Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Relationship of Serum Bile Acids with Fat Deposition in the Pancreas, Liver, and Skeletal Muscle
Authors Al-Ani Z, Ko J, Petrov MS
Received 19 June 2023
Accepted for publication 10 August 2023
Published 16 August 2023 Volume 2023:16 Pages 137—146
DOI https://doi.org/10.2147/CEG.S422995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Santosh Shenoy
Zena Al-Ani, Juyeon Ko, Maxim S Petrov
Department of Surgery, Faculty of Medical and Health Sciences, The University of Auckland, Auckland, New Zealand
Correspondence: Maxim S Petrov, Email [email protected]
Introduction: Ectopic fat deposition is well appreciated as a key contributor to digestive and liver diseases. Bile acids have emerged as pleiotropic signalling molecules involved in numerous metabolic pathways. The aim was to study the associations of bile acids with ectopic fat deposition and lipid panel.
Methods: A single 3.0 Tesla magnetic resonance imaging scanner was employed to measure fat deposition in the pancreas, liver, and skeletal muscle in 76 adults. Blood samples were drawn to determine total bile acids and lipid panel. Linear regression analyses were run, taking into account age, sex, body mass index, and other covariates.
Results: The studied ectopic fat depots were not significantly associated with levels of total bile acids in serum. Total bile acids were significantly associated high-density lipoprotein cholesterol – consistently in both the unadjusted (p = 0.018) and all adjusted models (p = 0.012 in the most adjusted model). Low-density lipoprotein cholesterol, total cholesterol, and triglycerides were not significantly associated with total bile acids in both the unadjusted and all adjusted models.
Conclusion: Fat deposition in the pancreas, liver, and skeletal muscle is not associated with circulating levels of total bile acids. High-density lipoprotein cholesterol is the only component of lipid panel that is associated with total bile acids.
Keywords: bile acids, cholesterol, triglycerides, intra-pancreatic fat, skeletal muscle fat, intra-hepatic fat
Introduction
Gastrointestinal, pancreatic, and liver diseases have a considerable global burden, which is projected to further increase.1–3 As complex, multi-faceted, and non-communicable diseases, their development often involves metabolic derangements and low-grade chronic inflammation.4,5 Excess fat deposition in the pancreas, liver, and skeletal muscle are exemplar metabolic derangements that occur in individuals with pancreatic and liver diseases.6–11 Further, ectopic deposition not infrequently plays an important role in the development of non-communicable diseases beyond the digestive system.12–14
Bile acids are amphipathic steroid molecules synthesised in the liver from cholesterol and excreted into bile.15 Studies into the beneficial effects of bariatric surgery consistently showed the importance of bile acid signaling in resultant metabolic improvements.16 There is growing appreciation that bile acids have the potential in the treatment of digestive and liver disorders, as signaling molecules acting through the Takeda G-protein receptor 5 and farnesoid X receptor.17 This is in addition to their classic role as an emulsifier of lipids and fat-soluble vitamins in the gastrointestinal tract.16 Given the detergent properties of bile acids, their overload can be harmful (eg, cholestatic liver disease) because of activation of pro-inflammatory and oxidative stress pathways.17 High circulating levels of total bile acids were reported in people with fatty liver disease.18 Changes in bile acids metabolism in people with excess deposition of fat in the pancreas or skeletal muscle have been sparsely investigated.4,19
The primary aim was to investigate the associations between total bile acids and fat deposition in the pancreas, liver, and skeletal muscle. The secondary aim was to study the associations between total bile acids and lipid panel.
