Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Repetitive Transcranial Magnetic Stimulation Improves Mild Cognitive Impairment Associated with Alzheimer’s Disease in Mice by Modulating the miR-567/NEUROD2/PSD95 Axis
Received 15 March 2021
Accepted for publication 17 April 2021
Published 2 July 2021 Volume 2021:17 Pages 2151—2161
DOI https://doi.org/10.2147/NDT.S311183
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Yongfeng Pang, Mingfei Shi
Department of Rehabilitation, Tiantai People’s Hospital of Zhejiang Province, Zhengjiang, 317200, People’s Republic of China
Correspondence: Mingfei Shi
Department of Rehabilitation, Tiantai People’s Hospital of Zhejiang Province, No. 1 Kangning Middle Road, Tiantai County, Zhengjiang, 317200, People’s Republic of China
Tel +86-13516877456
Email [email protected]
Background: Mild cognitive impairment (MCI) is a typical symptom of early Alzheimer’s disease (AD) and is driven by the dysfunction of microRNAs (miRs). Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive technique for handling neuropsychiatric disorders and has universally effects on the functions of miRs. In the current study, the improvement effects of rTMS on MCI associated with AD were explored by focusing on miR-567/NEUROD2/PSD95 axis.
Methods: MCI was induced in mice using scopolamine and was treated with rTMS of two frequencies (1 Hz and 10 Hz). The changes in cognitive function, brain structure, neurotrophic factor levels, and activity of miR-567/NEUROD2/PSD95 axis were assessed. The interaction between rTMS and miR-567 was further verified by inducing the level of miR-567 in AD mice.
Results: The administrations of rTMS improved the cognitive function of AD mice and attenuated brain tissue destruction, which were associated with the restored production of BDNF and NGF. Additionally, rTMS administrations suppressed the expression of miR-567 and up-regulated the expressions of NEUROD2 and PSD95, which contributed to the improved condition in central nerve system. With the induced level of miR-567, the effects of rTMS were counteracted: the learning and memorizing abilities of mice were impaired, the brain neuron viability was suppressed, and the production of neurotrophic factors was suppressed even under the administration of rTMS. The changes in brain function and tissues were associated with the inhibited expressions of NEUROD2 and PSD95.
Conclusion: The findings outlined in the current study demonstrated that rTMS treatment could protect brain against AD-induced MCI without significant side effects, and the function depended on the inhibition of miR-567.
Keywords: Alzheimer’s disease, mild cognitive impairment, miR-567, NEUROD2, repetitive transcranial magnetic stimulation
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder, and its prevalence is increasing worldwide with the progressive aging of the human population, thus constituting a significant burden to public health and patients’ caregivers.1 One of the symptoms associated with the early development of AD is mild cognitive impairment (MCI), which is attributed to multiple factors, including progressive neuronal loss, reduced levels of several crucial neurotransmitters, and altered forms of synaptic plasticity.2 In contrast to the memory loss in developed AD, MCI generally does not have an important impact on the daily life activity of the patients, and it is only characterized by the progressive decline of cognitive functions.3 Based on the domains influenced by the MCI, this symptom can be classified into several subtypes, including amnesic and non-amnesic forms. In certain cases (12–20%), patients with MCI develop a dementia syndrome; thus, MCI is considered to inevitably progress to fully developed AD over time.4 Currently, the diagnosis of MCI requires the positive expressions of several in vivo biomarkers of AD, including cerebrospinal fluid amyloid-β 1–42, total tau or 181-phosphorylated tau. Furthermore, recent studies have demonstrated that certain non-coding RNAs such as microRNAs (miRNAs/miRs) can also be employed as diagnostic predictors or treatment targets for MCI.
