Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Renal safety of tenofovir and/or entecavir in patients with chronic HBV monoinfection
Received 5 June 2017
Accepted for publication 13 September 2017
Published 26 September 2017 Volume 2017:13 Pages 1273—1285
DOI https://doi.org/10.2147/TCRM.S143286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Young-Mo Yang, Eun Joo Choi
Department of Pharmacy, College of Pharmacy, Chosun University, Gwangju, South Korea
Background: Tenofovir disoproxil fumarate (TDF) and entecavir (ETV) are recommended as the first-line therapy for chronic hepatitis B (CHB) due to their genetic barrier to resistance and effectiveness of virological suppression. TDF and ETV may cause renal toxicity through various mechanisms such as renal tubular injury, apoptosis, and mitochondrial toxicity. The aims of the current review were to assess the potential renal toxicity associated with the use of TDF and ETV in patients infected with chronic hepatitis B virus (HBV) and to provide clinical perspectives on these two agents in the treatment of CHB.
Methods: A literature search of clinical studies published in PubMed and posted on ClinicalTrials.gov website was implemented to find studies evaluating the potential renal toxicity of TDF and ETV.
Results: Twenty-one studies were examined in this review. The TDF dose used in the studies was 245 or 300 mg/day and that of ETV was 0.5 or 1 mg/day. Based on the markers of renal function, patients treated with TDF were not more likely to show changes in renal function than those treated with ETV; however, the estimated glomerular filtration rates (eGFRs) of patients receiving TDF tended to be more clearly reduced than those of patients receiving ETV. The eGFRs of patients treated with TDF decreased in a time-dependent manner, whereas those of patients treated with ETV increased or decreased across various time points.
Conclusion: The data shown in this study suggest that use of TDF and ETV could be at least associated with reductions in renal function in patients with chronic HBV infection. However, various risk factors, such as pre-existing renal failure and comorbidities, are also associated with decreased renal function during the treatment of TDF and ETV. Thus, studies of management strategies for HBV-infected patients with these risk factors are necessary in the near future.
Keywords: hepatitis B, tenofovir, entecavir, renal safety
Introduction
Hepatitis B virus (HBV) infection is considered as one of the most important global public health concerns; this potentially life-threatening infection damages the liver and can contribute to acute and chronic diseases. An estimated 240 million individuals are chronically infected with HBV worldwide, and over 686,000 individuals die annually because of end-stage chronic hepatitis B (CHB) and CHB-associated complications such as decompensated cirrhosis and hepatocellular carcinoma.1
Currently, two therapeutic options (ie, interferons [IFNs] and oral nucleos(t)ide analogs [NUCs]) are used to treat CHB; however, oral NUCs have been preferred for the treatment of CHB owing to their convenient regimen.2 In particular, the second-generation NUCs, such as entecavir (ETV) and tenofovir disoproxil fumarate (TDF), are recommended as the first-line therapy for CHB because of their high genetic barrier to resistance and effectiveness of virological suppression.3,4 The efficacy and safety of both these drugs were demonstrated through previous clinical trials.3 Safety should be particularly considered, since long-term treatment for CHB is usually required with ETV or TDF, although its ideal duration of treatment is not well determined.5
ETV and TDF may cause renal toxicity via various mechanisms such as renal tubular injury, apoptosis, and mitochondrial toxicity.5,6 Previous studies also reported an association between CHB and chronic kidney disease (CKD).7–11 Specifically, it was reported that glomerular diseases, such as membranous nephropathy and mesangiocapillary glomerulonephritis, might be the underlying causes of renal dysfunction in patients with CHB.12,13 Moreover, drug history except for NUCs, disease status of diabetes and/or hypertension (HTN), and baseline (BL) kidney function before starting NUCs may affect the potential nephrotoxicity caused by ETV and/or TDF. Consequently, renal safety is an important factor in choosing appropriate NUCs for the treatment of CHB because they are renally eliminated in an unchanged form, and this is particularly important in patients who have already had renal impairment or are at risk for it.7,14
The current review aimed to assess the potential renal toxicity associated with the use of ETV and TDF in patients infected with chronic HBV and to provide clinical perspectives on these two agents in the treatment of CHB.