Methods
Study Design
The present cross-sectional study was part of the ARIES project.20–23 Adults with a history of acute pancreatitis who gave informed consent to undergo follow-up with a view to identifying metabolic derangements after hospitalisation were eligible for the project. The exclusion criteria were detailed elsewhere.24–26
Measurement of Serum Bile Acids and Lipid Panel
The tertiary referral medical laboratory of Auckland City Hospital (Auckland, New Zealand) measured total bile acids and the lipid panel, which included total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides.27 Serum bile acids were measured in serum (derived from venous blood samples collected in a fasted state) and reported in umol/L. LDL cholesterol was calculated using the 2020 Sampson formula.28
Measurement of Ectopic Fat
Abdominal magnetic resonance images at 3.0 Tesla were acquired specifically for the purpose of the ARIES project, using the same MAGNETOM Skyra scanner (Siemens, Erlangen, Germany) for all study participants. Two assessors post-processed the mages and measured intra-pancreatic fat deposition (IPFD) and skeletal muscle fat deposition (SMFD) independently. Intra-hepatic fat deposition (IHFD) was measured by a single assessor. IPFD represented the average of intra-organ fat in two slices with the best visualisation of the whole pancreas in a series of 5 mm slices.29 A single axial slice at the lower endplate of L3 vertebra was used to measure intra-skeletal fat area of erector spinae muscles on T1-weighted images.30 Single-voxel spectroscopy (with no correction for relaxation effects) was used to measure intra-hepatic fat deposition, as described elsewhere.30,31
Measurement of Covariates
Data on smoking status and alcohol consumption were collected at the time of magnetic resonance image acquisition in the form of an online questionnaire.32–34 Weight and body mass index (BMI) were measured according to the standard protocol.35 Glycated haemoglobin was measered at the tertiary referral medical laboratory of Auckland City Hospital and reported in mmol/mol.
Statistical Analysis
Data were analysed statistically with the use of SPSS (SPSS Inc., Chicago, IL, USA). Continuous and categorical variables were presented as median (and interquartile range) and frequency (and percentage), respectively. If the normality assumption was not met, data were log-transformed. Two tiers of linear regression analyses were conducted. The first analysis investigated the associations of serum bile acids with IPFD, IHFD, and SMFD. A total of 4 models were built as follows: model 1 was the unadjusted model; model 2 was adjusted for age, sex, and ethnicity; model 3 was adjusted for age, sex, ethnicity, and BMI; and model 4 was adjusted for age, sex, ethnicity, BMI, glycated haemoglobin, and triglycerides. The second analysis investigated the associations of serum bile acids with the components of lipid panel. The same statistical models were built, with the exception of model 4 – triglycerides were omitted to avoid collinearity. Serum bile acids were used as the dependent variable in all the above analyses. Findings were reported as β coefficients (ie, the degree of change in the dependent variable for every 1-unit of change in the independent variable), along with 95% confidence intervals and p values.
Results
Study Population
A total of 76 participants met the eligibility criteria. Their detailed characteristics are presented in Table 1. The mean (± SE) value of serum bile acids was 8.6 umol/L (±0.6 umol/L). The inter-assessor concordance of IPFD measurements was 0.968 (95% confidence intervals, 0.951–0.981) whereas the inter-assessor concordance of SMFD measurements was 0.984 (95% confidence intervals, 0.975–0.990) (Figure 1).
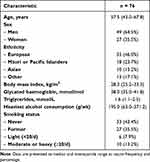 |
Table 1 Characteristics of Study Participants |
Relationship Between Serum Bile Acids and Ectopic Fat
The mean (± SE) percentages of ectopic fat depots in the study cohort were as follows: IPFD – 9.4% (± 0.2%), IHFD – 10.3% (± 1.2%), and SMFD – 15.5% (± 0.8%). There were no statistically significant associations between serum bile acids and IPFD, IHFD, and SMFD in both the unadjusted and adjusted models (Figure 2). Table 2 provides the detailed results.
 |
Table 2 Relationship of Bile Acids with Ectopic Fat |
Relationship Between Serum Bile Acids and Lipid Panel
The mean (± SE) levels of the components of the lipid panel were as follows: total cholesterol – 4.9 (± 0.16) mmol/L, HDL cholesterol – 1.3 (± 0.04) mmol/L, LDL cholesterol – 103.5 (± 4.7) mmol/L, and triglycerides – 2.5 (± 0.42) mmol/L. There was a statistically significant association between serum bile acids and HDL cholesterol in both the unadjusted (p=0.018) and all the adjusted models (p=0.014, p=0.013, and p=0.012 in models 2, 3, and 4, respectively). There were no statistically significant associations between serum bile acids and total cholesterol, LDL cholesterol, and triglycerides in both the unadjusted and adjusted models (Table 3).
 |
Table 3 Relationship of Bile Acids with the Components of Lipid Panel |
Discussion
The present study was the first to investigate the relationship between bile acids in serum and three ectopic fat depots (IPFD, IHFD, and SMFD) in the same individuals. Measurements of IPFD and SMFD were completed by two independent assessors, with excellent concordance demonstrated. Moreover, several covariates were adjusted for in the statistical analyses, including age, sex, ethnicity, BMI, glycated haemoglobin, and triglycerides. The main finding was the absence of statistically significant associations between total bile acids and the three ectopic fat depots. At the same time, a statistically significant positive association was observed between total bile acids and HDL cholesterol, consistently across all the statistical models.