MiRNAs are small non-coding RNAs that regulate the function of different genes by inducing either translational repression or mRNA destabilization of their target mRNA. MiRNAs play key roles in numerous neurobiological processes such as neurodevelopment, neuroplasticity and apoptosis.5 Furthermore, the abnormal expression profiles of certain miRNAs are involved in the development of various neurological deficits, including AD, Parkinson’s disease, amyotrophic lateral sclerosis, and vascular dementia.6–9 The data derived from these studies was also applied to the diagnosis and treatment of MCI pre-AD. In the study performed by Felice et al, the authors identified four dysregulated miRNAs in patients with MCI, of which miR-567 showed the highest fold-change and statistical significance.10 The authors also indicated that the downstream effectors of miR-567, including neurogenin 2 (NEUROG2), transcription factor (TCF) 3, TCF4, and T-box brain transcription factor 1 (TBR1), are likely to contribute to the dysfunction of the nervous system. For example, neuronal differentiation 2 (NEUROD2) is a transcriptional regulator implicated in neuronal differentiation, while TCF3 maintains the neural stem cell population during neocortical development.11,12 Therefore, miR-567-related signaling transduction can represent a potential and promising target for the diagnosis and treatment of MCI associated with AD progression. Thus, therapeutic strategies that modulate the activity of miR-567 can benefit patients with MCI long-term.
For decades, researches in academia and the pharmaceutical industry have been focused on the identification of therapies that can arrest or reverse memory loss in AD.13 However, these studies have been unsuccessful due to the complicated pathogenesis of AD or the delayed diagnosis of AD.14,15 Therefore, therapeutic intervention as early as possible (in the MCI phase) should enhance the opportunity of cognitive recovery.16
Single and paired-pulse transcranial magnetic stimulation (TMS) represent useful co-adjuvant diagnostic tools to assess in vivo neuroplastic changes, and have provided comprehensive information on local cortical excitability and functional connectivity between motor cortex and other cortical regions in combination with electroencephalography or functional magnetic resonance imaging. If delivered repetitively, repetitive TMS (rTMS) can induce long-lasting effects, and can be applied as a non-invasive treatment approach for neuropsychiatric disorders such as AD, depression, and Parkinson’s disease.17,18 Furthermore, the function of rTMS has also been attributed to its effects on the expression profile of multiple miRNAs, including miR-106b and miR-25.19,20 Thus, it is reasonable to explore the roles of miRNAs in the anti-AD effects of rTMS.
The present study explored the interaction between rTMS and the miR-567/NEUROD2 axis in the anti-AD effects of rTMS. AD symptoms were induced in mice using scopolamine, and then treated with rTMS of two frequencies (1 and 10 Hz). The effects of rTMS on the cognitive behaviors, nervous system function and structure, and the miR-567/NEUROD2 axis were assessed. The miR-567/NEUROD2 axis was then modulated by a specific agomir to provide additional information on the mechanisms of rTMS treatment in AD.
Materials and Methods
Agents
Scopolamine hydrobromide was obtained from SigmaAldrich (MO, USA) and dissolved in saline. In Situ Cell Death Detection Kit (11684817910) was purchased from Roche (Switzerland). Detection kits using enzyme-linked immune sorbent assay (ELISA) methods for brain derived neurotrophic factor (BDNF) (SEA011Mu) and nerve growth factor (NGF) (SEA105Mu) were purchased from USCN (China). Specific agomir for miR-567 and negative control (NC) agomir were synthesized by Sango Biotech (Shanghai, China). Agents for Western blotting assay, including RIPA lysis buffer and (P0013B), BCA Protein Concentration Kit (P0009), and ECL Plus reagent (P0018) were purchased from Beyotime Biotechnology (China). Agents for PCR, including RNA Purified Total RNA Extraction kit and Super M-MLV reverse transcriptase were purchased from BioTeke (Wuxi, China). The TUNEL in situ cell death detection kit was from Roche (Switzerland). Antibodies against Bax (#2772) were purchased from CST (USA). Antibodies against NeuroD2 (ab104430), PSD95 (ab238135), cleaved caspase 3 (ab49822), Bcl-2 (ab196495), and doublecortin (ab18723) were purchased from Abcam (UK). Antibody against GAPDH (bsm-33033M) was purchase from Bioss (China). Secondary IgG-HRP goat-anti rabbit (A0208) and goat anti-murine (A0216) antibodies were purchased from Beyotime Biotechnology (China). Secondary Cy3-labeled antibody goat anti-rabbit (A0277) was purchased from Beyotime Biotechnology (China).
