Back to Journals » OncoTargets and Therapy » Volume 15
Remarkable Response to the Triplet Combination of Olaparib with Pembrolizumab and Bevacizumab in the Third-Line Treatment of an Ovarian Clear Cell Carcinoma Patient with an ARID1A Mutation: A Case Report
Received 12 February 2022
Accepted for publication 28 March 2022
Published 2 April 2022 Volume 2022:15 Pages 323—328
DOI https://doi.org/10.2147/OTT.S362267
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Yingchao Zhao, Yao Jiang
Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Yao Jiang, Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China, Tel +86 13797011369, Fax +86-027-85873062, Email [email protected]
Abstract: Ovarian clear cell carcinoma (OCCC) is a highly aggressive malignancy with a poor prognosis, and most patients experience recurrence after primary treatment. Currently, there is no standard treatment option for recurrent OCCC. Herein, we report the case of a 32-year-old female patient with OCCC. The patient received primary cytoreductive surgery with adjuvant chemotherapy and remained disease-free for four months. She then experienced retroperitoneal lymph node recurrence and was treated with liposomal doxorubicin chemotherapy followed by secondary debulking surgery. The patient experienced a second relapse in the lower left lung 11 months later. Genomic profiling of tumor samples revealed a deleterious AT-rich interactive domain 1A (ARID1A) mutation and homologous recombination deficiency. Thus, the triplet combination of the poly (ADP-ribose) polymerase (PARP) inhibitor, olaparib; the PD-1 inhibitor, pembrolizumab; and the antiangiogenic agent, bevacizumab was administered. The patient achieved partial response, which was sustained for 12 months. Our study provides the first clinical evidence that the combination of olaparib with pembrolizumab and bevacizumab could be an effective treatment for patients with platinum-resistant, recurrent OCCC.
Keywords: ovarian clear cell carcinoma, PARP inhibitor, PD-1 inhibitor, antiangiogenic drug, next-generation sequencing
Introduction
Ovarian clear cell carcinoma (OCCC) is a highly aggressive subtype of epithelial ovarian cancer.1 The prevalence of OCCC varies widely according to the geographic region as it accounts for approximately 5% of epithelial ovarian cancers in Western countries compared with 11.1% of these cancers in Asia.2 In Japan, OCCC is the second most common histological subtype of epithelial ovarian cancer, with an incidence rate of 25%. Additionally, endometriosis is regarded as a major risk factor of OCCC.3,4 Currently, complete cytoreductive surgery with adjuvant chemotherapy is the standard primary treatment. However, OCCC is often chemoresistant and associated with poor prognosis, exhibiting a 5-year survival rate of 23.6% for stage III and 18.2% for stage IV OCCC.5 Thus, new therapies for OCCC are crucial.
Studies have revealed several genomic alterations in patients with OCCC. Particularly, deleterious AT-rich interactive domain 1A (ARID1A) mutations and oncogenic phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit α (PIK3CA) mutations are frequently detected in OCCC. Thus, these specific molecular changes hold potential as therapeutic targets. Preclinical studies showed that ARID1A deficiency impaired DNA damage checkpoint, increased tumor-infiltrating lymphocytes, elevated PD-L1 expression and, therefore, sensitized tumor cells to poly (ADP-ribose) polymerase (PARP) inhibitors and immune checkpoint inhibitors (ICIs).6,7 Moreover, strong VEGF expression was found in OCCC, and the antiangiogenic agent, bevacizumab demonstrated significant antitumor effects in the OCCC model.8 Clinically, several clinical trials have demonstrated that combination therapy of PARP inhibitors with ICIs and antiangiogenic agents displayed synergistic antitumor activity in recurrent ovarian cancer.9,10 However, due to the rarity of OCCC, consensus regarding recommendations for individualized treatment for this cancer has not yet been achieved. Herein, we report a case of platinum-resistant, recurrent OCCC in a patient with an ARID1A mutation. Treatment with the triplet combination of the PARP inhibitor, olaparib; the PD-1 monoclonal antibody, pembrolizumab; and bevacizumab was successful.
