Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Repeated intermittent ulipristal acetate in the treatment of uterine fibroids: a cost-effectiveness analysis
Authors Geale K , Saridogan E, Lehmann M, Arriagada P, Hultberg M, Henriksson M
Received 9 June 2017
Accepted for publication 13 September 2017
Published 1 November 2017 Volume 2017:9 Pages 669—676
DOI https://doi.org/10.2147/CEOR.S143557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Kirk Geale,1,2 Ertan Saridogan,3 Matthieu Lehmann,4 Pablo Arriagada,4 Marcus Hultberg,2 Martin Henriksson5
1Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden; 2PAREXEL International, Stockholm, Sweden; 3Women’s Health Division, University College London Hospital, London, UK; 4PregLem SA, Geneva, Switzerland; 5Department of Medical and Health Sciences, Linköping University, Linköping, Sweden
Abstract: There are limited treatment options available for women with moderate to severe symptoms of uterine fibroids (UFs) who wish to avoid surgery. For these women, treatment with standard pharmaceuticals such as contraceptives is often insufficient to relieve symptoms, and patients may require surgery despite their wish to avoid it. Clinical trials demonstrate that ulipristal acetate 5 mg (UPA) is an effective treatment for this patient group, but its cost-effectiveness has not been assessed in this population. A decision-analytic model was developed to simulate a cohort of patients in this population under treatment with UPA followed by surgery as needed compared to treatment with iron and non-steroidal anti-inflammatory drug (NSAID) followed by surgery as needed (best supportive care, BSC). The analysis took the perspective of the National Health Service (NHS) in England, UK, and was based on the published UPA clinical trials. Results were calculated for the long-term costs and quality-adjusted life years (QALYs) for each treatment arm and combined into an incremental cost-effectiveness ratio (ICER) as the primary outcome. The impact of parameter uncertainty on the results was assessed using scenario, deterministic, and probabilistic sensitivity analyses. The results show that treating patients with the UPA strategy, instead of the BSC strategy, results in an additional cost of £1,115 and a gain of 0.087 QALYs, resulting in an ICER of £12,850. Given commonly accepted cost-effectiveness thresholds in England, the use of UPA as a repeated, intermittent treatment for women with moderate to severe symptoms of UF wishing to avoid surgery is likely to be a cost-effective intervention when compared to BSC.
Keywords: uterine fibroids, cost-effectiveness, health economics, ulipristal acetate, economic evaluation
Introduction
Uterine fibroids (UFs) are benign tumors that affect 20%–40% of women of reproductive age, of which ~10%–20% are symptomatic.1 In clinical practice, detection of UF with ultrasound is often sufficient when the myomas are not numerous.2 Depending on their number, volume, and location in the uterus,3 UF can cause symptoms such as abnormal uterine bleeding, pelvic pressure and pain, and fertility disorders.4 Insufficient treatment can result in lowered quality of life (QOL) and increased economic burden borne by patients and society.5
The main goal of UF treatment is symptom reduction, and the choice of approach is usually determined by a number of medical and social factors such as age, childbearing aspirations, extent and severity of symptoms, fibroid characteristics (size, number, and location), associated medical conditions, possibility of malignancy, proximity to menopause, and desire for uterine preservation.1,6,7 When surgery is contraindicated or patients wish to avoid it, less-invasive pharmaceutical treatment options such as antifibrinolytics, oral contraceptives, and levonorgestrel intrauterine system are all potential treatment options in contemporary clinical practice. However, these alternatives do not treat the underlying UF, are often insufficient for patients with moderate to severe symptoms, and may lead to suboptimal treatment of UF or situations where patients are not treated at all. When standard pharmaceutical therapies do not sufficiently control symptoms of UF and surgery is contraindicated or undesirable, non-steroidal anti-inflammatory drugs (NSAIDs) and iron supplements may be given alone. In some cases, if symptoms become unbearable, the patient may undergo surgery despite a preference to avoid it. For these patients, best supportive care (BSC) consists of treatment with NSAIDs and iron supplements followed by surgery as needed. In reality, some patients may continue to receive standard pharmaceutical treatments in addition to NSAIDs and iron despite a lack of satisfactory effect. According to the definition of the patient population, these standard pharmaceuticals do not impact efficacy in a meaningful way and thus were not considered in this research.