Methods
A literature search was conducted to identify clinical studies in patients with HBV monoinfection, which assessed the safety of ETV and/or TDF. PubMed was searched from the inception of the database to March 2017, using “hepatitis B,” “entecavir,” and “tenofovir” as the search terms to find clinical trials written only in English. The reference lists of the selected articles and related reviews were utilized to find additional relevant articles. The data posted on ClinicalTrials.gov website were also used to identify the unpublished clinical outcomes. Two reviewers independently scanned the article titles and abstracts and identified relevant studies that met the following criteria: 1) retrospective or prospective clinical studies, 2) studies involving patients only with HBV infection, 3) studies in which ETV and/or TDF had to be administered for the treatment of HBV infection, and 4) studies whose results contained renal parameters, such as estimated glomerular filtration rate (eGFR), serum creatinine, and serum phosphorus, in order to evaluate the changes in renal function.
Results
Study characteristics
The literature search (Figure 1) identified 21 eligible studies that met the predetermined inclusion criteria. The main characteristics of the selected studies are presented in Table 1. The final eligible studies included in this review were conducted in the United States, Europe, Thailand, Taiwan, Korea, and China.3,15–34 In particular, 13 studies were conducted on Asian individuals.19–23,25–31,34 Most of the studies (61.9%), excluding five randomized clinical studies19,27,29,32,34 and three studies that did not accurately report study designs,24,25,28 were observational studies. Most studies were published in the last 2 years, although the articles dated back to 2011. Overall, 95.2% (20/21) of the studies were conducted on patients with mixed hepatitis B e antigen (HBeAg) status whereas only one study33 did not report HBeAg status. The TDF dose used in the studies was 245 or 300 mg/day and that of ETV was 0.5 or 1 mg/day.
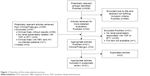  | Figure 1 Flowchart of the study selection process. |
Evaluation of renal safety of TDF and ETV
Information bias that may result from broad heterogeneity in the methodology among different studies was the major issue hindering meta-analyses. As presented in Table 2, various parameters were utilized in order to measure renal functions after administering TDF and ETV. The most common parameter used was eGFR calculated using modification of diet in renal disease (MDRD) and Cockcroft–Gault (CG) formulae.
An observational study comparing long-term renal functions reported eGFR in 424 patients with TDF-containing regimens and 187 patients with ETV according to the MDRD method.15 In the patients with TDF-containing regimens, the mean eGFR decreased from 90.8 mL/min at BL to 85.1 mL/min at 60 months. However, in the patients with ETV, the mean eGFR increased from 81.2 mL/min at BL to 90.7 mL/min at 60 months. A retrospective–prospective cohort study conducted in Taiwan determined a change in eGFR in 37 patients with TDF and 62 patients with ETV based on the MDRD method.20 In the patients with TDF, the mean eGFR changed from 78.3 mL/min/1.73 m2 at BL to 73.0 mL/min/1.73 m2 at 24 months, whereas in the patients with ETV, the mean eGFR increased from 75.6 mL/min/1.73 m2 at BL to 79.3 mL/min/1.73 m2 at 24 months. A similar change in eGFR calculated using the MDRD method was also observed in a retrospective cohort study conducted in Taiwan.23 The mean eGFR in 170 patients with TDF changed from 92 mL/min/1.73 m2 at BL to 86.3 mL/min/1.73 m2 at 24 months; however, in 233 patients with ETV, the mean eGFR changed from 86.1 mL/min/1.73 m2 at BL to 94.4 mL/min/1.73 m2 at 24 months.