In humans, bile acids are predominantly synthesised from cholesterol (accounting for approximately a half of daily cholesterol turnover in humans), subsequently conjugated in the liver with glycine or taurine.17 After being secreted into the duodenum, they are converted into secondary bile acids by intestinal bacteria, reabsorbed, and (mostly) recycled via the enterohepatic circulation.36 As the transfer of HDL cholesterol to hepatocytes is physiologically a key determinant of bile acids levels,17,36 the presence of a significant positive association between HDL cholesterol and bile acids in the present study (observed consistently in both the unadjusted and all the adjusted models) demonstrated the internal validity of our findings. Given that decreased (not increased) levels of HDL cholesterol have clinical implications as a risk factor for cardiovascular disease, decreased levels of bile acids could be viewed as a harbinger of metabolic derangements. A 2017 COSMOS meta-analysis showed decreased HDL cholesterol strongly correlated with increased IPFD (weighted mean correlation −0.33).19 A 2019 original COSMOS study demonstrated that the total cholesterol/HDL cholesterol ratio was significantly positively associated with IPFD, consistently across all statistical models.27 Although no significant association between bile acids and IPFD was observed in the present study, it is conceivable that the relationship between them is intricate and involves mediation by HDL cholesterol (and possibly by gut microbiota and other mediators).
Another explanation for the lack of statistically significant association between bile acids and IPFD (as well as IHFD and SMFD) is centred on the fact that total, not individual, bile acids were investigated in the present study. As the total pool includes distinct individual compounds (primary bile acids produced via the acidic or neutral pathway with the involvement of more than dozen liver enzymes, conjugated primary bile acids, secondary bile acids, conjugated secondary bile acids) with unique and at times opposite effects,36 a more nuanced analysis of individual bile acids and their derivatives may provide deeper insights into their relationship with ectopic fat depots.5 Two recent untargeted metabolomics studies (both using liquid chromatography–mass spectrometry) are worth mentioning in this regard.37,38 In one 2021 study,37 taurodeoxycholate – a bile acid conjugate – was significantly associated with IPFD (but not IHFD). This was independent of total body fat and over covariates. In the other 2021 study,38 sulfolithocholate – a major detoxified form of lithocholic bile acid – was significantly associated with IPFD (but not IHFD). Again, this was independent of total body fat and over covariates. Importantly, the above bile acids metabolites were not related to diabetes as either people with diabetes were excluded or fasting plasma glucose of study participants was statistically accounted for.37,38 The above findings suggest that there are likely to be differences in metabolomic signatures between ectopic fat depots and it is likely that this includes individual bile acids and their derivatives.4,5
Limitations of this study include its cross-sectional design, as no causal inference can be made from it.39–42 Longitudinal studies are now warranted to investigate temporal changes in bile acids and ectopic fat deposition in order to establish causality.43,44 As the study was the first to investigate total bile acids in the context of IPFD and SMFD,5 we were unable to estimate the required sample size prior to the commencement of the study. However, the presented findings will facilitate power calculation in future research. Also, the present study included people with a history of acute pancreatitis,45,46 as the project focusing on this population had received funding.47,48 Nevertheless, a complete clinical resolution was observed in all study participants at the time of magnetic resonance image acquisition.20–23 The protocol to measure IHFD in the present study might have been suboptimal as we did not use T1 and T2 correction of water signal and lipid signal from single echo magnetic resonance spectroscopy data. Future studies may need to do this with individually measured relaxation times (especially, if individuals with pancreatic fibrosis or iron overload are included). Last, bile acids were measured in serum, which is known to represent only a fraction of bile acids in the body.5 Moreover, the gut microbiota was not studied in the present study. The microbiota is known to transform bile acids (by means of deconjugation, dehydroxylation, epimerisation, and oxidation) and this may contribute to metabolic derangements.49,50 More comprehensive studies are warranted to investigate the role of bile acids in the context of ectopic fat deposition and the influence of gut dysbiosis.
