Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Renoprotection by Inhibiting Connexin 43 Expression in a Mouse Model of Obesity-Related Renal Injury
Authors An X, Li G, Wang S, Xie T, Ren X, Zhao Y
Received 28 March 2023
Accepted for publication 2 May 2023
Published 17 May 2023 Volume 2023:16 Pages 1415—1424
DOI https://doi.org/10.2147/DMSO.S412546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Xiaomin An,1,2,* Guohua Li,1,* Shu Wang,1 Tianrun Xie,1 Xiaoxiao Ren,1 Yongli Zhao1
1Department of Pediatrics, The Second Hospital of Dalian Medical University, Dalian, 116027, People’s Republic of China; 2Department of Nephrology, Xi’an Children’s Hospital, Xi’an, 710003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongli Zhao, Department of Pediatrics, The Second Hospital of Dalian Medical University, Dalian, 116027, People’s Republic of China, Tel +86 0411 84671291, Email [email protected]
Introduction: Our previous study conducted in an obesity-related renal injury rat model have established a connection between increased connexin 43 (Cx43) expression and renal injury. In this study, we investigated whether inhibiting Cx43 expression could provide renoprotection in a mouse model of obesity-related renal injury.
Methods: Five-week-old C57BL/6J mice were fed with a high-fat diet for 12 weeks to establish an obesity-related renal injury model, then they were treated with Cx43 antisense oligodeoxynucleotide (AS) or scrambled oligodeoxynucleotide (SCR) by an implanted osmotic pump for 4 weeks. Finally, the glomerular filtration function, the histological change in the glomeruli, and the markers of podocyte injury (WT-1, Nephrin) and inflammatory infiltration of renal tissue (CD68, F4/80 and VCAM-1) were examined respectively.
Results: The results showed that inhibiting Cx43 expression by AS in this mouse model of obesity-related renal injury can effectively improve glomerular filtration function, alleviate glomerular expansion and podocyte injury, and attenuate the inflammatory infiltration of renal tissue.
Conclusion: Our results demonstrated that inhibiting Cx43 expression by AS could provide renoprotection for the mouse model of obesity-related renal injury.
Keywords: childhood obesity, obesity-related renal injury, connexin 43, antisense oligodeoxynucleotide
Introduction
Obesity has many impacts on children’s health, which could lead to various metabolic disorders and increase the risks of diabetes, cardiovascular diseases, renal injury, and chronic kidney diseases in their adulthood,1 and the potential pathogenic mechanisms include chronic metabolic inflammation, oxidative stress, insulin resistance and others. It has been reported that microalbuminuria is the early clinical manifestation of obesity-related renal injury in children.2 The main pathological manifestations of obesity-related renal injury include glomerular hypertrophy and mesangial expansion with or without segmental sclerosis.3 The main ultrastructural pathological changes in the early stage include reduced numbers of podocytes, foot process fusion, and effacement,4 which is the main reason for the microalbuminuria.5
Connexin 43 (Cx43) is a subtype of the gap junction proteins of connexins,6 which is widely distributed in renal tissue,7,8 and has a critical role in maintaining normal glomerular filtration.7,8 In pathological situations, the alteration of Cx43 expression is related to renal injury.9,10 Our previous work carried out in an obesity-related renal injury model revealed that the increased Cx43 expression was connected to renal injury, which included glomerular expansion, podocyte foot process effacement, and other molecular pathological changes.11 Until now, it is unclear whether inhibiting Cx43 expression could provide renoprotection in the obesity-related renal injury model.
In this study, the Cx43 antisense oligodeoxynucleotide (AS) was used to reduce Cx43 expression in a mouse model of obesity-related renal injury, and then we investigated whether inhibiting Cx43 expression could provide renoprotection in this mouse model.
Materials and Methods
Animals
All animal experiments were approved by the Ethical Committee of the Second Hospital of Dalian Medical University. All animal experiments were carried out in compliance with the NIH guidelines for the care and use of laboratory animals (8th edition, NIH, 2011). All methods were carried out under relevant guidelines and regulations.
Four-week-old male C57BL/6J mice (n=48) were purchased from the Laboratory Animal Center of Dalian Medical University. Animals were housed doubly with a 12-h light-dark cycle by artificial light and at a constant room temperature of 20–22°C. All animals had free access to food and water.
