Back to Journals » Drug Design, Development and Therapy » Volume 18
Remifentanil is Superior to Propofol for Treating Emergence Agitation in Adults After General Anesthesia
Authors Li J, Zhu H , Wang Y, Chen J, He K, Wang S
Received 9 September 2023
Accepted for publication 25 January 2024
Published 7 February 2024 Volume 2024:18 Pages 341—350
DOI https://doi.org/10.2147/DDDT.S433155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Jun Li,1,2 Hongrui Zhu,2 Yu Wang,3 Jiaqi Chen,2 Keqiang He,2 Sheng Wang2
1Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Anesthesiology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China; 3Department of Anesthesiology, The First Affiliated Hospital of Soochow University, Soochow, People’s Republic of China
Correspondence: Sheng Wang, Department of Anesthesiology, The First Affiliated Hospital of USTC, No. 17, Lujiang Road, Luyang District, Hefei, Anhui, People’s Republic of China, Email [email protected]
Background: Emergence agitation (EA) is one of the most common complications in clinical general anesthesia during recovery in adults. Remifentanil and propofol can reduce the incidence of EA, but with no randomized controlled trial to evaluate their effectiveness for treating EA. This study aims to compare the effectiveness of remifentanil and propofol for treating EA following general anesthesia.
Patients and methods: Among 152 randomized patients with a mean of 49.5 years, and 99 (65.1%) of them being male, 149 were divided into two groups for subsequent analysis. The remifentanil group (Group R, n = 74) received a 0.5μg kg– 1 remifentanil infusion followed by a 0.05μg kg– 1 min– 1 infusion until 15 minutes, after the onset of agitation. The propofol group (Group P, n = 75) received a 1mg kg– 1 propofol infusion once agitation occurred. Emergence agitation was assessed using the Riker Sedation Agitation Score, with a score of ≥ 5 defining emergence agitation. During the post–anesthesia care unit (PACU), the recurrence of emergence agitation, time to extubation, and discharge from PACU were evaluated.
Results: The incidence of reoccurring emergence agitation was lower in Group R (29.7%) compared with Group P (49.3%), with an odds ratio of 0.44 (95% CI 0.22– 0.85; P=0.014). The time to extubation was shorter in Group R (mean 12min, range 8– 15 min) compared with Group P (mean 17min, range 13– 21 min) (P< 0.001), as was the time discharge from the PACU (mean 30.5 min, range 25– 40 min) vs Group P (mean 37.5 min, range 31– 50 min) (P=0.001).
Conclusion: Treatment of emergence agitation in adults with remifentanil infusion is more effective than propofol, with a shorter time to extubation and discharge from PACU.
Keywords: anesthesia, emergence agitation, remifentanil, propofol
Introduction
Emergence agitation (EA) is an acute, self–limited, and nonfluctuating state of psychomotor excitement.1 This hyperactive state is confined to the emergence period after general anesthesia. Although the incidence rate of EA in adults ranges from 0.25% to 21.3%2–5 and time of onset typically lasts for about 15 minutes.5 Serious consequences can happen if patients with EA are not treated promptly. For example, the agitated patients with uncontrolled combative limb movements can result in various tubes removal, airway obstruction, self-injury (self-extubation, bleeding, or surgical incision dehiscence), or even harm to healthcare workers.2–5 Increased risk of postoperative pulmonary complications and postoperative delirium is also closely associated with emergence agitation.2 EA also inevitably increases medical costs for patients and the workload of healthcare workers.
Various factors can lead to EA, and several studies have shown a strong association between emergence agitation with perioperative relevant influential factors such as pain, adverse stimuli (such as catheter, tracheal tubes, nasogastric tubes, and chest tubes), certain types of surgery (otolaryngologic surgery, oral, breast, and abdominal surgery), male gender, and inhalation anesthetics.2,4 A recent meta-analysis also indicated that smoking is a significant risk factor for EA in adults.6 Therefore, investigation of effective interventions for treating EA is of great urgency for anesthesiologists.