In conclusion, the present study demonstrated that reduced levels of HDL cholesterol are significantly associated with decreased circulating levels of total bile acids. This was independent of BMI, glycated haemoglobin, and other covariates. By contrast, other component of the lipid panel were not significantly associated with levels of total bile acids. Also, there were no significant associations of total bile acids with IPFD, IHFD, and SMFD (after accounting for BMI, glycated haemoglobin, and other covariates).
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the approved by the Health and Disability Ethics Committee (13/STH/182).
Acknowledgment
The study was part of the Clinical and epidemiOlogical inveStigations in Metabolism, nutritiOn, and pancreatic diseaseS (COSMOS) programme.
Funding
COSMOS is supported, in part, by the Royal Society of New Zealand (Rutherford Discovery Fellowship to Professor Max Petrov), which played no role in the study design; collection, analysis, or interpretation of data, or writing of the manuscript.
Disclosure
The authors declare no conflict of interest.
References
1. Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45–55. doi:10.1016/S2468-1253(16)30004-8
2. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2021. Gastroenterology. 2022;162:621–644. doi:10.1053/j.gastro.2021.10.017
3. Cho J, Petrov MS. Pancreatitis, pancreatic cancer, and their metabolic sequelae: projected burden to 2050. Clin Transl Gastroenterol. 2020;11(11):e00251. doi:10.14309/ctg.0000000000000251
4. Petrov MS, Taylor R. Intra-pancreatic fat deposition: bringing hidden fat to the fore. Nat Rev Gastroenterol Hepatol. 2022;19(3):153–168. doi:10.1038/s41575-021-00551-0
5. Petrov MS. Fatty change of the pancreas: the Pandora’s box of pancreatology. Lancet Gastroenterol Hepatol. 2023;8(7):671–682. doi:10.1016/S2468-1253(23)00064-X
6. Singh RG, Yoon HD, Wu LM, Lu J, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its clinical relevance: a systematic review, meta-analysis, and meta-regression. Metabolism. 2017;69:1–13. doi:10.1016/j.metabol.2016.12.012
7. Singh RG, Nguyen NN, Cervantes A, Kim JU, Stuart CE, Petrov MS. Circulating levels of lipocalin-2 are associated with fatty pancreas but not fatty liver. Peptides. 2019;119:170117. doi:10.1016/j.peptides.2019.170117
8. Singh RG, Nguyen NN, Cervantes A, Alarcon Ramos GC, Cho J, Petrov MS. Associations between intra-pancreatic fat deposition and circulating levels of cytokines. Cytokine. 2019;120:107–114. doi:10.1016/j.cyto.2019.04.011
9. Modesto EA, Ko J, Stuart CE, Bharmal SH, Cho J, Petrov MS. Reduced skeletal muscle volume and increased skeletal muscle fat deposition characterize diabetes in individuals after pancreatitis: a magnetic resonance imaging study. Diseases. 2020;8(3):E25. doi:10.3390/diseases8030025
10. Nguyen NN, Singh RG, Petrov MS. Association between intrapancreatic fat deposition and the leptin/ghrelin ratio in the fasted and postprandial states. Ann Nutr Metab. 2022;78(1):14–20. doi:10.1159/000520068
11. Ko J, Sequeira IR, Skudder-Hill L, Cho J, Poppitt SD, Petrov MS. Metabolic traits affecting the relationship between liver fat and intra-pancreatic fat: a mediation analysis. Diabetologia. 2023;66(1):190–200. doi:10.1007/s00125-022-05793-4
12. Kimita W, Ko J, Li X, Bharmal SH, Petrov MS. Associations between iron homeostasis and pancreatic enzymes after an attack of pancreatitis. Pancreas. 2022;51(10):1277–1283. doi:10.1097/MPA.0000000000002195
13. Bharmal SH, Ko J, Kimita W, Cho J, Petrov MS. Factors affecting the circulating levels of oxyntomodulin in health and after acute pancreatitis. Pancreas. 2022;51(7):774–783. doi:10.1097/MPA.0000000000002114
14. Petrov MS. Post-pancreatitis diabetes mellitus: prime time for secondary disease. Eur J Endocrinol. 2021;184:R137–R149. doi:10.1530/EJE-20-0468
15. Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi:10.1038/nature13135