A prospective cohort study conducted in Turkey reported a change in eGFR in 44 patients with TDF and 32 patients with ETV according to the Chronic Kidney Disease Epidemiology Collaboration and cystatin C (CKD-EPI-CysC) method.16 The mean eGFR in the patients with TDF decreased from 84.7 mL/min/1.73 m2 at BL to 76.9 mL/min/1.73 m2 at 24 months (p=0.004) and that in the patients with ETV decreased from 90.0 mL/min/1.73 m2 at BL to 84.5 mL/min/1.73 m2 at 24 months (p=0.46). However, the mean values of eGFR in both groups were different when the Chronic Kidney Disease Epidemiology Collaboration and creatinine plus cystatin C (CKD-EPI-Cr-CysC) method was used. The mean eGFR in the patients with TDF changed from 90.6 mL/min/1.73 m2 at BL to 73.6 mL/min/1.73 m2 at 24 months (p=0.05), and the mean eGFR in the patients with ETV changed from 93.5 mL/min/1.73 m2 at BL to 82.3 mL/min/1.73 m2 at 24 months (p=0.17). A study conducted by Koklu et al24 in Turkey reported eGFR calculated using the MDRD method. The mean eGFR in 273 patients with TDF changed from 100.72 mL/min/1.73 m2 at BL to 96.72 mL/min/1.73 m2 at 24 months (p=0.001), whereas the mean eGFR in 282 patients with ETV changed from 96.20 mL/min/1.73 m2 at BL to 95.94 mL/min/1.73 m2 at 24 months (p=0.535). In a study conducted by Hung et al28 in Taiwan, TDF and ETV showed decreased mean eGFR calculated using the MDRD method. The mean eGFR in 41 patients with TDF changed from 108 to 87 mL/min/1.73 m2 at 6 months (p=0.001), and the mean eGFR in 148 patients with ETV changed from 92 to 84 mL/min/1.73 m2 at 6 months (p=0.001).
Discussion
Close attention should be paid to the safety and efficacy of TDV and ETV for the long-term treatment of chronic HBV infection, because they are currently the most potent antiviral agents for treating HBV infection.3,5 TDF and ETV are likely to cause renal toxicity through various mechanisms including renal tubular injury, apoptosis, and mitochondrial toxicity.5,6 The present study reviewed the literature and provided a comprehensive summary of the renal safety of TDF and ETV for the treatment of patients with chronic HBV infection. The results based on the studies reviewed in this article indicated that TDF and ETV could be responsible at least for reduced kidney function in patients with chronic HBV infection.
In this study, the effects of TDF and ETV on renal function were assessed. Based on the markers of renal function, compared to patients treated with ETV, those treated with TDF were not more likely to show changes in renal function, although the eGFR of patients treated with TDF tended to be more clearly reduced than that of patients receiving ETV. The eGFRs of patients treated with TDF decreased in a time-dependent manner, whereas those of patients treated with ETV increased or decreased across various time points.15,16,20,23,24,28 Similar percentage of patients in both the treatment groups showed ≥20% decrease in eGFR during the treatment (based on CG, TDF 35.0% vs ETV 36.3%; based on MDRD, TDF 41.3% vs ETV 43.8%).33 A similar tendency was also observed in a recent clinical trial conducted in Thailand.19 After 36 months, 16.8% and 14.9% of patients receiving TDF and ETV, respectively, experienced ≥20% decrease in eGFR; however, the decrease was observed in more patients receiving TDF than in those receiving ETV at 12 and 24 months.19 Around 30% of patients in both TDF and ETV groups experienced a ≥0.2 mg/dL increase in creatinine from BL; however, creatinine increase of ≥0.5 mg/dL from BL occurred in more patients receiving ETV than in those receiving TDF (13.8% vs 3.8%; p=0.025).33 The frequencies of creatinine elevation by ≥0.3 mg/dL were similar in both groups (TDF/ETV 2.0% vs ETV 3.3%); however, creatinine elevation by ≥0.5 mg/dL was more frequent in patients treated with ETV alone (TDF/ETV 0.0% vs ETV 1.6%).32 These heterogeneous results may be partially attributed to different characteristics, such as comorbidities and co-administered drugs, of the study subjects.