Animal Experimental Design
All mice were allowed to acclimate a new environment for 1 week, and then randomly divided into two groups: the control (Con) group and the obesity-related renal injury (ORI) group. The control group was fed a normal-fat diet (10% kcal from fat; D12450, Research Diet, Inc.), and the ORI group was fed a high-fat diet (60% kcal from fat; D12492, Research Diet, Inc.). All mice were fed with a normal or high-fat diet continuously for 12 weeks. During this time, the body weight of the mice was measured weekly. In the 14th week, the partial mice in the control and ORI groups were treated with Cx43 antisense oligodeoxynucleotide (AS) or scrambled oligodeoxynucleotide (SCR) (concentration of AS or SCR: 10μmol/L, Takara, China) by an implanted osmotic pump at the posterior center skin of the neck (catalogue number: 2004, Alzet, USA). The sequence numbers of nucleotide: Cx43 AS: 5’-GTAATTGCGGCAGGAGGAATTGTTTCTGTC-3’, SCR: 5’-GACAGAAACAATTCCTCCTGCCGCAATTTAC-3’. In the 18th week, All the mice were fasted overnight and anesthetized by inhaling sevoflurane (Shanghai Hengrui Pharmaceutical co., Ltd.). The blood was taken by heart puncture, and then serum was immediately separated through centrifugation (3000 rpm for 10 min), and kept under −80°C until analysis; then the mice were perfused via heart with cold PBS at a pressure of 120 mmHg. Both sides of the kidneys were harvested and recorded weight, and then immediately dissected along the longitudinal section of the kidneys. Kidneys were fixed in 4% paraformaldehyde solution for the analysis of light microscopy or rapidly frozen in liquid nitrogen and stored at −80°C for real-time PCR assays.
Urine Samples
In the 18th week, the 24h urine samples of mice were collected. After centrifugation (2000 rpm for 10 min), the supernatant was collected and stored at −80°C until further use. Urinary creatinine concentrations were measured using an automatic biochemical analyzer (Dade Behring Marburg GmbH, Germany). Microalbuminuria was measured using ELISA kits according to the manufacturer’s instructions.
Serum Samples
The concentrations of cholesterol, triglyceride, serum creatinine, and blood urea nitrogen were measured using an automatic biochemical analyzer (Dade Behring Marburg GmbH, Germany). Fasting blood glucose (FBG) was measured by Accu-Chek Active glucometer (Roche).
Histology and Immunohistochemistry
Four-micrometer-thick sections were cut from the paraffin-embedded kidney tissues on the microtome. The sections were deparaffinized in xylene and rehydrated using a graded series of ethanol. For histological evaluation, the sections were stained with Periodic Acid Schiff (PAS) according to the manufacturer’s instruction (G1281 and G1343, Beijing Solarbio Technology co., Ltd.). Analysis of the glomerular area was blindly performed using ImageJ software (National Institutes of Health, USA), and at least 10 glomeruli on each slide were examined to determination of glomerular area changes. Immunohistochemistry (IHC) staining was performed using established procedures as described previously.12 Briefly, endogenous peroxidase activity was blocked for 10 min in 3% hydrogen peroxide and then the slides were incubated in a blocking solution for 10 min at room temperature. After that, the slides were overnight incubated with the primary antibody for anti-WT 1 (sc-192, dilution 1:250), anti-Nephrin (sc-32530, dilution 1:100), anti-F4/80 (sc-26643-R, dilution 1:250) (Santa Cruz Biotechnology, USA), anti-CD68 (ab125212, dilution 1:100) or anti-Connexin43 (ab11370, dilution 1:1000) (Abcam, Cambridge, USA) at 4°C, respectively. The immunoreaction was detected using the biotinylated secondary antibody and the streptavidin/HRP complex. The complex was detected using diaminobenzidine /H2O2 and hematoxylin. The number of stained positive podocytes with WT-1 and the percentage of stained positive area with Nephrin in the glomeruli was blindly analyzed using ImageJ software, and at least 10 glomeruli on each slide were examined for determined of the above changes.
