Back to Journals » Journal of Pain Research » Volume 10
Repeated administration of mazindol reduces spontaneous pain-related behaviors without modifying bone density and microarchitecture in a mouse model of complete Freund’s adjuvant-induced knee arthritis
Authors Robledo-González LE, Martínez-Martínez A, Vargas-Muñoz VM, Acosta-Gonzalez RI, Plancarte-Sánchez R, Anaya-Reyes M, Fernández del Valle-Laisequilla C, Reyes-García JG, Jiménez-Andrade JM
Received 9 March 2017
Accepted for publication 26 June 2017
Published 27 July 2017 Volume 2017:10 Pages 1777—1786
DOI https://doi.org/10.2147/JPR.S136581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
LE Robledo-González,1 A Martínez-Martínez,1 VM Vargas-Muñoz,1 RI Acosta-González,2 R Plancarte-Sánchez,3 M Anaya-Reyes,4 C Fernández del Valle-Laisequilla,5 JG Reyes-García,6 JM Jiménez-Andrade1
1Laboratorio de Farmacología, 2Departamento de Análisis Clínicos, Unidad Académica Multidisciplinaria Reynosa-Aztlán, UAT, Reynosa, Tamaulipas, Mexico; 3Departamento de Anestesiología, Terapia Intensiva y Clínica del Dolor, Instituto Nacional de Cancerología, Mexico City, Mexico; 4Investigación Clínica y Farmacovigilancia, 5Investigación Clínica y Farmacovigilancia, Productos Medix, S.A. de C.V., Mexico City, Mexico; 6Sección de Estudios de Posgrado e Investigación, Escuela Superior de Medicina, Instituto Politécnico Nacional, Mexico City, Mexico
Background: The role of dopaminergic system in the development of rheumatoid arthritis-related pain, a major symptom in this disease, has not been explored. Therefore, the antinociceptive effect of mazindol, a dopamine uptake inhibitor, was evaluated in a model of complete Freund’s adjuvant (CFA)-induced arthritis. Furthermore, as studies have shown that the dopaminergic system regulates bone metabolism, the effect of mazindol on bone mass and microarchitecture was determined.
Methods: Adult ICR male mice received intra-articular injections of either CFA or saline into the right knee joint every week. Spontaneous pain-like behaviors (flinching and guarding) and locomotor activity were assessed at day 26 post-first CFA, following which, a single intraperitoneally (i.p.) administered dose of mazindol was given (1, 3 and 10 mg/kg). Then, the antinociceptive effect of a repeated administration of 3 mg/kg mazindol (daily, i.p.; day 15–day 26) was evaluated. Additionally, at day 26, the participation of D1-like, D2-like or opioid receptors in the antinociceptive effect of mazindol was evaluated. The effect of mazindol on bone density and microarchitecture was evaluated by micro-computed tomography.
Results: Acute administration of mazindol decreased the spontaneous pain-like behaviors in a dose-dependent manner without reducing the knee edema. However, mazindol at 10 mg/kg significantly increased the locomotor activity; therefore, 3 mg/kg mazindol was used for further studies. Repeated administration of 3 mg/kg mazindol significantly decreased the pain-like behaviors without modifying locomotor activity. The antinociceptive effect of mazindol was blocked by administration of a D2-like receptor antagonist (haloperidol), but not by administration of D1-like receptor antagonist (SCH 23390) or an opioid receptor antagonist (naloxone). Repeated administration of mazindol did not significantly modify the density and microarchitecture of periarticular bone of the arthritic and nonarthritic knee joints.
Conclusion: Results suggest that mazindol via D2-like receptors has an antinociceptive role in mice with CFA-induced knee arthritis without modifying the bone health negatively.