Ulipristal acetate, a selective progesterone receptor modulator, is a treatment option for women with moderate to severe symptoms of UF, which can be given on a repeated intermittent basis. Although intermittent ulipristal acetate 5 mg (UPA) has been shown to effectively control bleeding and pain, reduce fibroid volume, and improve QOL in patients with symptomatic fibroids in a clinical trial context,8 the cost-effectiveness of such strategy has not been fully assessed. Only one previously published study has analyzed the cost-effectiveness of repeated intermittent UPA treatment in an Italian setting, where repeated intermittent use (4–10 courses) was found to be cost-effective compared to the pre-surgical use of UPA for two courses.9
This study aims to extend previous research by providing a cost-effectiveness analysis of the repeated intermittent UPA indication in the long term for women failing on standard pharmaceutical treatments with a wish to avoid surgery.
Methods
Analytical approach
The decision problem considered in this evaluation is whether it is cost-effective to treat patients with UPA followed by surgery as needed, instead of BSC, in England. A decision-analytic model was designed to estimate long-term costs and health outcomes, measured by quality-adjusted life years (QALYs), associated with both treatment strategies using data from the published clinical trials of UPA. Clinical trial data were synthesized with cost and QOL information from other sources to provide inputs to the model. The primary outcome of the analysis is the incremental cost-effectiveness ratio (ICER), calculated as the per-patient mean difference in cost to the health care sector divided by the per-patient mean difference in QALYs gained. Cost-effectiveness was assessed by comparing the ICER to the cost-effectiveness threshold range used by NICE in England, which is currently stated to be between 20,000 and 30,000 GBP.10
Patients and interventions
The patient population of interest in this evaluation includes women eligible for treatment with UPA who are contraindicated for, or wish to avoid, surgery and do not experience sufficient response to standard pharmaceutical treatments.
With the UPA strategy, 5 mg is taken orally once daily as a tablet and can be given on a repeated intermittent basis. Patients eligible for UPA are indicated to receive 3 months of UPA followed by a break of at least 2 months. Although presently unstudied, in theory, women exhibiting symptom control beyond 2 months may extend the break for as long as they are asymptomatic. Patients in the UPA strategy may withdraw to either BSC or directly to surgery if treatment does not sufficiently resolve UF symptoms.
With the BSC strategy, patients begin by receiving NSAIDs and iron supplements. In reality, some patients may also receive standard pharmaceutical treatments, despite a lack of satisfactory effect. It was assumed that these treatments do not have an impact on efficacy and thus were not explicitly modeled. As in the UPA arm, patients who experience unbearable symptoms may elect to undergo surgery, despite their wish to avoid it.
Decision-analytic model
A decision-analytic model was designed to estimate clinical pathways in both treatment arms11 by incorporating levels of pain/discomfort and bleeding, found in the published UPA clinical trials. In each monthly cycle, the number of patients remaining on treatment as well as the level of bleeding and pain is updated in the model. The model development followed the guidelines set by NICE.12 In the base-case analysis, the National Health Service (NHS) payer perspective in an English setting was used, and discount rates were applied at a rate of 3.5% per annum for both costs and QALYs.
Patients enter the model at 41.5 years of age, the average age in the PEARL IV13 trial. Treatment ends at the average age of menopause (51.4 years),14 where no significant differences between the two treatments are assumed due to the regression of symptoms of UF.15
Patients begin the model on treatment with either UPA or iron+NSAID and can transition according to the specification in Figure 1. Patients could withdraw from UPA to treatment with iron+NSAID and remain symptomatic, receive surgery, or die. Patients who receive surgery face a procedural risk of dying (red arrow in Figure 1). This risk was 0.038% and 0.02% for hysterectomy and uterine artery embolization (UAE), respectively. No increased mortality risk was applied to patients receiving myomectomies.16 Patients receiving a non-hysterectomy surgery are subsequently at risk of resurgery. Any patient who did not die during the month of surgery is subsequently assumed to have QOL equivalent to a member of the general population. Patients are also at risk of dying from any cause at all times, represented by orange arrows.