Case Presentation
On December 9, 2020, a 32-year-old female patient was admitted to our hospital presenting with a soft mass in the lower left lung. The treatment timeline is shown in Figure 1. The patient had received primary cytoreductive surgery and was diagnosed with FIGO stage IIB OCCC at another hospital on September 28, 2018. She denied any family history of cancer. The patient then received six cycles of adjuvant paclitaxel plus carboplatin chemotherapy. Four months after the first-line chemotherapy, a follow-up computed tomography (CT) scan showed recurrence in the retroperitoneal lymph nodes. The patient was then treated with four cycles of pegylated liposomal doxorubicin and achieved partial remission. She then underwent secondary cytoreductive surgery of the retroperitoneal lymph nodes. The postoperative pathology was consistent with the initial tumor. Immunohistochemistry staining demonstrated positive PAX8, CK7, NapsinA, and HNF1β; Ki67 (Li: 15%); P53 (wild-type) and negative WT1. Tumor tissues and blood samples were sent for next-generation sequencing using a 508-gene panel (Beijing Genomics institution, China). The results revealed a somatic ARID1A exon 20 nonsense mutation (c.5161C>T, p.R1721*15.25%), HER2 copy number amplification, and homologous recombination deficiency (HRD). Furthermore, next-generation sequencing results showed microsatellite stability, a low tumor mutation burden (2.56 Muts/Mb), and a heterozygous germline CHEK2 mutation (Table 1).
 |
Table 1 Next-Generation Sequencing Results |
 |
Figure 1 Treatment timeline. Abbreviations: CR, complete response; PR, partial response; *Means chemotherapy cycles. |
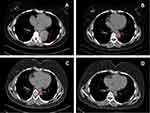
From October 2019 to January 2020, the patient was treated with four additional cycles of liposomal doxorubicin chemotherapy after secondary surgery. She then remained disease-free for 11 months. On December 4, 2020, a follow-up CT scan showed a soft, 5.3×4.4 cm mass in the lower lobe of the left lung (Figure 2A). Furthermore, the serum cancer antigen 125 (CA125) value was 625.8 U/mL, which was significantly higher than the reference value of 35 U/mL. After discussion, the patient refused chemotherapy. Because the molecular pathology revealed HRD and a deleterious ARID1A mutation, olaparib was considered a treatment option; however, single-agent PARP inhibitors have shown only modest effects in patients with platinum-resistant ovarian cancer (PROC).11,12 Furthermore, accumulating evidence has shown that a combination of PARP inhibitors with ICIs and antiangiogenic agents displays synergistic antitumor effects.13–15 Therefore, the triplet combination of olaparib with pembrolizumab and bevacizumab was administered on December 11, 2020. The patient received 200 mg of intravenous pembrolizumab with 7.5 mg/kg intravenous bevacizumab every three weeks and 300 mg of oral olaparib twice daily. On January 4, 2021, the serum CA125 value was found to have decreased to 143.7 U/mL after one cycle of treatment. After four cycles, CT imaging showed that the size of the tumor had reduced to 3.0×2.5 cm (Figure 2B), and the serum CA125 level returned to be within the normal range. As a result, the patient achieved partial remission according to RECIST v1.1 criteria. After eight treatment cycles, the tumor size reduced further to 1.4×1.2 cm (Figure 2C). Later, a follow-up CT scan showed sustained partial remission 12 months after treatment (Figure 2D). At the time of publishing this report, the patient is still in the partial remission stage and continuing combination therapy. During treatment, the patient experienced common adverse events, including mild nausea and vomiting, which resolved with supportive treatment, and grade 1 hypothyroidism. Thus, 150 µg of oral levothyroxine was administered daily, and the indices of thyroid function tests were restored. The patient maintained a good quality of life during treatment.