Various drugs and techniques have been proven effective in reducing the incidence of EA. In previous studies, many kinds of strategies were widely utilized, such as remifentanil,7 magnesium sulfate,8 dexmedetomidine,9–11 fentanyl,12,13 propofol,12,13 midazolam,12,13 ketamine,12,13 nalbuphine14, dizocine,15 and regional anesthesia techniques.16 Among them, there have also been reports that droperidol17,18 and dexmedetomidine19–21 can treat agitated adult patients. However, they both have a slow onset time and longer time of accumulation in the body. Droperidol can cause extrapyramidal disorders and also increase the risk of prolonging the QTs interval.22 Rapid injection of Dexmedetomidine can lead to severe hypertension and bradycardia. The side effects of the above drugs limit their application in the treatment of EA. However, propofol and remifentanil are the two alternative drugs for anesthesiologists because of rapid metabolism and shorter time of drug accumulation in the body. Previous studies have shown that continuous infusion of small doses of remifentanil until the tracheal catheter is removed can effectively avoid coughing and the occurrence rate of EA.7,23 Propofol is also the most commonly used medication for the prevention and treatment of EA in pediatric patients.24,25 However, only a few studies investigated the effect of a single injection of propofol on EA in adult patients.26
Therefore, clinical practice indicates that propofol and remifentanil are widely applied, and their benefit effects for patients have been proven in perioperative period. However, the lack of detailed application scenarios and concrete clinical trial evidence in treating EA in adults are two of the vital questions tackled. In this study, we aim to compare the therapeutic effect of propofol and remifentanil on adult patients diagnosed with EA after general anesthesia, providing several application values during clinical anesthesia practice.
Materials and Methods
Ethics and Participants
We conducted a prospective, randomized, controlled, single–blind trial at a single center with a 1:1 allocation ratio. The study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China (Ethics Approval Number: 2022KY [028]). This study was also registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR2200057412). The trial report strictly followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines and complied with the Declaration of Helsinki. Participants who met the inclusion criteria provided informed consent before the surgery.
Between March 22, 2022 and September 16, 2022, a total of 152 adult patients aged 18–65 years old, with a body mass index (BMI) of 18–30 kg m–2 and American Society of Anesthesiologists (ASA) physical status classification I–III, who underwent elective surgery and developed emergence agitation in the PACU, were enrolled in the study. Patients who were unable to communicate properly before surgery, had a history of mental disease, had second–degree or higher AV block, bradycardia (heart rate <50 beats/min), hypotension (blood pressure <90/60 mmHg), or poorly controlled hypertension (systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg), were allergic to propofol or opioid, or had a history of opioid addiction, or recent use of muscle relaxants within 30 minutes at the end of the surgery were all excluded from the study.
Randomization and Blinding
One investigator generated a randomization list based on a 1:1 allocation ratio and provided allocation concealment by using sequentially numbered, sealed opaque envelopes. Two standardized training evaluators would evaluate the patient’s level of agitation to decide whether the patient was eligible for enrollment or not. If there were any discrepancy in the assessment, the patient would not be enrolled correspondingly. Following enrollment, one evaluator would open the envelope and the trial medication was given intravenously. Subsequently, the two evaluators assessed the effectiveness of medication and collected all data.
Evaluators knew the patient group allocation because of the administration of drugs, so they did not participate in follow-up assessments and trial design. Participants, investigators, statisticians, and other study staff were blinded to the enrollment in this study.
Trial Procedure
Electrocardiogram (ECG), non-invasive blood pressure (NBP), pulse oxygen saturation (SpO2), mean arterial pressure (MAP), and heart rate (HR) were monitored once the eligible patient entered the operating room. General anesthesia would be administered with etomidate 0.2–0.4 mg kg−1, sufentanil 0.2–0.4 μg kg−1, and rocuronium 0.6–0.9 mg kg−1. After intubation, anesthesia was maintained with propofol, remifentanil, and sevoflurane according to the guidelines of bispectral index (BIS) target ranging from 40 to 60. All medications were stopped 5 minutes before the end of surgery, and the patient was transferred to the post-anesthesia care unit (PACU). After being admitted to the PACU, the patient was closely and dynamically monitored for ECG, NBP, SpO2, MAP, and HR, continuously.
A trained anesthesiologist and a trained PACU nurse evaluated the patient’s level of agitation during the emergence period using the Riker Sedation Agitation Scale (RSAS), respectively. Once agitation was identified, patients were divided into propofol or remifentanil groups according to previous design. Furthermore, patients allocated in Group R received remifentanil intravenously, with a dose of 0.5μg kg–1 at a concentration of 10μg mL–1, administered over 30 seconds, followed by continuous pumping for 15 minutes at a rate of 0.05 μg kg–1 min–1. The RSAS was assessed after 1 minute of continuous remifentanil pumping, and if the RSAS score of patients reached 5 or more, additional treatment with propofol at a dose of 1 mg kg–1 was administered correspondingly. Patients allocated to Group P received 1 mg kg–1 propofol.