Induction of an AD-Related MCI Model by Scopolamine and rTMS Administration
C57BL/6 mice (8-week-old) were purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd. and housed at 25±1°C with a humidity of 45–55% with food and water available. All the animal surgical processes were approved by the Institutional Animal Ethics Committee of Tiantai People’s Hospital of Zhejiang Province (approval no. 20190305) and were carried out in accordance with the Animal Welfare Law and the guidelines for animal care and use of the National Institutes of Health. The AD-related MCI model was induced using an intraperitoneal injection of scopolamine (1.5 mg/kg body weight) for 21 consecutive days. For the mice in the sham group, scopolamine was replaced by the same volume of sterile normal saline. For rTMS treatment, administration was performed 1 day following the first scopolamine injection, and it was completed along with the last injection: highly focusing magnetic-electric stimulator (High-Speed MES-10, Cadwell, Kennewick, WA, USA) with 9-centimeter diameter round coil was used to perform rTMS administrations. The coil was connected to MES-10 magnetic electric stimulator with monophasic current waveform, and it was held centered tangentially to the center of exposed head of rats (which were fixed in suitable cloth sleeves). In the current study, administrations of rTMS of two frequencies were conducted for exploring in detail the effects of the therapy. For treatment with low frequency rTMS, the mice were exposed to rTMS of 1 Hz with the magnetic stimulation intensity set at 30% of the maximum output (1.26 T). The treatment involved two sessions of rTMS consisting of 1000 pulses in 10 trains, and the interval between every two trains was 20 sec. The two sessions were separated by an interval of 2 min. For treatment with high frequency rTMS, the frequency was set to 10 Hz. Upon completion of the experiment, certain mice were sacrificed using 200 mg/kg body weight phenobarbital sodium, and their hippocampus CA1 tissues were collected and preserved at −80°C. The remaining mice were subjected to Morris water maze (MWM) tests to assess their learning and memorizing abilities.
MWM Assays
MWM assays were routinely performed to assess the effects of rTMS administration on the learning and memorizing abilities of mice. Briefly, the assay included a 4-day visible platform trial and a 1-day probe trial. For the visible platform trail in 60 sec, the mice were allowed to swim for 60 sec before reaching the platform four times each day. If the mice failed the test, they would be helped by investigators to reach the platform, where they stayed for 10 sec before being subjected to another test. For the probe trial in day 5, the platform was removed, and the mice were allowed to swim in the space. The number of times and animals that spent 60 sec in the former platform position were measured by two investigators blinded to the animal group.
TUNEL Staining
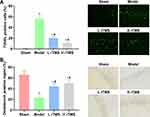
The changes in cell apoptotic rate in hippocampus CA1 region of brain were assessed using TUNEL staining. Brain sections were firstly permeabilized with 0.1% Triton X-100 at room temperature for 8 min, and then incubated in 3% H2O2 for 10 min. After three washes with PBS, brain sections were incubated with TUNEL reaction solution at 37°C for 1 h in dark. The results of staining were recorded using a microscope (BX53; Olympus Corporation) at x400 magnification. The number of the TUNEL positive cells was quantified in five anatomically consistent areas by two investigators who were blind to the experiment. The proportion of each area was then calculated in reference to the total neurons in the area.
Immunohistochemical (IHC) Detection
The level and distribution of doublecortin in hippocampus CA1 region of brain sections were determined by IHC detection. Briefly, 5-μm brain sections were incubated with different concentrations of alcohol and washed with PBS. Subsequently, a primary antibody against doublecortin (1:200) was incubated with the sections at 4°C overnight, followed by incubation of the sections with secondary Cy3-labeled antibody at 37°C for 30 min. Next, the sections were labeled with horseradish peroxidase-labeled avidin at 37°C for 30 min and incubated with DAB solution. Finally, the sections were re-stained with hematoxylin for 3 min and dehydrated using different concentrations of alcohol. The images were captured using a using a fluorescence microscope at x400 magnification.
Enzyme-Linked Immuno Sorbent Assay (ELISA)
The levels of BDNF and NGF in brain tissues were detected using corresponding kits following instructions for manufacturers. The levels of the two factors were calculated based on OD values at 450 nm measured by a Microplate Reader (ELX-800, BIOTEK, USA).