RNA Isolation and Real-Time PCR
The relative amounts of Cx43 mRNA and vascular cell adhesion molecule-1 (VCAM-1) in the kidneys were measured by real-time PCR on TaKaRa PCR Thermal Cycler Dice Real Time System Lite (TaKaRa Bio, Inc., Japan). Total RNA was extracted using a TaKaRa MiniBEST Universal RNA Extraction Kit (Code. 9767, TaKaRa Bio, Inc., Japan). The cDNA was synthesized using a primeScript RT reagent Kit with a gDNA Eraser Kit (TaKaRa Code. RR047) on TaKaRa PCR Thermal Cycler Dice (TaKaRa Bio, Inc., Japan). The PCR reaction mixture in a 25 μL volume contained 12.5 μL SYBR Premix Ex Taq II (Code. RR820, TaKaRa Bio, Inc., Japan), 2 μL RT product, 2 μL Primer F/R (each 10 μM) and 8.5 μL dH2O. The conditions of the PCR reaction: 95°C for 30s, then 95°C for 5s and 60°C for 30s, for 40 cycles. The GAPDH expression level was quantitated as an internal reference. The sequences of the primer used were as follows:
Cx43 Forward: 5’-AGGTCTGAGAGCCTGAACTCTCATT-3’
Reverse: 5’-GGCACTCCAGTCACCCATGT-3’
VCAM-1 Forward: 5’-TTCCGGCATTTATGTGTGTGAAG-3’
Reverse: 5’-GGCACATTTCCACAAGTGCAG-3’
GAPDH Forward: 5’-TGTGTCCGTCGTGGATCTGA-3’
Reverse: 5’-TTGCTGTTGAAGTCGCAGGAG-3’
The standard curve was constructed from series dilutions of template cDNA. The relative expression of mRNAs were calculated after normalizing with GAPDH.
Statistical Analysis
Statistical analysis was performed using the SPSS Software (Version 23.0, SPSS, Inc., Chicago IL, USA). All data are expressed as mean ± SEM. Comparison between the control group and the ORI groups before the intervention of nucleotide was made using independent sample t-test. All of the other data were analyzed by one-way ANOVA with LSD-t multiple comparison test. Differences were considered significant if the P value < 0.05.
Results
Cx43 as Reduced Cx43 Expression in the Kidney of the ORI and Control Mice
After four weeks of Cx43 AS or SCR treatment, there were no any adverse events occurred in all mice. The Cx43 expression in the mouse kidney was detected by IHC staining and real-time PCR. The expression of Cx43 in the renal tissue of the ORI group was significantly increased compared with the control group (Figure 1A and C). After a four-week inhibiting Cx43 by AS, the expression of Cx43 in both groups was decreased (Figure 1B and D). Also, the mRNA of Cx43 was decreased by 31% in the ORI group, and 29% in the control group, respectively (Figure 1E).
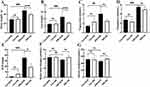
The Effect of Cx43 as on the Body Weight and Metabolic Disorders of ORI Mice
The body weight of five-week-old mice was no significant differences between the control and ORI mice. After being fed a high-fat diet for 12 weeks, the body weight of ORI mice was significantly increased compared with control mice (Figure 2A and B). There was no effect on the changing trend of the body weight of both groups after four-week SCR treatment (ORI-SCR vs Con-SCR, P<0.001) (Figure 3A). However, after four-week inhibiting Cx43 by AS, the body weight and liver weight of ORI mice were significantly decreased compared with SCR treatment (ORI-AS vs ORI-SCR, P<0.001) (Figure 3A and B). Unfortunately, the metabolism-related indicators of ORI mice, such as serum concentration of cholesterol and triglyceride were not improved after four-week AS treatment compared with SCR treatment (Figure 3C and D).
The Effect of Cx43 as on the Renal Function of ORI Mice
The ratio of microalbuminuria to urinary creatinine (ACR), blood urea nitrogen, and serum creatinine were used to assess the glomerular filtration function of mice. The level of serum creatinine and urea nitrogen in both groups did not have significant differences (ORI-SCR vs Con-SCR, P>0.05) (Figure 3F and G). However, the ACR of the ORI mice was significantly increased compared with control mice (ORI-SCR vs Con-SCR, P<0.05). After four-week inhibiting Cx43 by AS, the ACR of ORI mice was significantly decreased compared with SCR treatment (ORI-AS vs ORI-SCR, P<0.05) (Figure 3E).