The two evaluators in the PACU reassessed the patient’s agitation within 15 minutes after treatment. In case of inconsistent results, the assessment indicating the presence of agitation was taken into consideration. The tracheal catheter was removed when the patient was able to respond to commands clearly, had a tidal volume greater than 6 mL kg–1, and was breathing regularly.
Measurements and Study Outcomes
The primary outcome of the study was the recurrence rate of emergence agitation within 15 minutes after medication intervention. The agitation score of each patient was recorded on a scale from 1 to 7 as follows: 1 represents minimal or no response to noxious stimuli; 2 represents arousal to physical stimuli but does not communicate; 3 represents difficulty in arousal but awakens to verbal stimuli or gentle shaking; 4 represents calm and follows commands; 5 represents anxious or physically agitated and calms to verbal instructions; 6 represents requiring restraint and frequent verbal reminding of limits; and 7 represents pulling at tracheal tube, trying to remove catheters, or striking at staff.9 Emergence agitation was defined as a score of ≥5 on the RSAS.
The secondary outcomes included RSAS at 1 minute (T0), 15 minutes (T1), and 30 minutes (T2), as well as the patient’s vital signs (BP, HR, SpO2) before and after intervention. In addition, postoperative nausea and vomiting (PONV) was measured using a four–point score (0 = no nausea; 1 = mild nausea; 2 = severe nausea requiring antiemetics, and 3 = retching, vomiting, or both), and the coughing response during emergence was evaluated by a four–point scale (0 = no cough, 1 = single cough, 2 = persistent cough lasting 5 seconds, and 3 = persistent cough lasting ≥5 seconds or bucking).7
In addition to monitoring the patient’s agitation, pain was also assessed using the Numerical Rating Scale (NRS) in the PACU, and the total amount of sufentanil used as a rescue medication was recorded; If a patient’s score of NRS >4 or they requested analgesics, sufentanil 0.05 μg kg–1 was administered, and the total amount of sufentanil administered was recorded. The total amount of administration of vasoactive drugs (ephedrine, norepinephrine, atropine), propofol, and remifentanil was also recorded.
The time to remove the tracheal catheter (calculated from the moment of first agitation to the removal of the tracheal catheter) and the time to discharge from the PACU (calculated from the moment of first agitation to the moment of leaving PACU) were also recorded. Adverse events such as hypotension, bradycardia, hypoxemia, and chest wall stiffness were monitored in the PACU. Patients were discharged from the PACU when their Steward scores were more than 4.
Statistical Methods
According to previous studies, the sample size calculation was based on a literature report that the recurrent rate of EA after the intervention by propofol was approximately 20%.26 By contrast, the proposed EA recurrence rate for patients after remifentanil administration was 3.3%.7 To perform the sample size calculation with a power of 80% and a two–tailed significance level of 5%, a 10% dropout rate was also considered. As a result, the final sample size was 152 patients.
For the statistical analysis, SPSS software was used (version 23.0; SPSS Inc., IBM, Chicago, IL, USA). The data for continuous variables were presented either as mean ± standard deviation (SD) or median (interquartile range) depending on the normal distribution as determined by the Shapiro–Wilk test. The differences between the groups were analyzed using either an independent t-test or Mann–Whitney U-test depending on the data distribution. Meanwhile, categorical variables such as the recurrence rate of EA, and the incidence of adverse events were analyzed using the chi-squared test or Fisher’s exact test. For repeated measurement data, such as vital signs, repeated measurement ANOVA was used. Finally, a P value < 0.05 was regarded as statistically significant.