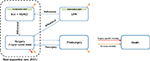  | Figure 1 Patient flow diagram. Abbreviations: NSAID, non-steroidal anti-inflammatory drug; UPA, ulipristal acetate 5 mg. |
The possibility of withdrawing to surgery with both treatment strategies reflects the fact that surgery may become an acceptable option, despite an initial wish to avoid a procedure, when symptoms worsen or become uncontrollable.
Data sources and input parameters
A series of Phase III randomized controlled trials investigating ulipristal acetate constitute the evidence base for UPA (PEARL I, II, III, and IV).8,17–19 The primary end points of these trials evaluated bleeding in terms of the pictorial bleeding assessment chart (PBAC). Secondary end points included comparative measures of pain and discomfort through the visual analog scale (VAS) and QOL through the Uterine Fibroid Symptom and Health-Related Quality of Life Questionnaire (PEARL II, III, and IV) and three-level, five-dimension EuroQoL instrument (EQ-5D, PEARL III and IV). PEARL I and II studied presurgical patients, while PEARL III and IV studied patients eligible for repeated intermittent use. PEARL IV was used as the primary source of data for UPA efficacy in this evaluation. The placebo arm of the PEARL I trial was the primary data source for BSC as patients were allowed to take both NSAIDs and iron.
UPA treatment break assumption
The UPA indication states that patients should receive 3 months of treatment followed by a minimum of 2 months off treatment before beginning the next treatment course. It was assumed that patients who did not withdraw after eight courses exhibited maintained symptom control beyond 2 months. In the model, patients adhere to a 3+2 treatment schedule for the first eight courses (~38 months of therapy), after which patients have an average of a 6-month treatment break (ie, 3+6) in the following courses.
Effectiveness and patient progression
Treatment effectiveness is operationalized as bleeding and pain/discomfort while on treatment with UPA or iron+NSAID, estimated from trial data as reported in the “QOL” section. Patients treated with UPA withdraw to surgery and iron+NSAID at variable rates during courses 1–4 according to the PEARL IV trial data (Table 1). After course four, patients are assumed to withdraw at a rate proportional to iron+NSAID (described in Supplementary materials). Patients treated with iron+NSAID were assumed to withdraw to surgery at a constant rate based on observational data.20 The BSC withdrawal rate and UPA withdrawal following course 4 are described in Supplementary materials.
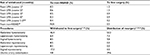  | Table 1 Summary of withdrawals Abbreviations: NSAID, non-steroidal anti-inflammatory drug; UPA, ulipristal acetate 5 mg. |
QOL
Patients’ QOL was measured through the instrument EQ-5D, using UK-based QOL weights associated with each of the five health dimensions defined in the EQ-5D instrument.25 While patients are on treatment with either UPA or iron+NSAID, EQ-5D QOL weights were established using a previously described function.26 In each cycle, the equation adjusts the underlying age-dependent, population-based QOL by the average levels of bleeding and pain in that cycle. Table 2 illustrates this relationship using a patient aged 42 years as an example.
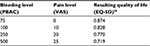  | Table 2 Sample quality of life (EQ-5D) weights given bleeding (PBAC) and pain (VAS) values Note: Average EQ-5D value for the UK population of age 42 years is equal to 0.883.27 Copyright © 2015. Elsevier. Reproduced from Geale K, Hultberg M, Henriksson M. Applying symptom-based utility functions in health economic modelling: a case study of uterine fibroids. Value Health. 2015;18(7):A728.26 Abbreviations: EQ-5D, three-level, five-dimension EuroQoL instrument; PBAC, pictorial bleeding assessment chart; VAS, visual analog scale. |
In the surgery and postsurgery health states, age-dependent QOL weights of the general population in the UK were applied.27
Extrapolation of UPA and iron+NSAID efficacy data was necessary as the time horizon of the analysis extends beyond the available trial data. For patients remaining on treatment with iron+NSAID, the last observed bleeding and pain values were assumed to stay constant. For UPA, the PBAC and VAS levels in the last observed treatment course were assumed to repeat indefinitely. Further details of the extrapolations are included as Supplementary materials.