Reverse Transcription Quantitative PCR (RT-qPCR)
Total RNA in brain tissues was extracted using the extraction kit according to the manufacturer’s instructions and then reversely transcribed into cDNA templates using Super M-MLV reverse transcriptase. The expression level of miR-567 (miR-567, forward:5ʹ-TAGGTACTAGGTGGAAGGAG-3ʹ; reverse: 5ʹ-CCACTGAAAGGCAATGGAAG-3ʹ. U6, forward:5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ; reverse: 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ) was detected using a Real-time PCR Detection System following routine protocol (Mx3000P, Agilent USA). The relative expression level of the miR was calculated using the formula of 2−ΔΔcq in reference to Sham group (normalized as 1).
Western Blotting
Total protein in the brain tissues was collected using RIPA lysis buffer and centrifuged at 1000g for 10 min. The protein samples (20 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 80V for 2.5 h. Then the proteins were transferred onto a PVDF membranes. After being blocked using 5% skim milk powder for one h, the membranes were incubated with primary antibodies against NeuroD2 (1:10,000), PSD95 (1:5000), and GAPDH (1:5000) at 4°C overnight. Afterwards, the membranes were incubated with secondary IgG-HRP antibodies (1:5000) at 37°C for 45 min. Blots were developed using ECL Plus reagent and the relative expression levels of proteins were calculated by Gel-Pro-Analyzer (Media Cybernetics, USA) in reference to Sham group (normalized as 1).
Modulation of miR-567 in vivo
To validate the key role of miR-567 inhibition in the cognitive improvement effects of rTMS, the level of the miR was induced in AD mice. Specific agomir of miR-567 and NC agomir were injected into mice via tail (5 mg/kg body weight) 24 h before scopolamine injection and at 14th day of scopolamine injection. Then the effects of miR-567 overexpression on the treatment effects of rTMS were determined using MWM, TUNEL, ELISA, IHC, and Western blotting assays as described above.
Statistical Analysis
All the data were expressed as mean ± standard deviation (SD) and the normal distribution of the data was justified with Shapiro test. To determine the overall effects of rTMS administrations, one-way analysis of variance (ANOVA) and post-hoc multiple comparisons using Tukey method were performed using GraphPad Prism version 6.0 (GraphPad Software, Inc., San Diego, CA). Statistical significance was accepted when the P value (two tailed) is smaller than 0.05.
Results
Treatment with rTMS Improves the Cognitive Behaviors of Mice with AD
The impairments associated with AD progression on the learning and memorizing functions of the brain have been well characterized. Thus, the present study firstly assessed the changes in the cognitive behaviors of the mice in the different groups using MWM tests. As shown in Figure 1, the establishment of the AD model showed a significant influence on the learning ability of mice in the 4-day visible platform trial. The latency of escaping time was increased in the Model group compared with that of the Sham group (P<0.01) (Figure 1A). Furthermore, the memorizing ability of the mice was also impaired in the AD model. A suppressed proportion of penetrating path in the platform area (P<0.01) (Figure 1B) and shortened duration of the mice in the platform quadrant (P<0.01) (Figure 1C) were recorded in the 1-day free-probe trial. The data collectively demonstrated the deficient cognitive functions of the mice with AD. Regarding mice treated with rTMS of two frequencies, the learning and memorizing abilities of the mice were significantly improved. The treatments decreased the escaping latency, increased the proportion of the penetrating path and lengthened the staying time (Figure 1). Furthermore, the effects of rTMS occurred in a frequency-dependent manner, with the high-frequency treatment exhibiting better improvement effects on the cognitive function than those of the low-frequency treatment (P=0.023) (Figure 1).
Treatment of rTMS Increases the Brain Neuron Activity in Mice with AD
In addition to evaluating the brain protective effects of the rTMS treatments by assessing the cognitive behaviors of mice with AD, the changes in brain tissue were also detected directly to confirm the benefits of the rTMS treatments. The apoptotic rate in the hippocampus regions of mice with AD was determined with TUNEL staining. The induction of AD symptoms significantly increased the proportion of apoptotic neurons (stained blue) in the brain tissues compared with that of tissues in the Sham group (P<0.01) (Figure 2A). The impaired viability of neurons was further supported by the restricted expression and distribution of doublecortin observed in mice with AD compared with those of mice in the Sham group (P<0.01) (Figure 2B). However, for mice treated with rTMS, the proportion of apoptotic cells was markedly suppressed (Figure 2A), and the level and distribution of doublecortin were reversed (Figure 2B), which collectively inferred the protective effects of rTMS on the brain tissues of mice with AD. In contrast to the effects of rTMS on the cognitive functions, the improvements in brain structure observed in the current study did not exhibit a frequency-dependent pattern.