Results
Between March 22, 2022, and September 16, 2022, a total of 8664 patients were assessed for eligibility; at last, 8452 adult patients were eligible for the study, with 152 patients (1.8%) experiencing emergence agitation in the PACU. A total of 152 agitated patients were randomized to Group P or Group R. Three patients were excluded from the analyses due to the use of neostigmine, and none lost to follow–up. Finally, 149 patients [Group R (n = 74) and Group P (n = 75)] completed the study (as shown in Figure 1). The baseline characteristics between the two groups were similar (as shown in Table 1).
 |
Table 1 Patient Characteristics and Type of Operation to Group R and Group P |
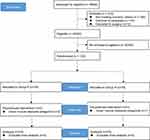 |
Figure 1 Flowchart of the trail diagram. |
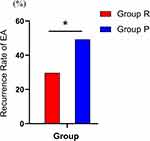
The recurrence rate of emergence agitation within 15 minutes after drug intervention was found to be lower in Group R compared with Group P (29.7 vs 49.3%, with an odds ratio of 0.44, 95% CI 0.22–0.85; P=0.014), as shown in Figure 2.
Other parameters related to the emergence from the PACU are represented in Table 2. The time from emergence agitation to extubation and discharge from the PACU was shorter in Group R compared with Group P (12 min in Group R virus 17min in Group P, P<0.001 and 30.5 min in Group R versus 37.5 min in Group P, P=0.001). The coughing severity was also different between the two groups during emergence (1 [0–1] in Group R vs 1 [1–2] in Group P, P<0.001). The incidence rate of hypoxemia and PONV was similar between the two groups (2 cases in the remifentanil group versus 3 cases in the propofol group, P=0.660, and 8 cases in the remifentanil group vs 6 cases in the propofol group, P=0.906). The pain NRS scores and the use of sufentanil as a pain reliever in the PACU were comparable between the two groups (Table 2).
 |
Table 2 Recovery Characteristics in PACU |
The MAP and HR during emergence are shown in Figure 3. The MAP and HR were similar in both groups, and the changes in vital signs at the same time points were not statistically significant between the groups (MAP, P=0.267, HR, P=0.526). Based on baseline vital signs, the treatment of hypotension and bradycardia was determined by the attending physician, and no patient was deemed to require drug intervention.
No adverse events such as hypotension, bradycardia, and chest wall stiffness were observed. Two patients in Group R and three patients in Group P experienced hypoxemia.
Discussion
Our findings showed that when emergence agitation occurs in adults, it can be treated with intravenous propofol or remifentanil infusion, with remifentanil being more effective than propofol. Furthermore, the use of remifentanil infusion can shorten extubation time and duration of PACU stay, without increasing the risk of other complications.
Various medications have been studied to reduce the incidence of EA. Among them, two meta-analyses showed that compared to the placebo, propofol or opioids could reduce the incidence of EA.27,28 Kim et al29 compared the use of propofol or fentanyl at the end of sevoflurane anaesthesia in children who underwent inguinal hernia repair and showed that propofol or fentanyl had a similar prophylactic effect on EA. However, fentanyl caused a higher incidence of PONV compared to propofol. A survey report on EA in children showed that the satisfaction of propofol treatment for EA was higher than that of opioids,30 which was different from our results. The possible reason is that children are more prone to crying and anxiety in unfamiliar environments, and opioids cannot effectively eliminate the status caused by psychological factors. Propofol has an anti-anxiety effect and is more suitable for pediatric agitated patients. However, there were only a few studies that explored the treatment of EA in adults. Recently, Feng et al26 conducted a randomized controlled clinical trial to compare the effects of dexmedetomidine versus propofol for treating EA in adults. The clinical outcomes are also promising.
Remifentanil is an ultra-short–acting agent, which offers rapid pain relief and no accumulation. Lee et al31 found that different remifentanil loading doses injected over 30 seconds (0.25μg kg–1, 0.5μg kg–1, 0.75μg kg–1) had varying onset times for preventing injection pain from propofol and no desaturation in the three groups. Although the onset time was lower in the remifentanil 0.75μg kg–1 group than in the 0.5μg kg–1 group, remifentanil 0.5μg kg–1 given within 10 seconds provided a decrease in ventilator, according to Gelberg et al.32 So, we chose a 0.5μg kg–1 remifentanil for over 30 seconds as loading dose. Previous studies have also shown the efficacy of remifentanil infusion in reducing emergence agitation. Cavaliere et al23 found that a remifentanil infusion of up to 0.05μg kg–1 min–1 reduced agitation without affecting respiration in critically ill patients. Aouad et al7 also observed a less non-purposeful movement, as well as a lower incidence and severity of coughing, in patients receiving remifentanil infusion at a rate of 0.014±0.011μg kg–1 min–1 compared with the control group. The dosage of 0.05μg kg–1 min–1 is more operable, with better analgesic effects than the dosage of 0.014±0.011μg kg–1 min–1, so we chose the dosage of 0.05μg kg–1 min–1 as maintenance dose.