Patients undergoing surgery receive a QOL decrement from the procedure, differentiated by the type and approach of surgery.28 QOL decrements relating to surgical events last for a total of 1 year, which are summarized in Table 3. The details of adverse event QOL decrements values are included as Supplementary materials.
  | Table 3 Summary of QOL and corresponding decrements Abbreviations: EQ-5D, three-level, five-dimension EuroQoL instrument; QOL, quality of life; UAE, uterine artery embolization. |
Decrements to QOL were also applied for adverse events due to surgery, UPA treatment, and iron+NSAID treatment. The frequencies of adverse events were based on published trial data8,29,30 and were assumed to last for 1 month. Short-term adverse events from surgery occur within 1 year of surgery. Persistent adverse events are also applied for hysterectomy patients, which are present 12 months after the intervention.
Costs
In the base-case analysis, costs for UPA, iron+NSAID, surgery, adverse events, and patient follow-up were included (Table 4).
  | Table 4 Summary of costs Notes: aOn average over the abdominal, laparoscopic, and vaginal approaches. bDetails of adverse event costs are included as Supplementary materials. Abbreviations: GP, general practitioner; NSAID, non-steroidal anti-inflammatory drug; UPA, ulipristal acetate 5 mg. |
Analysis
The estimated ICERs are compared to the cost-effectiveness thresholds of £20,000–30,000 currently employed by NICE in England.
The model’s input parameters are subject to uncertainty, which was quantified through probabilistic sensitivity analysis (PSA). In this analysis, uncertainty (represented by probability distributions) in input parameters associated with sampling uncertainty was propagated through the model using simulation methods. Based on 10,000 simulations, the probability of UPA being cost-effective at different threshold values is assessed and reported. Distributional assumptions regarding input parameters are included as Supplementary materials.
Scenario analyses were also conducted by varying model inputs not associated with statistical uncertainty to investigate their impact on the cost-effectiveness results.
Results
In the base-case analysis, the UPA strategy is associated with 6.696 QALYs and a cost of £6,669. The BSC strategy is associated with 6.610 QALYs and a cost of £5,555. Incrementally, UPA followed by surgery as needed is estimated to add 0.087 QALYs at a cost of £1,115, resulting in a cost per QALY gained of £12,850, compared with BSC.
A scenario analysis including costs of productivity loss41 falling outside the health care budget was calculated, where patients treated with iron+NSAID were estimated to be off work for an additional 1.5 days per month compared with UPA, while those receiving abdominal surgery, laparoscopic or vaginal surgery, or uterine artery embolization spent 74, 56, and 7 days away from work, as a one-time event. Other scenarios varying withdrawal rates, duration of UPA treatment break, age of incident patients, and the age of menopause are reported in Table 5.
Further sensitivity analyses are presented in Supplementary materials.
PSA
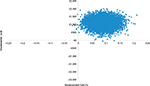
The results of the PSA are presented graphically as a plot on the cost-effectiveness plane (Figure 2).
  | Figure 2 PSA iterations plotted on a cost-effectiveness plane. Abbreviations: PSA, probabilistic sensitivity analysis; QALYs, quality-adjusted life years. |
For cost-effectiveness thresholds of £20,000 and £30,000, the probability of UPA strategy being cost-effective was ~85% and 96%, respectively. In order for UPA strategy to have a 50% (75%) [95%] probability of being cost-effective compared with BSC, the threshold must be at least £12,850 (£16,950) [£27,800]. The probability of cost-effectiveness at different threshold values was plotted as a cost-effectiveness acceptability curve (Supplementary materials).
Discussion
The base-case ICER of £12,850 per QALY gained is below the commonly accepted threshold of £20,000-30,000 in England for the NHS payer, implying that UPA followed by surgery as needed is cost-effective compared to BSC for patients wishing to avoid surgery. The QALY loss over the duration of the model in the scenario where UPA is not available is approximately equivalent to having pneumonia continuously for one year.42 The results of the analysis presented in this paper expand the overall evaluation of UPA as a treatment for women with the prevalent condition of UF.
Probabilistic and scenario sensitivity analyses showed that the conclusion of cost-effectiveness holds in many cases. An analysis of particular note is the inclusion of productivity losses (sometimes called the societal perspective), where treating patients with the UPA strategy instead of the BSC strategy results in health gains and provides additional value to the wider economy. These values are not likely to impact the NHS budget directly and therefore the total cost to the NHS is still positive. Although it is not clear how to explicitly take these potential benefits into account in a constrained NHS perspective, the results indicate that there may be wider social benefits associated with UPA treatment. Furthermore, in some jurisdictions a societal perspective is advocated (eg, Sweden and Norway), and these gains would be considered in an economic evaluation of UPA.