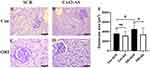
The Effect of Cx43 as on the Cross-Section Area of the Glomeruli of ORI Mice
The cross-section area of the glomeruli was used to assess glomerular hypertrophy in mice. Compared with the control group, The mean cross-section area of the glomeruli of ORI mice was significantly increased (ORI-SCR vs Con-SCR, P<0.05) (Figure 4A, C and E). After four-week inhibiting Cx43 by AS, the mean cross-section area of the glomerulus of ORI mice was significantly decreased compared with SCR treatment (ORI-AS vs ORI-SCR, P<0.05) (Figure 4B, D and E).
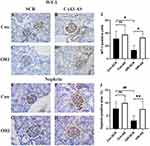
The Effect of Cx43 as on Podocyte Injury in ORI Mice
Nephrin and WT-1 were used as markers of podocyte injury. Immunohistochemistry analysis showed that the expression of nephrin was significantly decreased in the glomeruli of ORI mice than that of control mice (ORI-SCR vs Con-SCR, P<0.05) (Figure 5E, G, and J), after four-week inhibiting Cx43 by AS, the expression of nephrin in the glomeruli of ORI mice was significantly improved compared with SCR treatment (ORI-AS vs ORI-SCR, P<0.05) (Figure 5F, H and J). WT-1 was used to track the quantity change of podocytes in the glomeruli. The WT-1-positive cells were significantly reduced in the glomerulus of ORI mice than that of control mice (ORI-SCR vs Con-SCR, P<0.05) (Figure 5A, C and I), after four-week inhibiting Cx43 by AS, the WT-1-positive cells in the glomeruli of ORI mice were significantly increased compared SCR treatment (ORI-AS vs ORI-SCR, P<0.05) (Figure 5B, D and I).
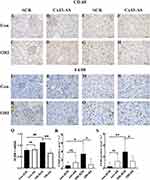
The Effect of Cx43 as on the Inflammatory Infiltration in the Glomeruli and Mesenchyme of ORI Mice
The CD68, F4/80 and VCAM-1 were used as the marker of chronic inflammation to determine the inflammatory infiltration in the glomeruli and mesenchyme of mice. Immunohistochemistry analysis showed that the expression of CD68 and F4/80 were significantly increased in the glomeruli and mesenchyme of ORI mice than that of control mice (ORI-SCR vs Con-SCR, P<0.05) (Figure 6A, C, I, K, E, G, M, O, R and S), after inhibiting Cx43 by AS, their expression in the glomerulus of ORI mice was significantly decreased compared with SCR treatment (ORI-AS vs ORI-SCR, P<0.05) (Figure 6B, D, J, L, F, H, N, P, R and S). The result of real-time PCR showed that VCAM-1 mRNA was significantly increased in the renal tissue of ORI mice than that of control mice (ORI-SCR vs Con-SCR) (Figure 6Q), after four-week inhibiting Cx43 by AS, its mRNA in the renal tissue of ORI mice was significantly decreased compared with SCR treatment (ORI-AS vs ORI-SCR) (Figure 6Q).
Discussion
In this study, after four-week targeting Cx43 with the antisense oligodeoxynucleotide (AS) by an implanted osmotic pump, we found that the body weight and liver weight of ORI mice were significantly improved, and the ACR, glomerular expansion and podocyte injury of ORI mice were also improved. Furthermore, targeting Cx43 with AS can alleviate the inflammatory infiltration and fatty infiltration in the renal tissue of ORI mice. These results demonstrate that decreased Cx43 expression could provide renal protection for the mouse model of obesity-related renal injury.
As many clinical investigations have found that microalbuminuria is the early marker of renal injury of childhood ORI. At this stage, the renal function of most children patients is normal.11 Some studies conducted renal biopsies in the patients with obesity revealed that the main pathological features of the kidney include glomerular hypertrophy and mesangial expansion with or without segmental sclerosis.4,13 Furthermore, several rodent studies also observed similar pathological changes.14–16 In this study, our findings were alone with these results observed in patients and mice. Therefore, our mice model is an appropriate animal model for the relevant studies of childhood obesity -related renal injury.