Our study found that remifentanil was effective in preventing emergence agitation in 70.3% of patients. This efficacy is lowered compared with 97.3% reported in a previous study on ENT surgery.7 The difference in results could be due to the complex influence factors contributing to emergence agitation in our study, compared with previous research that only focused on one specific type of surgery or limited risk factors for emergence agitation. However, in our study, those influencing factors are more complex resulting in a lower effect of treatment on EA than in previous studies. Our findings align with a recent meta-analysis, which reported an efficacy of 63.85% for remifentanil in preventing agitation.12
Propofol is one of the most used drugs to prevent and treat emergence agitation in children.24,25 In our study, the treatment efficacy of propofol for agitation was 50.7%, which was lower than the previous results.26 One possible reason for the lower efficacy of propofol in our study may be due to the shorter observation period of only within 15 minutes, and the influence of multiple risk variables affecting agitation than previously described in the literature.
The incidence of PONV in our study was 9.2% (14/152), which was lower than reported in previous studies.33 This may be due to the administration of dexamethasone during anesthesia induction and dexmedetomidine during the intraoperative procedure. Our study showed that PONV was not statistically significant between the remifentanil and propofol groups. The incidence of PONV was associated with the dosage of remifentanil,34 and low-dose remifentanil has not been found to increase the incidence of nausea and vomiting.35,36
In this study, both propofol and remifentanil showed the potential to cause hypoxemia. Three cases of hypoxemia occurred in the propofol group and two cases in the remifentanil group after the removal of the tracheal catheter. Hypoxemia can be alleviated by holding up the jaw or using an oxygen mask. One case in the propofol group had a low SpO2 of 85%, but it was quickly corrected by supporting the mandible support and inhaling oxygen through a mask. Because the residual effects of other drugs had synergistic effects with propofol, the possibility of hypoxemia increased.
However, our study has several limitations. Firstly, we did not use the double-blind method to compare the effects of two groups of drugs on emergence agitation, which could introduce bias. To reduce bias, we made efforts to evaluate the treatment’s effect by two medical staff members who received rigorous training in emergence agitation assessment. Secondly, according to our results, we did not observe the side effects (bradycardia, hypotension, chest wall stiffness, etc.) with either medication, which may be related to our slower medication administration speed. However, they may still happen in our clinical practice. So, we should actively evaluate the patient’s respiratory and hemodynamic status, and be prepared with vasoactive drugs and emergency airway equipment. Thirdly, in this study, we only investigated the preliminary dosage of the two medications to treat EA. Due to the side effects of respiratory depression, the dosage of the two medications for elderly patients needs to be treated carefully. Recently, Lee et al37 found that a repeated verbal reminder of orientation to patients during the postoperative recovery period could significantly reduce the incidence of EA. The possibility of using a multimodal approach to treat EA is worth being investigated further.
Conclusion
In essence, remifentanil (0.5μg kg–1 intravenous and 0.05μg kg–1 min–1 continuous infusion for 15 minutes) or propofol (1mg kg–1) infusion can be used to treat emergence agitation in adults, with remifentanil being a more efficient medication.
Abbreviations
EA, emergence agitation; IQR, interquartile range; BIS, bispectral index; PACU, post–anesthesia care unit; BMI, body mass index; ASA, American Society of Anesthesiologists; ECG, electrocardiogram; SpO2, pulse oxygen saturation; HR, heart rate; NBP, non-invasive blood pressure; MAP, mean arterial pressure; RSAS, Riker Sedation-Agitation Scale; PONV, postoperative nausea and vomiting; NRS, Numerical Rating Scale; IQR, interquartile range; CI, confidence interval; ENT, ears, nose, and throat.
Data Sharing Statement
All data used or analyzed during the study were included in this article. Further inquiries about the datasets are available from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge the invaluable assistance of our PACU nurses in outcome evaluation and data collection.
Funding
This study was funded by the National Natural Science Foundation of China (82272225, 81860249), wujieping medical foundation (320.6750.2021-03-1).
Disclosure
The authors declare that there are no potential conflicts of interest in this study.