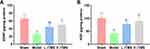
Treatment with rTMS Increases the Neurotrophic Factor Levels in Mice with AD
The level of neurotrophic factor is another indicator representing the health of the nervous system. Thus, the levels of brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF), two classical neurotrophic factors, in the brain tissues were determined by ELISA. The results showed that the levels of both factors were suppressed by scopolamine in the Model group, while they were increased by rTMS treatments (P<0.05) (Figure 3). Similar to the effects on cognitive behaviors, the effects of rTMS on neurotrophic factor levels were strengthened with frequency (Figure 3).
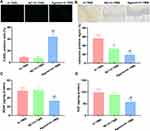
Treatment with rTMS Inhibits the Expression of miR-567, While It Increases the Expression of NEUROD2 and Discs Large MAGUK Scaffold Protein 4 (PSD95)
The current study focused on the interaction between rTMS and miRNAs to explain the potential mechanisms driving the protective effects of rTMS against AD progression. Thus, miR-567, a well-characterized pro-AD miR, and its downstream effectors, NEUROD2 and PSD95, were selected as the research focus. As shown in Figure 4A, the establishment of the AD model induced the expression of miR-567, which was then suppressed by rTMS treatment (both frequencies) (Figure 4A). Correspondingly, the expression of NEUROD2 was suppressed in mice with AD, while it was restored in mice treated with rTMS and it contributed to the changes in PSD95 expression (Figure 4B). NEUROD2 and PSD95 are important for the development of the hippocampus region of the brain. Thus, the changes in the miR-567/NEUROD2/PSD95 axis may provide a preliminary explanation of the anti-AD effects of rTMS.
Protective Effects of rTMS Against AD Symptoms are Dependent on the Inhibition of miR-567
Although the aforementioned results provided certain evidence on the role of miR-567 in the anti-AD function of rTMS, whether this miR is indispensable for the treatment strategy remains unclear. Thus, additional assays were performed by overexpressing the levels of miR-567 in mice with AD using a specific agomir, and the mice were then subjected to rTMS of 10 Hz treatment to further evaluate the interaction between the rTMS effects and miR-567. For mice injected with agomir, the benefits from rTMS were generally impaired. In MWM tests, the latency of escaping time was increased, and the proportion of penetrating path in the platform area as well as the duration in the platform quadrant were suppressed by agomir injection (Figure 5), although the changes were not statistically significant. The neuron viability, and the production of BDNF and NGF were also inhibited by miR-567 agomir (Figure 6). The deterioration of the function and structure of brain tissues in mice with AD was associated with the inhibited expression of NEUROD2 and PSD95 (Figure 7), which was attributed to the restored levels of miR-567. Collectively, the obvious interferences on rTMS treatment by miR-567 agomir indicated that the effects of rTMS on AD symptoms were dependent on the inhibition of miR-567.
Discussion
As a non-invasive stimulation strategy developed from the in vivo neuroplastic change mapping technique, rTMS can conduct brief electric currents in brain tissues via an inductive coil and induce long-term effects on the central nervous system.21 According to the literature, these currents can cause the depolarization of corticospinal tract neurons at the axon hillock or via the depolarization of interneurons.22 The effects of rTMS in the modulation of the functions of central nervous system have attracted interest in the potential of rTMS for treating neurodegenerative disorders. In the last decades, numerous studies have been performed to verify the potential and outcomes of rTMS, and the treatment effects of rTMS on neuropsychiatric disorders, including AD, depression, and Parkinson’s disease have been demonstrated by various studies.23 The mechanisms associated with the aforementioned treatment strategy have been partially revealed. It has been proposed that treatment with rTMS can affect Ca2+ metabolism, cell hydration, and γ-aminobutyric acid production,24,25 inferring that the technique can benefit the central nervous system long-term. The current study aimed to provide additional information on the mechanism driving the protective effects of rTMS against MCI induced by AD by focusing on the interaction between rTMS and miRNAs. Based on previous reports, the miR-567/NEUROD2/PSD95 axis was selected as the current research focus.