According to multivariate analyses, various risk factors, such as advanced age, preexisting renal failure, comorbidities, history of transplant, concomitant nephrotoxic drugs, advanced HIV coinfection, and male gender, were associated with eGFR reductions by TDF or ETV.23,26,33,35,36 Especially, preexisting renal insufficiency was a major independent risk factor for deterioration of renal function during the treatment of chronic HBV infection.23,26,33 Moreover, previous studies have reported an association of CHB with CKD, and ~15%–30% of patients with CHB showed BL renal insufficiency or comorbidities that were likely to cause CKD, such as diabetes mellitus (DM) and HTN.7–11 However, TDF therapy was not significantly associated with changes in renal function when compared with ETV therapy.26,33 Large proportions of TDF and ETV are also renally excreted in their unchanged forms.37,38 Thus, NUCs other than TDF and ETV may be considered to prevent the progression of renal decline in patients with CHB and decreased renal functions.
Compared with TDF, tenofovir alafenamide (TAF), a novel prodrug of tenofovir, led to approximately four times higher intracellular concentrations of tenofovir diphosphate, an active metabolite, which may result in much lower doses of TAF than those of TDF.39,40 Consequently, ~90% lower systemic exposure of tenofovir was expected in patients treated with TAF than in those treated with TDF.40 This is likely to reduce the risk for tenofovir-associated renal toxicity. According to a clinical trial conducted in patients infected with HIV-1, decreases or slight increases from BL to Week 48 in total urinary protein, albumin, retinol-binding protein, and β2-microglobulin to urine creatinine ratios were observed in the TAF group; however, increases from BL to Week 48 in the protein to urine creatinine ratios were reported in the TDF group.40 Two recent randomized clinical trials conducted in patients with HBeAg-negative or -positive chronic HBV infection reported that TAF not only was non-inferior to TDF but also improved the negative effect of tenofovir on renal function.41,42 In patients with HBeAg-negative chronic HBV infection, a small mean increase in creatinine from BL to Week 48 was reported in both TAF and TDF groups, and at Week 48, a median decrease in eGFR by CG was lower in patients treated with TAF than in those treated with TDF.41 Significantly smaller increases from BL to Week 48 in the markers of proximal tubular dysfunction, retinol-binding protein, and β2-microglobulin to urine creatinine ratios were noted in the TAF group than in the TDF group.41 Similar tendencies were observed in patients with HBeAg-positive chronic HBV infection.42 A network meta-analysis conducted by Chan et al7 reported that telbivudine (LdT) consistently improved renal functions measured by eGFR independent of measuring methods. In particular, tenofovir monotherapy caused decreases in eGFR, but combinational therapy of tenofovir with LdT improved renal functions.7
According to the WHO guidelines for the treatment of CHB in 2015, measuring BL renal function and assessing BL risks for renal dysfunction are recommended before commencing antiviral therapy.43 In cases where BL patients have eGFR <50 mL/min or risk factors for renal insufficiency, such as long-term DM, uncontrolled HTN, and severe bone-related diseases, tenofovir should be avoided, its dose should be adjusted, or ETV should be used.43 Thus, as shown in previous randomized clinical studies,40,41 TAF could be considered as the first drug of choice for the treatment of CHB in patients with reduced renal function or in those with risk factors for renal dysfunction. In addition, LdT monotherapy or combinational therapy with TAF could be another option for these patients; however, well-organized, randomized clinical trials are necessary to prove renal safety when TAF + LdT or LdT alone is administered to these patients.
This study had some limitations that should be addressed. Two electronic databases (ie, PubMed and ClinicalTrials.gov website) were utilized to search relevant clinical trials, although various databases are available. This limited database utilization also likely limited our opportunities to search additional valuable and relevant clinical trials. Almost all of the selected clinical trials mentioned that TDF and ETV were not likely to have significantly negative effects on renal functions. However, consistent results were not shown partially owing to the different characteristics of study subjects and various markers used to measure renal functions, which made the conducting of further meta-analysis difficult.