Keywords: dopamine, analgesia, arthritic pain, micro-computed tomography
Background
Rheumatoid arthritis (RA) is an autoimmune disease affecting ~1% of the world’s population.1,2 RA is characterized by chronic and symmetric inflammation of synovial joints, leading to joint destruction, chronic pain, loss of function and disability.3 Although RA-induced joint pain exerts a significant toll on the society in terms of both quality of life and functional disabilities, there are relatively few effective therapies for fully controlling joint pain that are not accompanied by significant unwanted side effects.4–8 In light of this, there is a clinical need to develop mechanism-based therapies with a better efficacy and with fewer side effects to treat joint pain in patients with RA.
Recent studies have shown that dopamine acting through its receptors is involved in the pathogenesis of RA.9,10 Dopamine receptors are seven-transmembrane G-coupled receptors and, so far, five subtypes have been reported to exist.11–13 D1 and D5 receptors are D1-like receptors, whereas D2–D4 are D2-like receptors.11–13 Anatomic studies have demonstrated the expression of dopamine in dendritic cells in the synovial tissue from patients with RA, as well as the expression of D1–D5 receptors on B cells and fibroblasts from patients with RA.10,14 Interestingly, a study14 has shown an increased expression of D2-like receptors on B cells in synovial fluids from RA patients, and administration of a D2-like receptor agonist decreased arthritic scores in mice with collagen-induced RA.10,15 Moreover, both administration of D2-like receptor antagonist and genetic ablation of this receptor exacerbate arthritic scores in a collagen-induced RA mouse model and in a human RA/ severe combined immunodeficiency (SCID) mouse chimera model, respectively.10,16 Conversely, administration of a D1-like receptor antagonist suppressed the disease progression both in a collagen-induced RA and in a human RA/SCID mouse chimera model.16,17
While these studies suggest a role of dopaminergic signaling in the pathogenesis of RA, there have been no studies evaluating the role of dopaminergic signaling in the development or maintenance of arthritic joint pain, one of the most frequent symptoms in patients with RA. Therefore, the first goal of this study was to evaluate the antinociceptive effect of mazindol, a dopamine uptake inhibitor (an anorectic drug widely used in Mexico and Brazil, but not in the USA). The second goal was to then determine which dopamine receptors mediate the effect of mazindol using mice with unilateral complete Freund’s adjuvant (CFA)-induced knee arthritis, a model that mimics some features of RA.
RA is also associated with periarticular osteopenia, generalized bone mineral density (BMD) loss and an increased risk of osteoporotic-related fractures.18,19 In line with clinical data, mice with CFA-induced arthritis exhibit a significant periarticular bone loss.20 Recent studies have shown that dopaminergic signaling is not only involved in several functions of the central nervous system (CNS), but also in the regulation of bone metabolism.21,22 Knock-out mice lacking the dopamine transporter have a decreased bone mass and bone strength.21 Furthermore, in vitro studies have demonstrated that dopamine acting through D2-like receptors inhibits human osteoclastogenesis.23 Based on this, the third major goal of this study was to evaluate the effect of repeated administration of mazindol on the bone mass and microarchitecture using micro-computed tomography (micro-CT) analysis in mice with CFA-induced arthritis.
Methods
Reagents
The following compounds were used in this study (obtained from the sources indicated): CFA (catalog # F5881; Sigma-Aldrich Co., Toluca, Mexico); mazindol (a gift from MEDIX Pharmaceutical, Mexico City, Mexico); haloperidol (D2-like receptor antagonist, catalog # H1512; Sigma-Aldrich); naloxone (opioid receptor antagonist, catalog # N7758; Sigma-Aldrich); and R(+)-SCH 23390 (D1-like receptor antagonist, catalog # D054-5MG; Sigma-Aldrich Co.). Mazindol was dissolved in 0.025% carboxymethyl cellulose (CMC). Other drugs were dissolved in saline solution.
Animals
Experiments used a total of 87 male ICR mice (Envigo, Mexico City, Mexico) weighing 18–20 g (8 weeks old) at the beginning of the experiments. Mice were housed in groups of four animals per cage at a constant temperature of 22°C±2°C and a 12 h light/dark cycle, with access to food and water ad libitum. All experiments were conducted in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals.24 This study was approved by the Comité de Ética Institucional de la Unidad Académica Multidisciplinaria Reynosa Aztlán de la Universidad Autónoma de Tamaulipas (CEI-UAMRA-2015-10). At the end of the study, mice were sacrificed in a CO2 chamber.