The MCI symptom was induced using scopolamine in the present study, as previously reported.26 Based on the results of MWM assays, the injection of scopolamine impaired the learning and memorizing abilities of the mice, while mice with AD subjected to rTMS showed improved cognitive function. The effects of rTMS were strengthened with frequency. Whether treatment with rTMS should be employed at high or low frequency has been long-term debated. Certain studies indicate that, although high-frequency rTMS has different benefits on the central nervous system, it may also induce adverse reactions such as stroke and seizure;27 thus, low-frequency rTMS may be a more favorable option for the application of rTMS. However, the detection of histological changes in the current study did not show a significant difference in terms of affecting the brain structure between high and low frequencies. The results of TUNEL staining and IHC assay showed that both frequencies could attenuate scopolamine-induced apoptosis and increase the production of doublecortin in brain tissues. Regarding the levels of neurotrophic factors, both frequencies of rTMS restored the levels of BDNF and NGF that were suppressed by scopolamine injection in the present mouse model. Thus, no significant difference was detected regarding the side effects of high and low-frequency treatments in the present study. Furthermore, the current findings support the improvement effects of rTMS on the impaired cognitive function associated with AD or other neurodegenerative disorders reported in previous studies.2,21,28
The protective effects of rTMS on the central nervous system can be exerted via multiple mechanisms. Of these, miRNAs have attracted increased attention in recent years. For example, the clinical trial performed by Cao et al inferred that rTMS could attenuate attention deficit hyperactivity disorder in children by suppressing let-7d levels.29 The study by Liu et al showed that rTMS promoted the proliferation of neural progenitor cells by increasing the levels of miR-106.20 These results suggested that miRNAs may be crucial for the function of rTMS. Thus, the current study explored the changes in the miR-567/NEUROD2/PSD95 axis under rTMS treatment. The data showed that rTMS treatments of both frequencies inhibited the expression of miR-567, which subsequently induced the upregulation of the downstream effectors NEUROD2 and PSD95. The potential involvement of miR-567 in the progression of AD was firstly proposed by Felice et al, who demonstrated that miR-567 was abnormally expressed at high levels in patients with MCI-AD.10 Based on target prediction analysis, the authors further indicated that several downstream effectors of miR-567, including NEUROG2, TCF3, TCF4 and TBR1, were important to the functional maintenance of the nervous system.11,12,30,31 Since the effects of rTMS on the levels of miR are ubiquitous, it was hypothesized that the administration of rTMS would influence the expression and function of miR-567, and subsequently modulate the downstream effectors of this miR.
The detection of the miR-567 levels in mice with AD showed that the injection of scopolamine markedly increased the level of this miRNA, which was associated with the inhibited expression of NEUROD2 an PSD95. According to previous studies, NEUROD2 is critical for the development of hippocampal mossy fiber synapses.12 The molecule exerts its function by regulating the level of the synaptic scaffolding molecule PSD95 in the developing hippocampus. The in vitro function of PSD95 on spine morphogenesis remains controversial.32,33 Regarding their role in AD, PSD95 levels are diminished in ageing-related and neurodegenerative disorders, including AD and Huntington’s disease.34,35 Thus, the changes in miR-456/NEUROD2/PSD95 in model mice solidly supported the development of AD symptoms in the mouse model used in the current study. The changes in the miR-456/NEUROD2/PSD95 axis were all reversed by rTMS at both frequencies. The effects of rTMS on the miR-456/NEUROD2/PSD95 axis was synchronized with its effects on cognitive function, brain structure, and neurotrophic factors, indicating that the cognition improvement function of the rTMS technique was associated with the activity of the miR-456/NEUROD2/PSD95 axis. However, whether this axis plays a central in the treatment needs further verification. Therefore, additional groups were set up by injecting miR-456 agomir along with AD induction and rTMS treatment. The induced in vivo level of miR-456 evidently counteracted the effects of rTMS, as represented by the deteriorated performance in MWM tests, destructed brain structure, and reduced levels of BDNF and NGF in mice with AD even under treatment with rTMS. Based on the data of mice injected with miR-456 agomir, the current study concluded that the improvement effects of rTMS treatment on the cognitive function of mice with AD depended on the inhibition of the miR-456 level. As a miR normally studied in cancer,36 the present study is the first report connecting its function with therapies for neurodegenerative disorders.