References
1. Tolly B, Waly A, Peterson G, et al. Adult emergence agitation: a veteran-focused narrative review. Anesth Analg. 2021;132(2):353–364. doi:10.1213/ANE.0000000000005211
2. Fields A, Huang J, Schroeder D, et al. Agitation in adults in the post-anaesthesia care unit after general anaesthesia. Br J Anaesth. 2018;121(5):1052–1058. doi:10.1016/j.bja.2018.07.017
3. Card E, Pandharipande P, Tomes C, et al. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br J Anaesth. 2015;115(3):411–417. doi:10.1093/bja/aeu442
4. Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2000 patients. Can J Anaesth. 2010;57(9):843–848. doi:10.1007/s12630-010-9338-9
5. Lepousé C, Lautner CA, Liu L, et al. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747–753. doi:10.1093/bja/ael094
6. Wei B, Feng Y, Chen W, et al. Risk factors for emergence agitation in adults after general anesthesia: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2021;65(6):719–729. doi:10.1111/aas.13774
7. Aouad MT, Al-Alami AA, Nasr VG, et al. The effect of low-dose remifentanil on responses to the endotracheal tube during emergence from general anesthesia. Anesth Analg. 2009;108(4):1157–1160. doi:10.1213/ane.0b013e31819b03d8
8. Elsersy HE, Metyas MC, Elfeky HA, Hassan AA. Intraoperative magnesium sulphate decreases agitation and pain in patients undergoing functional endoscopic surgery: a randomised double-blind study. Eur J Anaesthesiol. 2017;34(10):658–664. doi:10.1097/EJA.0000000000000642
9. Kim SY, Kim JM, Lee JH, et al. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111(2):222–228. doi:10.1093/bja/aet056
10. Kim JA, Ahn HJ, Yang M, et al. Intraoperative use of dexmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial. Can J Anaesth. 2019;66(4):371–379. doi:10.1007/s12630-019-01299-7
11. Aouad MT, Zeeni C, Al Nawwar R, et al. Dexmedetomidine for improved quality of emergence from general anesthesia: a dose-finding study. Anesth Analg. 2019;129(6):1504–1511. doi:10.1213/ANE.0000000000002763
12. Jiao H, Wang H, Jiang Z, Hu J. Comparative efficacy of ancillary drugs in sevoflurane-related emergence agitation after paediatric adenotonsillectomy: a Bayesian network meta-analysis. J Clin Pharm Ther. 2020;45(5):1039–1049. doi:10.1111/jcpt.13133
13. Chen J, Li W, Hu X, Wang D. Emergence agitation after cataract surgery in children: a comparison of midazolam, propofol and ketamine. Paediatr Anaesth. 2010;20(9):873–879. doi:10.1111/j.1460-9592.2010.03375.x
14. He J, Zhang L, Tao T, et al. Nalbuphine reduces the incidence of emergence agitation in children undergoing Adenotonsillectomy: a prospective, randomized, double-blind, multicenter study. J Clin Anesth. 2023;85:111044. doi:10.1016/j.jclinane.2022.111044
15. An LJ, Zhang Y, Su Z, et al. A single dose of dezocine suppresses emergence agitation in preschool children anesthetized with sevoflurane-remifentanil. BMC Anesthesiol. 2017;17(1):154. doi:10.1186/s12871-017-0446-8
16. Kaçar CK, Uzundere O, Salık F, et al. Effects of adding a combined infraorbital and infratrochlear nerve block to general anaesthesia in septorhinoplasty. J Pain Res. 2020;13:2599–2607. doi:10.2147/JPR.S255720
17. Hatzakorzian R, Shan WL, Côté AV, et al. The management of severe emergence agitation using droperidol. Anaesthesia. 2006;61(11):1112–1115. doi:10.1111/j.1365-2044.2006.04791.x
18. Taylor DM, Yap CYL, Knott JC, et al. Midazolam-droperidol, droperidol, or olanzapine for acute agitation: a randomized clinical trial. Ann Emerg Med. 2017;69(3):318–326.e1. doi:10.1016/j.annemergmed.2016.07.033
19. O’Reardon JP, Takieddine N, Datto CJ, et al. Propofol for the management of emergence agitation after electroconvulsive therapy: review of a case series. J ECT. 2006;22(4):247–252. doi:10.1097/01.yct.0000235929.46903.67
20. Tzabazis A, Schmitt HJ, Ihmsen H, et al. Postictal agitation after electroconvulsive therapy: incidence, severity, and propofol as a treatment option. J ECT. 2013;29(3):189–195. doi:10.1097/YCT.0b013e3182887b5b