WT-1 and nephrin are often used as the markers of podocyte injury in many kidney diseases.17,18 In our study, the WT-1 was used to estimate the change in the numbers of the podocytes in the glomerulus, and the nephrin expression was used to assess the injury degree of podocytes. In this study, we found the decreased expression of nephrin and a decline in the number of WT-1-positive cells in the glomerulus of ORI mice, which were the markers of podocyte injury. Similar results were also found in our previous study.11 Our results also revealed that inflammatory infiltration was alone with podocyte injury, which was the possible reason for podocyte injury. Many other studies conducted in various obesity models have found similar results, which revealed that there was a positive relationship between inflammatory infiltration and podocyte injury. Furthermore, the study by Saisudha Koka et al revealed that activation of NLRP3 inflammasomes was an early mechanism of podocyte injury in obese mice.19
The typical ultrastructural pathological change of childhood ORI is podocyte injury, which include a reduced number of podocytes, foot process fusion, and effacement.4 Cx43, which is one type of gap junctional protein, may be involved in the regulation of podocyte injury of ORI. Many studies have found that the downregulation of Cx43 expression has a protective effect on podocytes. Abed et al found downregulation of Cx43 expression significantly improved the filtration function of microalbuminuria and glomeruli hypertrophy in a mice model of hypertension-induced nephropathy.20 Additionally, Kavvadas et al found downregulation of Cx43 expression not only improved albuminuria and glomerular expansion in the mice model of nephrotoxic serum-inducted glomerulonephritis (NTS-GN), but also alleviated podocyte injury (the loss of WT-1 and Nephrin upregulated).21 As well, an in vitro study of aldosterone-induced podocyte damage, Yang et al found downregulation of Cx43 expression improved the loss of WT-1 and alleviated the injury of podocytes.22 In this study, our results further confirmed that inhibiting Cx43 could also alleviate podocyte injury of ORI and provide certain renal protection for this kidney disease model.
In this study, we found that Cx43 expression in the glomeruli and mesenchyme was significantly increased along with podocyte injury and inflammatory infiltration. Furthermore, inhibiting Cx43 by AS not only alleviated podocyte injury, but also reduced inflammatory infiltration. Similar results were also found in many previous studies, which showed that specific downregulation of Cx43 expression by congenital (using Cx43 ± heterozygous mice) or acquired methods (using Cx43 antisense oligonucleotides or Cx43 specific blocker: GAP26 mimetic peptide) can improve podocyte injury and inhibit inflammatory infiltration.20,21 Recently, many studies have indicated that inflammation was one of the important causes of podocyte injury in ORI.21 Whereas Cx43 was involved in the inflammatory response, in which the NLRP3 inflammasome was an important regulatory factor.23 Further study by Koka et al found that activation of NLRP3 inflammasomes was a mechanism of podocyte injury in the early stage of ORI.19 Therefore, we speculated that inhibiting C43 by AS could reduce inflammatory infiltration by the inactivation of NLRP3 inflammasomes, which then alleviates podocyte injury associated with obesity.