Collectively, the current findings highlighted the role of miR-567 in the cognitive improving function of rTMS. The treatment strategy protected mice against AD-induced MCI and brain tissue destruction by upregulating miR-567, which subsequently restored the function of NEUROD2 and PSD95. However, the current study only provided a preliminary explanation for connecting the effects of rTMS on AD with the function of miR. It failed to explicitly reveal the regulatory pattern of miR-567 by rTMS. Additionally, the current study only performed in vivo assays, thus, the conclusion of the current study might be substantially influenced by the complicated condition of in vivo system. There are numerous miRs whose expression is influenced by rTMS, and miR-567 might not be the only miR that contributes to the function of rTMS. More comprehensive studies are needed to explain in detail interaction between miRs and rTMS treatment in order to promote the application of this technique for treating neurodegenerative disorders.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Amani H, Kazerooni H, Hassanpoor H, Akbarzadeh A, Pazoki-Toroudi H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: a review. Artifi Cell Nanomed B. 2019;47(1):3524–3539. doi:10.1080/21691401.2019.1639723
2. Nardone R, Tezzon F, Höller Y, Golaszewski S, Trinka E, Brigo F. Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurolo Scand. 2014;129(6):351–366. doi:10.1111/ane.12223
3. Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm. 2010;117(1):105–122. doi:10.1007/s00702-009-0333-7
4. Manenti R, Cotelli M, Calabria M, Maioli C, Miniussi C. The role of the dorsolateral prefrontal cortex in retrieval from long-term memory depends on strategies: a repetitive transcranial magnetic stimulation study. Neuroscience. 2010;166(2):501–507. doi:10.1016/j.neuroscience.2009.12.037
5. Manenti R, Tettamanti M, Cotelli M, Miniussi C, Cappa SF. The neural bases of word encoding and retrieval: a fMRI-guided transcranial magnetic stimulation study. Brain Topogr. 2010;22(4):318–332.
6. Freitas C, Mondragón-Llorca H, Pascual-Leone A. Noninvasive brain stimulation in Alzheimer’s disease: systematic review and perspectives for the future. Exp Gerontol. 2011;46(8):611–627. doi:10.1016/j.exger.2011.04.001
7. Boggio PS, Valasek CA, Campanhã C, et al. Non-invasive brain stimulation to assess and modulate neuroplasticity in Alzheimer’s disease. Neuropsychol Rehabil. 2011;21(5):703–716. doi:10.1080/09602011.2011.617943
8. Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Tonali PA. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease: evidence of impaired glutamatergic neurotransmission? Ann Neurol. 2003;53(6):824. doi:10.1002/ana.10600
9. Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109(1):127–135. doi:10.1007/BF00228633
10. De Felice B, Montanino C, Oliva M, Bonavita S, Di Onofrio V, Coppola C. MicroRNA expression signature in mild cognitive impairment due to Alzheimer’s Disease. Mol Neurobiol. 2020;57(11):4408–4416. doi:10.1007/s12035-020-02029-7
11. Kuwahara A, Sakai H, Xu Y, Itoh Y, Hirabayashi Y, Gotoh Y. Tcf3 represses Wnt-β-catenin signaling and maintains neural stem cell population during neocortical development. PLoS One. 2014;9(5):e94408. doi:10.1371/journal.pone.0094408
12. Wilke SA, Hall BJ, Antonios JK, et al. NeuroD2 regulates the development of hippocampal mossy fiber synapses. Nat Med. 2012;7:9.