Intra-articular CFA injection
A version of a previously validated model of arthritic inflammation of the knee joint was produced by administering four intra-articular injections of 10 μL of CFA (catalog no. F5881; Sigma-Aldrich Co.) at days 0, 7, 14 and 21 unilaterally into the right knee joint. Mice were anesthetized using a mixture of ketamine/xylazine (100/5 mg/kg) administered intraperitoneally (i.p.).25–28
Evaluation of pain-related behaviors
Spontaneous behavioral measures of arthritic joint pain, including number of flinches and time spent guarding the affected extremity, were quantified during a 5 min time period as described previously.25,27,28 Briefly, mice were placed in open Plexiglas observation chambers (cylinders: height of 30 cm and inside diameter of 20 cm) for 20 min to allow them to acclimatize to their surroundings. Mirrors were placed behind the chambers to enable unhindered observation. Flinches were defined as the number of times the animal raised its hind paw, which was used as a measure of spontaneous arthritic pain. The spontaneous guarding of the ipsilateral hind limb was defined as the amount of time the animals held the hind paw aloft while stationary. Although spontaneous flinching or guarding is not present in patients with RA, this behavior may reflect the spontaneous pain that arthritic patients experience without movement (pain at rest) of the affected extremity.29–31
Once spontaneous behaviors had been simultaneously evaluated, horizontal exploratory activity (locomotor activity) was quantified using a modified open-field paradigm. Mice were placed in the center of an acrylic rectangular chamber (18×27×15 cm). The chamber was divided into a grid of equally sized rectangles (nine in total, measuring 9×6 cm each) using lines drawn on the chamber floor for visual quantification. The total number of each line crossed in a 2 min time period was quantified by the experimenter.27,32,33
In all cases, behavioral analysis was performed between 09:00 and 13:00 h by an observer blinded to the experimental conditions of the animals.
Evaluation of knee edema
The diameter of the knee joint just below the level of the patella was measured in the anesthetized mice (isoflurane in O2: 4% for induction and 2% for maintenance) using a digital vernier caliper. In all instances, knee edema was measured 1 day after the intra-articular CFA injection and was determined after behavioral evaluation in order to minimize alterations in behavioral responses due to handling. The measurement was repeated three times and the average value was used. The results are expressed as percentage of inflammation compared to that in the contralateral knee joint.27
Micro-CT analysis
Micro-CT images of tibiofemoral knee joints were analyzed using a desktop micro-CT system (Skyscan 1272; Bruker, Brussels, Belgium). At the end of the experiment, mice with CFA-induced knee arthritis were repeatedly injected with saline or mazindol were euthanized in a CO2 chamber and the hind limbs were removed and stored at a temperature of –20°C in 0.1 M PBS (pH 7.4) until their analysis. The scanning process was conducted at a voxel size of 10 µm, an X-ray power of 60 kVp and 166 µA with an integration time of 627 ms, according to the guidelines for micro-CT analysis of rodent bone structure.34 All the scanner images were reconstructed using NRecon Software (Bruker). The region of interest was bordered laterally by the cortex in a 2 mm band in the metaphysis, starting 0.5 mm from the growth plate. A hydroxyapatite calibration phantom was used to calibrate bone density values (250 and 750 mg/cm3). Trabecular bone volume rate (BV/TV), trabecular thickness, trabecular number, trabecular separation, degree of anisotropy and trabecular bone mineral density were determined using CT analyzer software (Bruker). The acquisition settings for cortical analysis in the diaphyseal femur were the same as in metaphyseal region. In order to calculate the morphologic cortical parameters, a region of interest was chosen inside the diaphyseal femur, selecting a band of 1 mm, starting 4 mm and extending distally from the growth plate. Cortical bone analyses were performed in the CT Analyzer software, calculating the three-dimensional parameter cortical thickness, two-dimensional cross-sectional cortical area and cortical bone mineral density. To determinate whether mazindol by itself modified bone structural parameters, contralateral hind limbs were removed and analyzed by micro-CT.
