ||
Gosepath J, Amedee RG, Mann WJ. Nasal provocation testing as an international standard for evaluation of allergic and nonallergic rhinitis. Laryngoscope. 2005;115(3):512–516. | ||
Shelton DM, Eiser NM. Evaluation of active anterior and posterior rhinomanometry in normal subjects. Clin Otolaryngol Allied Sci. 1992;17(2):178–182. | ||
Scadding GW, Calderon MA, Bellido V, et al. Optimisation of grass pollen nasal allergen challenge for assessment of clinical and immunological outcomes. J Immunol Methods. 2012;384(1-2):25–32. | ||
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6(Suppl 16):5–40. | ||
Baroody FM, Ford S, Proud D, et al. Relationship between histamine and physiological changes during the early response to nasal antigen provocation. J Appl Physiol. 1999;86(2):659–668. | ||
Proud D, Riker DK, Togias A. Reproducibility of nasal allergen challenge in evaluating the efficacy of intranasal corticosteroid treatment. Clin Exp Allergy. 2010;40(5):738–744. | ||
Hilberg O. Objective measurement of nasal airway dimensions using acoustic rhinometry: methodological and clinical aspects. Allergy. 2002;57(s70):5–39. | ||
Riechelmann H, Bachert C, Goldschmidt O, et al; Working Team for Clinical Immunology. Application of the nasal provocation test on diseases of the upper airways. Position paper of the German Society for Allergology and clinical immunology (ENT Section) in cooperation with the working team for clinical immunology. Laryngo-Rhino-Otologie. 2003;82(3):183–188. | ||
Houghton CM, Woodcock AA, Singh D. A comparison of lung function methods for assessing dose-response effects of salbutamol. Br J Clin Pharmacol. 2004;58(2):134–141. | ||
Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York: Wiley; 1981. | ||
Cicchetti DV, Sparrow SS. Developing Criteria for Establishing the Interrater Reliability of Specific Items in a Given Inventory. American Journal of Mental Deficiency. 1981;86:127–137. | ||
Dvoracek J, Hillis A, Rossing R. Comparison of sequential anterior and posterior rhinomanometry. J Allergy Clin Immunol. 1985;76(4):577–582. | ||
Frølund L, Madsen F, Mygind N, et al. Comparison between different techniques for measuring nasal patency in a group of unselected patients. Acta Otolaryngol. 1987;104(1-2):175–179. | ||
Jones AS, Lancer JM, Stevens JC, Beckingham E. Rhinomanometry: do the anterior and posterior methods give equivalent results? Clin Otolaryngol Allied Sci. 1987;12(2):109–114. | ||
Austin CE, Foreman JC. Acoustic rhinometry compared with posterior rhinomanometry in the measurement of histamine- and bradykinin-induced changes in nasal airway patency. Br J Clin Pharmacol. 1994;37(1):33–37. | ||
Kim YH, Yang TY, Lee DY, et al. Evaluation of acoustic rhinometry in a nasal provocation test with allergic rhinitis. Otolaryngol Head Neck Surg. 2008;139(1):120–123. | ||
Sim TC, Grant JA, Hilsmeier KA, Fukuda Y, Alam R. Proinflammatory cytokines in nasal secretions of allergic subjects after antigen challenge. Am J Respir Crit Care Med. 1994;149(2 Pt 1):339–344. | ||
Erin EM, Leaker BR, Zacharasiewicz AS, et al. Single dose topical corticosteroid inhibits IL-5 and IL-13 in nasal lavage following grass pollen challenge. Allergy. 2005;60(12):1524–1529. | ||
Boot JD, Chandoesing P, de Kam ML, et al. Applicability and reproducibility of biomarkers for the evaluation of anti-inflammatory therapy in allergic rhinitis. J Investig Allergol Clin Immunol. 2008;18(6):433–442. | ||
Terada N, Hamano N, Kim WJ, et al. The kinetics of allergen-induced eotaxin level in nasal lavage fluid: its key role in eosinophil recruitment in nasal mucosa. Am J Respir Crit Care Med. 2001;164(4):575–579. | ||
Semik-Orzech A, Barczyk A, Wiaderkiewicz R, Pierzchała W. Eotaxin, but not IL-8, is increased in upper and lower airways of allergic rhinitis subjects after nasal allergen challenge. Allergy Asthma Proc. 2011;32(3):230–238. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.