Conclusion
The data reported in this study suggest that use of TDF and ETV could be associated with reductions in kidney function in patients with chronic HBV infection. The eGFRs of patients treated with TDF were reduced in a time-dependent manner, whereas the eGFRs of patients treated with ETV increased or decreased across various time points. TAF as the first drug of choice for the treatment of chronic HBV infection could be used in patients with decreased renal function or in those with risk factors for renal dysfunction, and TAF + LdT or LdT alone could also be considered for these patients. However, well-organized, prospective, large-scale, randomized clinical trials are necessary to determine the renal safety of TAF + LdT or LdT alone for the treatment of such patients. In addition, studies on management strategies for HBV-infected patients with various risk factors (eg, advanced age, pre-existing renal failure, comorbidities, history of transplant, and concomitant nephrotoxic drugs) associated with reduction in eGFR are warranted in the near future.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (No 2016R1C1B1015938).
Disclosure
The authors report no conflicts of interest in this work.
References
WHO [webpage on the Internet]. WHO Fact Sheet on Hepatitis B. 2016. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. Accessed March 14, 2017. | ||
Shin JH, Kwon HJ, Jang HR, et al. Risk factors for renal functional decline in chronic hepatitis B patients receiving oral antiviral agents. Medicine (Baltimore). 2016;95(1):e2400. | ||
Rodríguez-Nóvoa S, García-Samaniego J, Prieto M, et al. Altered underlying renal tubular function in patients with chronic hepatitis B receiving nucleos(t)ide analogs in a real-world setting: the MENTE study. J Clin Gastroenterol. 2016;50(9):779–789. | ||
Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in ‘real-life’ settings: from clinical trials to clinical practice. J Viral Hepat. 2012;19(6):377–386. | ||
Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J. 2016;13:64. | ||
Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16–34. | ||
Chan HL, Shaikh J, Gupta S, Hamed K. Renal function in nucleos(t)ide analog-treated patients with chronic hepatitis B: a systematic literature review and network meta-analysis. Adv Ther. 2016;33(5):862–875. | ||
Chacko EC, Surrun SK, Mubarack Sani TP, Pappachan JM. Chronic viral hepatitis and chronic kidney disease. Postgrad Med J. 2010;86(1018):486–492. | ||
Bhimma R, Coovadia HM. Hepatitis B virus-associated nephropathy. Am J Nephrol. 2004;24(2):198–211. | ||
Yi Z, Jie YW, Nan Z. The efficacy of anti-viral therapy on hepatitis B virus-associated glomerulonephritis: a systematic review and meta-analysis. Ann Hepatol. 2011;10(2):165–173. | ||
Fabrizi F, Dixit V, Martin P. Meta-analysis: anti-viral therapy of hepatitis B virus-associated glomerulonephritis. Aliment Pharmacol Ther. 2006;24(5):781–788. | ||
Lai KN, Li PK, Lui SF, et al. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324(21):1457–1463. | ||
Appel G. Viral infections and the kidney: HIV, hepatitis B, and hepatitis C. Cleve Clin J Med. 2007;74(5):353–360. | ||
Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology. 2009;49(5 suppl):S185–S195. | ||
Riveiro-Barciela M, Tabernero D, Calleja JL, et al. Effectiveness and safety of entecavir or tenofovir in a Spanish cohort of chronic hepatitis B patients: validation of the page-G score to predict hepatocellular carcinoma. Dig Dis Sci. 2017;62(3):784–793. | ||
Koksal AR, Alkim H, Boga S, et al. Value of cystatin C-based e-GFR measurements to predict long-term tenofovir nephrotoxicity in patients with hepatitis B. Am J Ther. Epub 2016 Oct 4. | ||
López Centeno B, Collado Borrell R, Pérez Encinas M, Gutiérrez García ML, Sanmartin Fenollera P. Comparison of the effectiveness and renal safety of tenofovir versus entecavir in patients with chronic hepatitis B. Farm Hosp. 2016;40(4):279–286. | ||
Zoulim F, Białkowska-Warzecha J, Diculescu MM, et al. Entecavir plus tenofovir combination therapy for chronic hepatitis B in patients with previous nucleos(t)ide treatment failure. Hepatol Int. 2016;10(5):779–788. | ||