Experimental design
For behavioral analysis, mice were randomly assigned to treatment groups of six to eight animals according to their weight and received intra-articular sterile saline or intra-articular CFA. In order to determine the efficacy of single administration of mazindol in CFA-induced arthritic nociception and impaired functional outcomes, a dose–response curve was constructed. For this, CFA-injected mice at day 26 post-initial injection of CFA were acutely administered (i.p.) with mazindol at doses of 1, 3 and 10 mg/kg 15 min before behavioral evaluation, because mazindol has a Tmax of 15 min in plasma after i.p. administration in mice.35 One group received saline (i.p.) at the same schedule of administration (vehicle group). From the dose–response curve, the dose of 3 mg/kg was determined as the effective dose that does not affect locomotor activity in the open-field test. Thus, it was used for further studies. Day 26 was selected as the time point for drug testing because we36,37 and others26 have reported that pain behaviors and knee edema reach a plateau at this time. At the end of the experiments, mice were euthanized with CO2 (day 26 after the first CFA injection).
In a separate experiment, CFA-injected mice were repeatedly injected (i.p.) with mazindol starting at day 15 until day 26 (i.p. 12 daily injections). Behavioral analysis was performed at 15, 19, 22 and 26 days following initial CFA injection. Once behavioral analysis was terminated at day 26, mice were euthanized with CO2 and knee joints were harvested and processed for micro-CT analysis as described above. Mazindol was repeatedly administered for 12 days due to its peak effect on locomotor activity that has been previously reported to occur after 10 repeated administrations.38
To determine whether the antinociceptive effect of mazindol was receptor specific, at day 26 post-CFA injection, mice with CFA-induced arthritis were randomly divided into five groups of six animals: CFA-injected mice + 0.025% CMC (i.p.; vehicle of mazindol) + distilled water (i.p.; vehicle for antagonists); CFA-injected mice + mazindol (i.p.; 3 mg/kg) + distilled water (i.p.); CFA-injected mice + mazindol (i.p.; 3 mg/kg) + haloperidol (i.p.; 0.2 mg/kg; antagonist D1); CFA-injected mice + mazindol (i.p.; 3 mg/kg) + R(+)-SCH 23390 (i.p.; 0.03 mg/kg; antagonist D2); CFA-injected mice + mazindol (i.p.; 3 mg/kg) + naloxone (i.p.; 3 mg/kg; opioid antagonist) and CFA-injected mice + haloperidol (i.p.; 0.2 mg/kg; antagonist D2). Mazindol was given daily from day 15 until day 26 after the first CFA injection. At day 26, 30 min before behavioral evaluation, all antagonists were administered; then, 15 min later, mazindol was administered. Finally, behavioral analysis was performed at 30 min post-antagonist administration to ensure that mice were tested within the therapeutic time window of the antagonist.39–41
Statistical analysis
All values are presented as the mean ± standard error of the mean. A two-way repeated-measures analysis of variance followed by Bonferroni’s post hoc test was used to compare the behavioral measures between the experimental groups at different time points. Student’s t-test followed by Mann–Whitney post hoc was used to compare exploratory activity and bone parameters. A one-way analysis of variance was used to compare spontaneous flinches and time spent guarding, followed by Dunnett posttest. Significance level was set at p<0.05. Statistical comparisons were performed using SigmaPlot 12.0 package.
Results
Effect of acute administration of mazindol on CFA-induced spontaneous pain-like behaviors
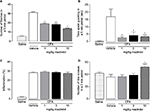
Mice that received intra-articular injections of saline showed minimal spontaneous nociceptive behavior or impairment of functional outcomes. In contrast, on day 26, CFA-injected mice exhibited pain-like behaviors, showing both higher number of flinches (Figure 1A) and time spent guarding (Figure 1B), as compared to saline-injected mice. The number of line crosses in the open-field test was not significantly different in CFA-injected mice as compared to saline-injected mice (Figure 1D).