Discussion
OCCC is an aggressive ovarian cancer characterized by insensitivity to chemotherapy, with a response rate of 25% to first-line chemotherapy and <10% to second-line chemotherapy.16–18 This patient experienced a relapse four months after first-line chemotherapy and was classified as having PROC. The treatment options for patients with PROC are currently limited. The patient underwent liposomal doxorubicin chemotherapy, followed by secondary cytoreductive surgery, and remained disease-free for 11 months until she suffered a second relapse.
OCCC has unique biological profiles distinct from other histological subtypes of epithelial ovarian cancer. Multiple driver mutations have been discovered in OCCC. The most frequent genomic alterations in OCCC are ARID1A deleterious mutations in approximately 50% of cases.19,20 ARID1A is a tumor suppressor that plays an important role in both non-homologous end-joining and homologous recombination repair of DNA double-strand breaks. Thus, synthetic lethal strategies were widely investigated in ARID1A-mutant tumors.21 Preclinical research has demonstrated that ARID1A deficient tumors are sensitive to PARP inhibitors.6 In this case, genomic sequencing revealed a deleterious ARID1A mutation and HRD, which indicates that PARP inhibitors could be a promising therapeutic option. However, PARP inhibitor monotherapy has exhibited only a modest effect in PROC.11,12 Therefore, PARP inhibitor-based combination treatment was suggested.
Recently, ICIs have been gaining attention for the treatment of OCCC. Okamura et al reported that ARID1A alterations predicted longer PFS after ICI treatment, independently of microsatellite instability or tumor mutation burden.22 A Phase I study of patients with recurrent ovarian cancer who received the PD-L1 inhibitor, avelumab showed that this treatment resulted in partial remission in two patients with OCCC.23 Another Phase II trial evaluated the PD-1 inhibitor, nivolumab, in patients with PROC. The results showed that two patients (10%) achieved complete remission, one of whom had OCCC.24,25 In the KEYNOTE-100 study, pembrolizumab monotherapy demonstrated an increased response rate of 15.8% for recurrent OCCC.26 Moreover, in a recent Phase III study of nivolumab compared with chemotherapy for patients with PROC (NINJA), nivolumab induced a response rate of 7.6%. Subgroup analysis demonstrated a numerically longer overall survival with nivolumab compared with chemotherapy among patients with OCCC.27,28 Taken together, these findings suggest that ICI monotherapy can induce responses in only a small subset of patients with OCCC. Several studies have proposed that PARP inhibitors can induce immunogenic tumor cell death, increase tumor neoantigens, upregulate PD-L1 expression, and, therefore, enhance the response to ICIs.29–31 Thus, the combination therapy of ICIs and PARP inhibitors is being explored. In a phase I study of the PD-L1 inhibitor, durvalumab combined with olaparib for recurrent ovarian cancer, one patient with OCCC was recruited who achieved partial remission.32
Angiogenesis plays a crucial role in tumor growth. High levels of VEGF expression have been detected in patients with early and advanced stage OCCC and are associated with poor prognosis. In models of parental and cisplatin-refractory clear cell carcinoma cell-derived tumors, bevacizumab, a humanized monoclonal antibody against VEGF, markedly inhibited tumor growth.8 Furthermore, Lampert et al reported that the VEGF/VEGFR pathway might counteract the immunostimulatory effects of PARP inhibitors and act as a therapeutic target to further improve the efficacy of PARP and PD-1 inhibitors.14 A clinical trial of the antiangiogenic agent, anlotinib combined with the PARP inhibitor, niraparib for platinum-resistant OCCC is ongoing (NCT05130515, not yet recruiting). Moreover, in the MEDIOLA phase II study, the triplet combination of olaparib plus durvalumab and bevacizumab achieved a significantly higher progression-free survival and objective response rate than the doublet combination of olaparib plus durvalumab for platinum-sensitive relapsed ovarian cancer.9
Based on the studies mentioned above, the triplet combination of olaparib, pembrolizumab, and bevacizumab was administered. The patient achieved partial remission after four treatment cycles and continued to demonstrate a remarkable and sustained remission after 12 months. This combination treatment was well-tolerated with few mild adverse events that did not cause drug discontinuation or dose reduction. Recently, in the OPAL phase II study of niraparib in combination with the PD-1 inhibitor, dostarlimab, and bevacizumab for PROC, the response rate was 17.9%.10 However, no patients with OCCC were enrolled in this study. The current study findings may indicate that this triplet combination is effective for platinum-resistant, recurrent OCCC.