Sriprayoon T, Mahidol C, Ungtrakul T, et al. Efficacy and safety of entecavir versus tenofovir treatment in chronic hepatitis B patients: a randomized controlled trial. Hepatol Res. 2017;47(3):E161–E168. | ||
Tsai MC, Chen CH, Tseng PL, et al. Does nucleos(t)ide analogues treatment affect renal function in chronic hepatitis B patients who have already decreased eGFR? A longitudinal study. PLoS One. 2016;11(3):e0149761. | ||
Park JY, Kim CW, Bae SH, et al. Entecavir plus tenofovir combination therapy in patients with multidrug-resistant chronic hepatitis B: results of a multicentre, prospective study. Liver Int. 2016;36(8):1108–1115. | ||
Wang HM, Hung CH, Lee CM, et al. Three-year efficacy and safety of tenofovir in nucleos(t)ide analog-naïve and nucleos(t)ide analog-experienced chronic hepatitis B patients. J Gastroenterol Hepatol. 2016;31(7):1307–1314. | ||
Tsai MC, Chen CH, Tseng PL, et al. Comparison of renal safety and efficacy of telbivudine, entecavir and tenofovir treatment in chronic hepatitis B patients: real world experience. Clin Microbiol Infect. 2016;22(1):95.e1–e7. | ||
Koklu S, Gulsen MT, Tuna Y, et al. Differences in nephrotoxicity risk and renal effects among anti-viral therapies against hepatitis B. Aliment Pharmacol Ther. 2015;41(3):310–319. | ||
Kim BG, Jung SW, Kim EH, et al. Tenofovir-based rescue therapy for chronic hepatitis B patients who had failed treatment with lamivudine, adefovir, and entecavir. J Gastroenterol Hepatol. 2015;30(10):1514–1521. | ||
Ha NB, Ku K, Ha NB, Chaung KT, Trinh HN, Nguyen MH. Renal function in chronic hepatitis B patients treated with tenofovir disoproxil fumarate or entecavir monotherapy: a matched case-cohort study. J Clin Gastroenterol. 2015;49(10):873–877. | ||
Lim YS, Yoo BC, Byun KS, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in adefovir-resistant chronic hepatitis B patients with multiple drug failure: results of a randomised trial. Gut. 2016;65(6):1042–1051. | ||
Hung CH, Hu TH, Lu SN, et al. Tenofovir versus entecavir in treatment of chronic hepatitis B virus with severe acute exacerbation. Antimicrob Agents Chemother. 2015;59(6):3168–3173. | ||
Lim YS, Byun KS, Yoo BC, et al. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in patients with entecavir-resistant chronic hepatitis B with multiple drug failure: results of a randomised trial. Gut. 2016;65(5):852–860. | ||
Qi X, Wang JY, Mao RC, Zhang JM. Impact of nucleos(t)ide analogues on the estimated glomerular filtration rate in patients with chronic hepatitis B: a prospective cohort study in China. J Viral Hepat. 2015;22(1):46–54. | ||
Tien C, Xu JJ, Chan LS, et al. Long-term treatment with tenofovir in Asian-American chronic hepatitis B patients is associated with abnormal renal phosphate handling. Dig Dis Sci. 2015;60(2):566–572. | ||
Lok AS, Trinh H, Carosi G, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology. 2012;143(3):619–628. | ||
Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941–946. | ||
Liaw YF, Sheen IS, Lee CM, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53(1):62–72. | ||
Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. | ||
Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21:1273–1281. | ||
Product Information: VIREAD(R) oral tablets, tenofovir disoproxil fumarate oral tablets. Foster City, CA: Gilead Sciences, Inc.; 2010. | ||
Product Information: BARACLUDE(R) oral tablets, solution, entecavir oral tablets, solution. Princeton, NJ: Bristol-Myers Squibb Company; 2009. | ||
Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–455. | ||
Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615. | ||
Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. | ||
Chan HL, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):185–195. | ||
WHO [webpage on the Internet]. WHO Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. 2015. Available from: http://www.who.int/hepatitis/publications/hepatitis-b-guidelines/en/. Accessed May 30, 2017. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