At day 26 after the first CFA injection, mice with arthritis were administered (i.p.) vehicle (0.025% CMC) or mazindol at doses of 1, 3 and 10 mg/kg. Administration of mazindol reduced the number of spontaneous flinches in mice with CFA-induced arthritis in a dose-dependent manner (Figure 1A). Mazindol also resulted in a significant reduction of time spent guarding (Figure 1B), reaching a ceiling effect at the dose of 10 mg/kg. In contrast, a single administration of mazindol did not reduce CFA-induced knee edema (Figure 1C). Finally, the effect of mazindol on locomotor activity was evaluated to determine its toxicologic profile. A single administration of mazindol at the highest dose evaluated (10 mg/kg) significantly increased the number of line crosses in the open-field test (Figure 1D). Based on this, 3 mg/kg of mazindol was used in subsequent experiments.
Effect of repeated mazindol administration on spontaneous pain-like behaviors and weight of mice
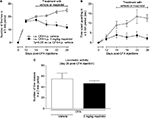
Repeated administration of mazindol (3 mg/kg; i.p.) from day 15 to day 26 after the first CFA injection significantly decreased the number of spontaneous flinches at day 19, 22 and 26 (Figure 2A) and the time spent guarding at day 15, 19, 22 and 26 (Figure 2B). Furthermore, repeated treatment of mazindol at a dose of 3 mg/kg did not significantly affect the locomotor activity in the open-field paradigm (Figure 2C). As previous studies have shown that repeated administration of mazindol decreases body weight at doses of 25 and 10 mg/kg,42,43 we evaluated the effect of mazindol on body weight. At day 15, vehicle-treated mice had a body weight of 33.88±1.18 g and CFA group mice had a body weight of 35.62±1.18 g. After repeated treatment with mazindol, vehicle-treated mice had a body weight of 34±1.21 g, which was not significantly different as compared to the CFA group’s weight of 35.03±1.30 g, indicating that mazindol at this dose and schedule of administration did not induce weight loss.
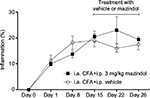
Effect of repeated administration of mazindol on CFA-induced knee edema
In this study, repeated injections of CFA resulted in knee edema, which significantly increased in magnitude as time progressed (data not shown). Repeated treatment with mazindol (3 mg/kg, i.p.; daily from day 15 until day 26) did not significantly modify the CFA-induced knee edema as compared to CFA-injected mice treated with vehicle (Figure 3).
Participation of dopaminergic and opioid receptors in the antinociceptive effect of mazindol
In order to know the mechanism involved in the antinociceptive effect of mazindol, a D1 antagonist (R(+)-SCH 23390 HCl), a D2 antagonist (haloperidol) or an opioid antagonist (naloxone) was injected i.p. 15 min before mazindol administration (3 mg/kg; i.p.). Antagonism of D2 receptors with haloperidol reversed the antinociceptive effect of mazindol (Figure 4A, B). In contrast, administration of D1 receptor or opioid antagonist failed to block the antinociceptive effect of mazindol (Figure 4A, B). Administration of haloperidol alone (without mazindol) did not affect the CFA-induced pain-like behaviors (Figure 4A, B).
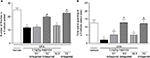
Effect of repeated administration of mazindol on BMD and trabecular microarchitecture
Repeated administration of mazindol (3 mg/kg; i.p.), from day 15 to day 26 after the first CFA injection, did not significantly affect trabecular or cortical bone parameters at the distal femoral metaphysis or proximal tibia metaphysis from the ipsilateral extremity (Table 1). In order to determine whether mazindol altered the bone metabolism in the noninjured joint (contralateral), cortical and trabecular bone parameters were examined in the left knee joint. Mazindol did not significantly modify the parameters of cortical and trabecular bone in the nonarthritic knee joint (Table 1).