13. Gravitz L. Drugs: a tangled web of targets. Nature. 2011;475(7355):S9–11. doi:10.1038/475S9a
14. Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med. 2011;17(9):1060–1065. doi:10.1038/nm.2460
15. Arendash GW, Millard WJ, Dunn AJ, Meyer EM. Long-term neuropathological and neurochemical effects of nucleus basalis lesions in the rat. Science. 1987;238(4829):952–956. doi:10.1126/science.2890210
16. Khachaturian ZS. Revised criteria for diagnosis of Alzheimer’s disease: national Institute on Aging-Alzheimer’s Association diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):253–256. doi:10.1016/j.jalz.2011.04.003
17. Barker AT. Electricity, magnetism and the body: some uses and abuses. J R Soc Health. 1994;114(2):91–97. doi:10.1177/146642409411400210
18. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi:10.1016/S0140-6736(85)92413-4
19. Liu H, Li G, Ma C, Chen Y, Wang J, Yang Y. Repetitive magnetic stimulation promotes the proliferation of neural progenitor cells via modulating the expression of miR-106b. Int J Mol Med. 2018;42(6):3631–3639. doi:10.3892/ijmm.2018.3922
20. Guo F, Han X, Zhang J, et al. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation via the regulation of MiR-25 in a rat model of focal cerebral ischemia. PLoS One. 2014;9(10):e109267. doi:10.1371/journal.pone.0109267
21. Arendash GW. Transcranial electromagnetic treatment against Alzheimer’s disease: why it has the potential to trump Alzheimer’s disease drug development. J Alzheimer Dis. 2012;32(2):243–266. doi:10.3233/JAD-2012-120943
22. Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93(1):59–98.
23. Herrero Babiloni A, Bellemare A, Beetz G, et al. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: a systematic review. Sleep Med Rev. 2020;55:101381. doi:10.1016/j.smrv.2020.101381
24. Danielyan AA, Mirakyan MM, Grigoryan GY, Ayrapetyan SN. The static magnetic field effects on ouabain H3 binding by cancer tissue. Physiol Chem Phys Med NMR. 1999;31(2):139–144.
25. Blackman CF, Benane SG, Kinney LS, Joines WT, House DE. Effects of ELF fields on calcium-ion efflux from brain tissue in vitro. Radiat Res. 1982;92(3):510–520. doi:10.2307/3575923
26. Manral A, Meena P, Saini V, Siraj F, Shalini S, Tiwari M. DADS analogues ameliorated the cognitive impairments of Alzheimer-like rat model induced by scopolamine. Neurotox Res. 2016;30(3):407–426. doi:10.1007/s12640-016-9625-5
27. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7,1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi:10.1016/S0168-5597(97)00096-8
28. Ni Z, Chen R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl Neurodegener. 2015;4:22. doi:10.1186/s40035-015-0045-x
29. Cao P, Wang L, Cheng Q, et al. Changes in serum miRNA-let-7 level in children with attention deficit hyperactivity disorder treated by repetitive transcranial magnetic stimulation or atomoxetine: an exploratory trial. Psychiat Res. 2019;274:189–194. doi:10.1016/j.psychres.2019.02.037
30. Vegas N, Cavallin M, Kleefstra T, et al. Mutations in TBR1 gene leads to cortical malformations and intellectual disability. Eur J Med Genet. 2018;61(12):759–764. doi:10.1016/j.ejmg.2018.09.012
31. Crux S, Herms J, Dorostkar MM. Tcf4 regulates dendritic spine density and morphology in the adult brain. PLoS One. 2018;13(6):e0199359. doi:10.1371/journal.pone.0199359
32. Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. P Natl Acad Sci USA. 2007;104(10):4176–4181. doi:10.1073/pnas.0609307104
33. Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52(2):307–320. doi:10.1016/j.neuron.2006.09.012
34. de Bartolomeis A, Latte G, Tomasetti C, Iasevoli F. Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches. Mol Neurobiol. 2014;49(1):484–511. doi:10.1007/s12035-013-8534-3
35. Arbuckle MI, Komiyama NH, Delaney A, et al. The SH3 domain of postsynaptic density 95 mediates inflammatory pain through phosphatidylinositol-3-kinase recruitment. EMBO Rep. 2010;11(6):473–478. doi:10.1038/embor.2010.63
36. Liu D, Zhang C, Li X, Zhang H, Pang Q, Wan A. MicroRNA-567 inhibits cell proliferation, migration and invasion by targeting FGF5 in osteosarcoma. EXCLI J. 2018;17:102–112. doi:10.17179/excli2017-932
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.