Conclusion
This case study provides the first clinical evidence that the combination of olaparib, pembrolizumab, and bevacizumab could be an effective therapy in platinum-resistant, recurrent OCCC, especially in patients with ARID1A mutations. Prospective clinical trials are warranted to further investigate the efficacy of this triple combination strategy in OCCC.
Abbreviations
ARID1A, AT-rich interactive domain 1A; CA125, cancer antigen 125; CT, computed tomography; HRD, homologous recombination deficiency; ICIs, immune checkpoint inhibitors; OCCC, ovarian clear cell carcinoma; PARP, poly (ADP-ribose) polymerase; PIK3CA, phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit α; PROC, platinum-resistant ovarian cancer.
Ethics Approval
This study was approved by the Ethics Committee of the Union Hospital of Huazhong University of Science and Technology (20220023).
Patient Informed Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images in an anonymized manner.
Acknowledgments
We thank the patient for her participation in this study. We would like to thank Editage for English language editing.
Author Contributions
Yao Jiang and Yingchao Zhao were responsible for the conception and design of the study. Yao Jiang reviewed and revised the manuscript. Yingchao Zhao drafted the manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Grant NO. 81902854 and 81974463). The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. The sponsors had no role in the design and conduct of the study, the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jang JYA, Yanaihara N, Pujade-Lauraine E, et al. Update on rare epithelial ovarian cancers: based on the rare ovarian tumors young investigator conference. J Gynecol Oncol. 2017;28(4):e54. doi:10.3802/jgo.2017.28.e54
2. Anglesio MS, Carey MS, Köbel M, Mackay H, Huntsman DG. Vancouver ovarian clear cell symposium speakers. Clear cell carcinoma of the ovary: a report from the first ovarian clear cell symposium, June 24th, 2010. Gynecol Oncol. 2011;121(2):407–415. doi:10.1016/j.ygyno.2011.01.005
3. Kobayashi H. Ovarian cancer in endometriosis: epidemiology, natural history, and clinical diagnosis. Int J Clin Oncol. 2009;14(5):378–382. doi:10.1007/s10147-009-0931-2
4. Pearce CL, Templeman C, Rossing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385–394. doi:10.1016/S1470-2045(11)70404-1
5. Mizuno M, Kikkawa F, Shibata K, et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. J Surg Oncol. 2006;94(2):138–143. doi:10.1002/jso.20251
6. Shen J, Peng Y, Wei L, et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5(7):752–767. doi:10.1158/2159-8290.CD-14-0849
7. Shen J, Ju Z, Zhao W, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24(5):556–562. doi:10.1038/s41591-018-0012-z
8. Mabuchi S, Kawase C, Altomare DA, et al. Vascular endothelial growth factor is a promising therapeutic target for the treatment of clear cell carcinoma of the ovary. Mol Cancer Ther. 2010;9(8):2411–2422. doi:10.1158/1535-7163.MCT-10-0169
9. Drew Y, Penson RT, O’Malley DM, et al. 814MO Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol. 2020;31:S615–S616. doi:10.1016/j.annonc.2020.08.953
10. Liu J, Gaillard S, Hendrickson AW, et al. An open-label phase II study of dostarlimab (TSR-042), bevacizumab (bev), and niraparib combination in patients (pts) with platinum-resistant ovarian cancer (PROC): cohort A of the OPAL trial [abstract]. Gynecol Oncol. 2021;162(suppl1):S17–S18. doi:10.1016/S0090-8258(21)00680-6