Discussion
This study shows for the first time the effect of repeated administration of mazindol on pain-related behaviors, BMD and bone microarchitecture in a murine model of unilateral CFA-induced arthritis. While 12 daily administrations of mazindol decreased the CFA-induced spontaneous related-pain behaviors, it neither decreased the knee edema nor significantly modified the parameters of cortical and trabecular bone at the tibiofemoral joint. The antinociceptive effect of mazindol reported in our study is consistent with previous studies which have shown that mazindol reduced pain-related behaviors induced by intraplantar formalin (a mouse model of acute inflammatory pain).44,45 Moreover, the antinociceptive effect of mazindol was blocked by a D2-like receptor antagonist, but not by a D1-like receptor antagonist or an opioid receptor antagonist. In line with these findings, different studies have shown that spinal46,47 and supraspinal48,49 administration of D2-like receptor agonists, but not D1-like receptor agonists,49 resulted in antinociceptive effects in different experimental pain models.
While we do not yet know the exact mechanisms behind the antinociceptive effect of mazindol in this arthritic chronic pain model, we propose the following mechanisms. Mazindol is a non-phenylethylamine anorectic drug known to act primarily, although not exclusively, by inhibiting dopamine uptake in the CNS.50 Thus, increased levels of dopamine in the CNS after mazindol administration51 may preferentially activate D2-like receptors, the activation of which has been reported to 1) hyperpolarize the membrane potentials of spinal dorsal horn neurons by G-protein–mediated activation of K+ channels47 and 2) attenuate the discharge of the ON cell-like rostroventromedial medulla neurons (cells that have a pronociceptive role) resulting in analgesia.52
Previous studies have demonstrated that pretreatment with naloxone prevents the antinociceptive effect of mazindol after i.p. administration in formalin-induced nociception (an acute inflammatory pain model), suggesting that mazindol exerts an antinociceptive effect via activation of an endogenous opioidergic mechanism.44 In contrast, our experiments demonstrated that administration of naloxone (using a previously validated dose and schedule of administration)27,53 did not reverse the antinociceptive effect of mazindol on CFA-induced pain-like behaviors. The reason(s) for these differences are unclear; however, it is possible that mechanisms underlying mazindol antinociception in acute pain models may be different from those involved in chronic pain states and/or there may be a decrease in the levels of endogenous endorphins in chronic arthritic conditions. In support of this, a clinical study demonstrated that there is an inverse correlation between the serum levels of β-endorphins with rheumatoid disease activity score and the duration of RA.54
Another possibility by which mazindol decreases CFA-induced nociception in the arthritic joint is that it reduces the arthritis-induced bone resorption by causing apoptosis of osteoclasts and thus inhibiting acidosis in areas with major bone resorption. CFA injection into the hind paw increases the number of osteoclasts in areas with significant bone resorption in the rat metatarsal bones.55 Then, protons or acid originating from the osteoclasts may activate and/or sensitize the primary afferent neurons expressing acid-sensing ion channels such as TRPV1,56,57 resulting in pain sensation. In support of this, mechanical and thermal hyperalgesia are significantly reduced in TRPV1-knockout mice as compared to wild-type mice after intra-articular injection of CFA.58,59 Furthermore, dopamine and D2-like receptor agonists,23 but not D1-like receptor agonist,23 suppress human osteoclastogenesis, and chronic treatment with haloperidol (a D2-like receptor antagonist) induces a loss of trabecular bone of the femur in female rats.22 In contrast with these studies, we found that daily administration of 3 mg/kg mazindol for 12 days did not significantly prevent the CFA-induced bone loss at the ipsilateral tibiofemoral joint. Moreover, mazindol did not alter the parameters of cortical and trabecular bone in the mouse nonarthritic joint (contralateral joint). The reasons for these discrepancies are unknown; however, species-related (human and rat in previous studies) differences and time of exposure of mazindol (6 months in previous studies) could partially explain these differences.