Conclusion
Our results demonstrated that inhibiting Cx43 can significantly alleviate podocyte injury of ORI, which was along with the improvement of glomerular expansion, and glomerular filtration function. Moreover, our results also revealed that inhibiting Cx43 expression can reduce inflammatory infiltration of the kidney in this mouse model. Collectively, these results suggest that inhibiting Cx43 expression could provide renoprotection for this mouse model of obesity-related renal injury via decreased renal chronic inflammatory infiltration.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81402694) and the Natural Science Foundation of Liaoning Province (20180530104).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gunta SS, Mak RH. Is obesity a risk factor for chronic kidney disease in children? Pediatr Nephrol. 2013;28(10):1949–1956. doi:10.1007/s00467-012-2353-z
2. Savino A, Pelliccia P, Giannini C, et al. Implications for kidney disease in obese children and adolescents. Pediatr Nephrol. 2011;26(5):749–758. doi:10.1007/s00467-010-1659-y
3. Fowler SM, Kon V, Ma L, Richards WO, Fogo AB, Hunley TE. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009;24(4):851–855. doi:10.1007/s00467-008-1024-6
4. Chen HM, Liu ZH, Zeng CH, Li SJ, Wang QW, Li LS. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis. 2006;48(5):772–779. doi:10.1053/j.ajkd.2006.07.025
5. Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5(8):463–468. doi:10.1038/nrneph.2009.108
6. Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012;524(1):2–15. doi:10.1016/j.abb.2012.03.008
7. Abed AB, Kavvadas P, Chadjichristos CE. Functional roles of connexins and pannexins in the kidney. Cell Mol Life Sci. 2015;72(15):2869–2877. doi:10.1007/s00018-015-1964-5
8. Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1143–1155. doi:10.1152/ajpregu.00808.2009
9. Hillis GS, Duthie LA, Brown PA, Simpson JG, MacLeod AM, Haites NE. Upregulation and co-localization of connexin43 and cellular adhesion molecules in inflammatory renal disease. J Pathol. 1997;182(4):373–379. doi:10.1002/(SICI)1096-9896(199708)182:4<373::AID-PATH858>3.0.CO;2-B
10. Toubas J, Beck S, Pageaud AL, et al. Alteration of connexin expression is an early signal for chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(1):F24–32.
11. Zhao Y, Li G, Wang Y, Liu Z. Alteration of Connexin43 expression in a rat model of obesity-related glomerulopathy. Exp Mol Pathol. 2018;104(1):12–18. doi:10.1016/j.yexmp.2017.11.017
12. Huang Y, Mao Z, Zhang Z, et al. Connexin43 contributes to inflammasome activation and lipopolysaccharide-initiated acute renal injury via modulation of intracellular oxidative status. Antioxid Redox Signal. 2019;31(16):1194–1212. doi:10.1089/ars.2018.7636
13. D’Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–471. doi:10.1038/nrneph.2016.75
14. Luo M, Luo P, Zhang Z, et al. Zinc delays the progression of obesity-related glomerulopathy in mice via down-regulating P38 MAPK-mediated inflammation. Obesity. 2016;24(6):1244–1256. doi:10.1002/oby.21463
15. Sun YB, Qu X, Howard V, et al. Smad3 deficiency protects mice from obesity-induced podocyte injury that precedes insulin resistance. Kidney Int. 2015;88(2):286–298. doi:10.1038/ki.2015.121
16. Yan Z, Ni Y, Wang P, et al. Peroxisome proliferator-activated receptor delta protects against obesity-related glomerulopathy through the P38 MAPK pathway. Obesity. 2013;21(3):538–545. doi:10.1002/oby.20103
17. Marshall CB, Krofft RD, Pippin JW, Shankland SJ. CDK inhibitor p21 is prosurvival in Adriamycin-induced podocyte injury, in vitro and in vivo. Am J Physiol Renal Physiol. 2010;298(5):F1140–1151. doi:10.1152/ajprenal.00216.2009
18. Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69(12):2131–2147. doi:10.1038/sj.ki.5000410
19. Koka S, Xia M, Zhang C, Zhang Y, Li PL, Boini KM. Podocyte NLRP3 inflammasome activation and formation by adipokine visfatin. Cell Physiol Biochem. 2019;53(2):355–365.
20. Abed A, Toubas J, Kavvadas P, et al. Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice. Kidney Int. 2014;86(4):768–779. doi:10.1038/ki.2014.108
21. Kavvadas P, Abed A, Poulain C, et al. Decreased expression of connexin 43 blunts the progression of experimental GN. J Am Soc Nephrol. 2017;28(10):2915–2930. doi:10.1681/ASN.2016111211
22. Yang M, Wang B, Li M, Jiang B. Connexin 43 is involved in aldosterone-induced podocyte injury. Cell Physiol Biochem. 2014;34(5):1652–1662. doi:10.1159/000366367
23. Wang X, Chen H, Zhang M, Liu Z. Roles of mast cells and monocyte chemoattractant protein-1 in the renal injury of obesity-related glomerulopathy. Am J Med Sci. 2013;346(4):295–301. doi:10.1097/MAJ.0b013e31827559f8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.