11. Vanderstichele A, Van Nieuwenhuysen EV, Han S, et al. Randomized phase II CLIO study on olaparib monotherapy versus chemotherapy in platinum-resistant ovarian cancer. J Clin Oncol. 2019;37(suppl 15):5507. doi:10.1200/JCO.2019.37.15_suppl.5507
12. Moore KN, Secord AA, Geller MA, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (Quadra): a multicentre, open-label, single-arm, Phase 2 trial. Lancet Oncol. 2019;20(5):636–648. doi:10.1016/S1470-2045(19)30029-4
13. Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–1214. doi:10.1016/S1470-2045(14)70391-2
14. Lampert EJ, Zimmer A, Padget M, et al. Combination of PARP inhibitor Olaparib, and PD-L1 inhibitor Durvalumab, in recurrent ovarian cancer: a proof-of-concept Phase II study. Clin Cancer Res. 2020;26(16):4268–4279. doi:10.1158/1078-0432.CCR-20-0056
15. Li AP, Yi M, Qin S, Chu Q, Luo S, Wu K. Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol. 2019;12(1):98. doi:10.1186/s13045-019-0784-8
16. Tan DSP, Rye T, Barrie C, et al. Analysis of outcomes in patients (pts) with recurrent ovarian clear cell carcinoma (ROCCC): time to rethink our approach to treatment. J Clin Oncol. 2014;32(suppl 15):5548. doi:10.1200/jco.2014.32.15_suppl.5548
17. Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol. 2007;105(2):404–408. doi:10.1016/j.ygyno.2006.12.024
18. Miyamoto M, Takano M, Goto T, et al. Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol. 2013;24(1):37–43. doi:10.3802/jgo.2013.24.1.37
19. Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. doi:10.1056/NEJMoa1008433
20. Chandler RL, Damrauer JS, Raab JR, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6:6118. doi:10.1038/ncomms7118
21. Williamson CT, Miller R, Pemberton HN, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun. 2016;7:13837. doi:10.1038/ncomms13837
22. Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer. 2020;8(1):e000438. doi:10.1136/jitc-2019-000438
23. Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN solid tumor phase Ib Trial: safety and clinical activity. J Clin Oncol. 2016;34(15_suppl):5533. doi:10.1200/JCO.2016.34.15_suppl.5533
24. Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, Nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi:10.1200/JCO.2015.62.3397
25. Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management. Gynecol Oncol. 2018;151(2):381–389. doi:10.1016/j.ygyno.2018.09.001
26. Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–1087. doi:10.1093/annonc/mdz135
27. Hamanishi J, Takeshima N, Katsumata N, et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA). J Clin Oncol. 2021;39(33):3671–3681. doi:10.1200/JCO.21.00334
28. Porter RL, Matulonis UA. Checkpoint blockade: not yet NINJA status in ovarian cancer. J Clin Oncol. 2021;39(33):3651–3655. doi:10.1200/JCO.21.01886
29. Wu Z, Cui P, Tao H, et al. The synergistic effect of PARP inhibitors and immune checkpoint inhibitors. Clin Med Insights Oncol. 2021;15:1179554921996288. doi:10.1177/1179554921996288
30. Shen J, Zhao W, Ju Z, et al. PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res. 2019;79(2):311–319. doi:10.1158/0008-5472.CAN-18-1003
31. Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. doi:10.1158/1078-0432.CCR-16-3215
32. Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor Durvalumab in combination with poly (ADP-ribose) polymerase inhibitor Olaparib or vascular endothelial growth factor Receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation, Phase I study. J Clin Oncol. 2017;35(19):2193–2202. doi:10.1200/JCO.2016.72.1340
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.