Our results show that mazindol is more effective in guarding than flinching behaviors. Guarding (withdrawing and holding) and repetitive paw withdrawal (flinching) behaviors in the ipsilateral limb are commonly observed in the mono-arthritic animal. In fact, we do not know the exact neural mechanisms of guarding vs flinching. In our group, we consider, speculatively, that the sensitized joint and local tissues generate an exaggerated afferent barrage when the animal bears weight and guarding is an effort to avoid this peripherally driven event. In contrast, we interpret the flinching behavior as an episodic reflex response reflecting the presence of aberrant afferent activity arising from the inflamed joint innervated by the so-called “silent nociceptors”, where local inflammation initiates spontaneous afferent discharges and sensitization.60 Such an episodic activity would initiate a stimulus leading to abrupt and episodic withdrawal.61 Though a different model, such an explanation involving episodic afferent input is commonly used to explain the flinching observed after intraplantar formalin. Accordingly, we can only speculate that the modest differences observed between guarding and flinching reflect upon the intensity of the stimulus conditions generating the two responses: bursting in the silent nociceptors vs occasional gradual afferent traffic generated by progressive load bearing of the inflamed joint. We also observed that anti-NGF therapy is also more effective at reducing guarding behavior than flinching behavior in a murine model of fracture pain.62 This would provide one explanation for the difference that accounts for the increased effect of mazindol on guarding than flinching. An intriguing and nonexclusive possibility for these observations is that D2 activation may mediate an effect (nonspinal) on emotion/anxiety and the guarding response being an example of avoiding an anticipated pain state vs a behavior reflecting ongoing afferent traffic (as in flinching) might account for the important effect of D2 antagonism on the guarding.
This study has some limitations. First, mazindol is a psychostimulant that, like cocaine and amphetamine, increases locomotor activity.63–65 It is currently not marketed in the USA. In our study, acute administration of 10 mg/kg, but not 3 mg/kg, mazindol increased spontaneous locomotor activity in mice. Thus, the latter dose was used for repeated administration. While our results showed that 12 daily administrations of 3 mg/kg mazindol did not significantly increase the spontaneous locomotor activity, we cannot rule out the possibility that periods of exposure longer than 12 days may increase this parameter. Second, our results showed that 12 daily administrations of mazindol did not affect the bone metabolism as evaluated by micro-CT. However, it is unknown if longer time of administration of this drug may negatively alter bone health. Finally, mazindol is also a potent compound inhibitor of norepinephrine uptake63,64 (not evaluated in this study); thus, a noradrenergic component may also be involved in the antinociceptive effect of mazindol. Future studies need to be performed to explore the cellular and molecular targets of mazindol in painful arthritic conditions.
Conclusion
Our results show that mazindol has an antinociceptive role in mice with CFA-induced knee arthritis, through activation of D2-like receptors, and does not modify negatively the bone density and microarchitecture of femur and tibia. These results suggest that mazindol may represent a new therapeutic candidate for further testing in humans with arthritic pain.
Acknowledgments
The authors kindly thank Aleyda Arianne Loredo-Perez and Carlos Enrique Montalvo-Blancothe for the technical support. The authors wish to thank Sarah Woller who assisted in the proof-reading of the manuscript. This work was partially supported by CONACyT (CB-2014/240829 and INFR-2016/270549), Universidad Autónoma de Tamaulipas (PFI2016-34) and LERG CONACyT fellowship (No. 581277).
Author contributions
LERG, RIAG and JMJA designed the study, executed the experiments, analyzed the data as well as wrote the manuscript. AMM and VMVM performed micro-CT analysis. RIAG, RPS, and JGRG assisted in the statistical analysis and interpretation of data, as well as revised the manuscript. MAR and CFDVL revised the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
MAR and CFDVL work for Productos Medix, S.A de C.V., which is a pharmaceutical company that sells mazindol as an anorectic in Mexico. However, Productos Medix, S.A de C.V. were not involved in the recollection and analysis of data. The authors report no conflicts of interest in this work.
References
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.