21. Read MD, Maani CV, Blackwell S. Dexmedetomidine as a rescue therapy for emergence delirium in adults: a case series. A a Case Rep. 2017;9(1):20–23. doi:10.1213/XAA.0000000000000510
22. Perkins J, Ho JD, Vilke GM, DeMers G. American Academy of emergency medicine position statement: safety of droperidol use in the emergency department. J Emerg Med. 2015;49(1):91–97. doi:10.1016/j.jemermed.2014.12.024
23. Cavaliere F, Antonelli M, Arcangeli A, et al. A low-dose remifentanil infusion is well tolerated for sedation in mechanically ventilated, critically-ill patients. Can J Anaesth. 2002;49(10):1088–1094. doi:10.1007/BF03017909
24. Rosen HD, Mervitz D, Cravero JP. Pediatric emergence delirium: Canadian Pediatric Anesthesiologists’ experience. Paediatr Anaesth. 2016;26(2):207–212. doi:10.1111/pan.12812
25. Urits I, Peck J, Giacomazzi S, et al. emergence delirium in perioperative pediatric care: a review of current evidence and new directions. Adv Ther. 2020;37(5):1897–1909. doi:10.1007/s12325-020-01317-x
26. Feng Z, Shi X, Yan X, et al. Comparing the effects of dexmedetomidine versus propofol on the treatment of emergence agitation in adult patients after general anesthesia: study protocol for a randomized, superiority, controlled trial (DP-TEA Trial). Trials. 2021;22(1):811. doi:10.1186/s13063-021-05743-2
27. Jiang S, Liu J, Li M, Ji W, Liang J. The efficacy of propofol on emergence agitation--a meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand. 2015;59(10):1232–1245. doi:10.1111/aas.12586
28. Tan Y, Shi Y, Ding H, Kong X, Zhou H, Tian J. μ-Opioid agonists for preventing emergence agitation under sevoflurane anesthesia in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2016;26(2):139–150. doi:10.1111/pan.12815
29. Kim MS, Moon BE, Kim H, Lee JR. Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth. 2013;110(2):274–280. doi:10.1093/bja/aes382
30. Huett C, Baehner T, Erdfelder F, et al. Prevention and therapy of pediatric emergence delirium: a National Survey. Paediatr Drugs. 2017;19(2):147–153. doi:10.1007/s40272-017-0212-x
31. Lee SH, Lee SE, Chung S, et al. Impact of time interval between remifentanil and propofol on propofol injection pain. J Clin Anesth. 2016;34:510–515. doi:10.1016/j.jclinane.2016.06.029
32. Gelberg J, Jonmarker C, Stenqvist O, Werner O. Intravenous boluses of fentanyl, 1 μg kg⁻¹, and remifentanil, 0.5 μg kg⁻¹, give similar maximum ventilatory depression in awake volunteers. Br J Anaesth. 2012;108(6):1028–1034. doi:10.1093/bja/aes029
33. Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting [published correction appears in Anesth Analg. 2020 Nov;131(5):e241]. Anesth Analg. 2020;131(2):411–448. doi:10.1213/ANE.0000000000004833
34. Hozumi J, Egi M, Sugita S, Sato T. Dose of intraoperative remifentanil administration is independently associated with increase in the risk of postoperative nausea and vomiting in elective mastectomy under general anesthesia. J Clin Anesth. 2016;34:227–231. doi:10.1016/j.jclinane.2016.04.018
35. Watanabe T, Moriya K, Tsubokawa N, Baba H. Effect of remifentanil on postoperative nausea and vomiting: a randomized pilot study. J Anesth. 2018;32(5):781–785. doi:10.1007/s00540-018-2550-4
36. Massoth C, Schwellenbach J, Saadat-Gilani K, et al. Impact of opioid-free anaesthesia on postoperative nausea, vomiting and pain after gynaecological laparoscopy - A randomised controlled trial. J Clin Anesth. 2021;75:110437. doi:10.1016/j.jclinane.2021.110437
37. Lee S, Sohn JY, Hwang IE, et al. Effect of a repeated verbal reminder of orientation on emergence agitation after general anaesthesia for minimally invasive abdominal surgery: a randomised controlled trial. Br J Anaesth. 2023;130(4):439–445. doi:10.1016/j.bja.2022.12.009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.