There is a lack of robust data regarding certain key model parameters. For example, if patients remain on a 3+2 treatment schedule indefinitely in combination with conceivable changes in other parameters, scenarios can arise where the ICER is above the standard cost-effectiveness threshold range. Conversely, extending the break, or applying it earlier than the ninth treatment cycle, increases the strength of the cost-effective result. Second, patients receiving iron+NSAID may not be in regular contact with gynecological specialists, leading to uncertainty in the input parameters relating to this patient group. If these patients are treated less often than assumed in the analysis, the cost of this treatment arm will decrease.
In the analysis, there are more patients receiving surgery with the BSC strategy compared with the UPA strategy. Therefore, the model may be conservative as it excludes potential quality-of-life decrements that may be relevant to the patient population wishing to avoid surgery, such as feelings of lost femininity and anxiety before surgery.
Efficacy data for UPA were limited in time compared to the duration of the analysis, and trial-based follow-up was not as frequent as required to inform monthly disease progression parameters. Therefore, interpolation and extrapolation were necessary in order to model patients over the time horizon and are a source of uncertainty. This source of uncertainty is unavoidable in most health economic assessments, and sensitivity analyses show that the conclusion that UPA is cost-effective appears valid when investigating the importance of these parameters.
The results generally show that intermittent treatment with UPA followed by surgery as needed is cost-effective compared to BSC in patients wishing to avoid surgery. Future research should analyze the cost-effectiveness of UPA in alternative patient populations, health care perspectives, and compared to different interventions. In particular, the cost-effectiveness of using UPA as a replacement for surgery in perimenopausal patients should be studied, as UPA may allow these patients to reach menopause (at which time many experience natural symptom reduction), avoiding an invasive surgical procedure. From a clinical point of view, it would be beneficial to understand more about the impact of patients’ preferences for avoiding surgery as well as the patient group treated with BSC in terms of demographics, QOL, and resource use.
Acknowledgments
The authors thank Helen Saunders of PregLem SA for her involvement in this analysis and her support together with Urmi Bapat from Gedeon Richter (UK) Ltd with the preparation of the manuscript. The research was funded by PregLem SA.
Disclosure
KG, MHe, and MHu were employed by PAREXEL International when the model was developed. KG and MHu were employed by PAREXEL International when the manuscript was drafted. ML and PA were employed by PregLem SA when the model was developed and the manuscript was drafted. ES received honoraria for provision of training to health care professionals and consultancy fees from Gedeon Richter. KG was employed by Quantify Research when the manuscript was published. The authors report no other conflicts of interest in this work.
References
Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104(1):393–406. | ||
Battista C, Capriglione S, Guzzo F, et al. The challenge of preoperative identification of uterine myomas: is ultrasound trustworthy? A prospective cohort study. Arch Gynecol Obstet. 2016;293(6):1235–1241. | ||
Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:615–626. | ||
Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. | ||
Downes E, Sikirica V, Gilabert-Estelles J, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol. 2010;152:96–102. | ||
Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2):CD000547. | ||
Viswanathan M, Hartmann K, McKoy N, et al. Management of uterine fibroids: an update of the evidence. Evid Rep Technol Assess (Full Rep). 2007;(154):1–122. | ||
Donnez J, Donnez O, Matule D, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril. 2016; 105(1):165–173. | ||
Maratea D. Repeated-intermittent use of ulipristal acetate for the management of uterine fibroids: an Italian pharmacoeconomic evaluation. Minerva Ginecol. 2016;68(1):15–20. | ||
National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. 2013. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed July 6, 2015. | ||
Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15:1295–1310. | ||
National Institute for Health and Care Excellence [webpage on the Internet]. The Guidelines Manual – 07 Assessing Cost Effectiveness. 2012. Available from: http://www.nice.org.uk/article/pmg6/chapter/7-assessing-cost-effectiveness. Accessed July 6, 2015. | ||
Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519–527. | ||
Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. | ||
Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. BMJ. 1986;293(6553):359–362. | ||
Zowall H, Cairns JA, Brewer C, Lamping DL, Gedroyc WM, Regan L. Cost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroids. BJOG. 2008;115(5):653–662. | ||
Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. | ||
Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420. | ||
Donnez J, Vazquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101(6):1565–1573. | ||
Fernandez H, Schmidt T, Powell M, Costa APcF, Arriagada P, Thaler C. Real world data of 1473 patients treated with ulipristal acetate for uterine fibroids: Premya study results. Eur J Obstet Gynecol Reprod Biol. 2017;208:91–96. | ||
Carls GS, Lee DW, Ozminkowski RJ, Wang S, Gibson TB, Stewart E. What are the total costs of surgical treatment for uterine fibroids? J Womens Health. 2008;17(7):1119–1132. | ||
HES. Hospital Episode Statistics Database. England: NHS. 2013. | ||
Edwards RD, Moss JG, Lumsden MA, et al. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370. | ||
Moss JG, Cooper KG, Khaund A, et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011;118:936–944. | ||
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. | ||
Geale K, Hultberg M, Henriksson M. Applying symptom-based utility functions in health economic modelling: a case study of uterine fibroids. Value Health. 2015;18(7):A728. | ||
Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539–545. | ||
Sculpher M, Manca A, Abbott J, Fountain J, Mason S, Garry R. Cost effectiveness analysis of laparoscopic hysterectomy compared with standard hysterectomy: results from a randomised trial. BMJ. 2004;328(7432):134. | ||
Brummer TH, Jalkanen J, Fraser J, et al. FINHYST, a prospective study of 5279 hysterectomies: complications and their risk factors. Hum Reprod. 2011;26(7):1741–1751. | ||
Manyonda IT, Bratby M, Horst JS, Banu N, Gorti M, Belli AM. Uterine artery embolization versus myomectomy: impact on quality of life: results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) Trial. Cardiovasc Intervent Radiol. 2012;35(3):530–536. | ||
Stein MB, Roy-Byrne PP, Craske MG, et al. Functional impact and health utility of anxiety disorders in primary care outpatients. Med Care. 2005;43(12):1164–1170. | ||
BNF. British National Formulary. 2015. Available from: https://www.medicinescomplete.com/. Accessed September, 2014. | ||
Mysupermarket [homepage on the Internet]. Price Database. 2015. Available from: http://www.mysupermarket.co.uk/. Accessed July 6, 2015. | ||
de Vries CJ, Wieringa-de Waard M, Vervoort CoLA, Ankum WM, Bindels PJ. Abnormal vaginal bleeding in women of reproductive age: a descriptive study of initial management in general practice. BMC Womens Health. 2008;8:7. | ||
Hart J, Chatzipapas IK, Magos A. The cost of a hysterectomy in a UK hospital. J Obstet Gynaecol. 1999;19:522–525. | ||
HES. Hospital Episode Statistics Database. England: NHS. 2014. | ||
NHS [webpage on the Internet]. Reference Cost Collection: National Schedule of Reference Costs – Year 2013-14 – NHS Trusts and NHS Foundation Trusts. 2015. Available from: https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014. Accessed July 6, 2015. | ||
PSSRU [webpage on the Internet]. Unit Costs of Health & Social Care 2014. 2014. Available from: http://www.pssru.ac.uk/project-pages/unit-costs/2014/. Accessed July 6, 2015. | ||
Moraloglu O, Tapisiz OL, Moroy P, et al. Functional results and quality-of-life after hysterectomy in Turkish women. Gazz Med Ital Arch Sci Med. 2007;166:97. | ||
ONS. ASHE 1997 to 2014 Selected Estimates. 2014. Available from: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/ashe1997to2015selectedestimates. Accessed July 6, 2015. | ||
Van den Hout WB. The value of productivity: human-capital versus friction-cost method. Ann Rheum Dis. 2010;69(suppl 1):i89–i91. | ||
Jit M, Cromer D, Baguelin M, Stowe J, Andrews N, Miller E. The cost-effectiveness of vaccinating pregnant women against seasonal influenza in England and Wales. Vaccine. 2010;29(1):115–122. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