16. Cole AJ, Teigen LM, Jahansouz C, Earthman CP, Sibley SD. The influence of bariatric surgery on serum bile acids in humans and potential metabolic and hormonal implications: a systematic review. Curr Obes Rep. 2015;4(4):441–450. doi:10.1007/s13679-015-0171-x
17. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–191. doi:10.1152/physrev.00010.2008
18. Ferslew BC, Xie G, Johnston CK, et al. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60(11):3318–3328. doi:10.1007/s10620-015-3776-8
19. Singh RG, Yoon HD, Poppitt SD, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its biomarkers: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(8):e2918. doi:10.1002/dmrr.2918
20. Ko J, Kimita W, Skudder-Hill L, et al. Dietary carbohydrate intake and insulin traits in individuals after acute pancreatitis: effect modification by intra-pancreatic fat deposition. Pancreatology. 2021;21(2):353–362. doi:10.1016/j.pan.2021.01.018
21. Ko J, Skudder-Hill L, Cho J, Bharmal SH, Petrov MS. The relationship between abdominal fat phenotypes and insulin resistance in non-obese individuals after acute pancreatitis. Nutrients. 2020;12(9):2883. doi:10.3390/nu12092883
22. Ko J, Skudder-Hill L, Tarrant C, Kimita W, Bharmal SH, Petrov MS. Intra-pancreatic fat deposition as a modifier of the relationship between habitual dietary fat intake and insulin resistance. Clin Nutr. 2021;40(7):4730–4737. doi:10.1016/j.clnu.2021.06.017
23. Ko J, Skudder-Hill L, Priya S, Kimita W, Bharmal SH, Petrov MS. Associations between intra-pancreatic fat deposition, pancreas size, and pancreatic enzymes in health and after an attack of acute pancreatitis. Obes Facts. 2022;15(1):70–82. doi:10.1159/000519621
24. Singh RG, Nguyen NN, DeSouza SV, Pendharkar SA, Petrov MS. Comprehensive analysis of body composition and insulin traits associated with intra-pancreatic fat deposition in healthy individuals and people with new-onset prediabetes/diabetes after acute pancreatitis. Diabetes Obes Metab. 2019;21(2):417–423. doi:10.1111/dom.13523
25. Singh RG, Cervantes A, Kim JU, et al. Intrapancreatic fat deposition and visceral fat volume are associated with the presence of diabetes after acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2019;316(6):G806–G815. doi:10.1152/ajpgi.00385.2018
26. DeSouza SV, Priya S, Cho J, Singh RG, Petrov MS. Pancreas shrinkage following recurrent acute pancreatitis: an MRI study. Eur Radiol. 2019;29(7):3746–3756. doi:10.1007/s00330-019-06126-7
27. Singh RG, Nguyen NN, Cervantes A, Cho J, Petrov MS. Serum lipid profile as a biomarker of intra-pancreatic fat deposition: a nested cross-sectional study. Nutr Metab Cardiovasc Dis. 2019;29(9):956–964. doi:10.1016/j.numecd.2019.06.003
28. Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5(5):540–548. doi:10.1001/jamacardio.2020.0013
29. Skudder-Hill L, Sequeira IR, Cho J, Ko J, Poppitt SD, Petrov MS. Fat distribution within the pancreas according to diabetes status and insulin traits. Diabetes. 2022;71(6):1182–1192. doi:10.2337/db21-0976
30. Stuart CE, Ko J, Modesto AE, et al. Implications of tobacco smoking and alcohol consumption on ectopic fat deposition in individuals after pancreatitis. Pancreas. 2020;49(7):924–934. doi:10.1097/MPA.0000000000001600
31. Crane JC, Olson MP, Nelson SJ. SIVIC: open-source, standards-based software for DICOM MR spectroscopy workflows. Int J Biomed Imaging. 2013;2013:169526. doi:10.1155/2013/169526
32. Petrov MS. Panorama of mediators in postpancreatitis diabetes mellitus. Curr Opin Gastroenterol. 2020;36(5):443–451. doi:10.1097/MOG.0000000000000654
33. Stuart CE, Singh RG, Alarcon Ramos GC, et al. Relationship of pancreas volume to tobacco smoking and alcohol consumption following pancreatitis. Pancreatology. 2020;20(1):60–67. doi:10.1016/j.pan.2019.10.009
34. Stuart CE, Ko J, Alarcon Ramos GC, Modesto AE, Cho J, Petrov MS. Associations between cannabis use, abdominal fat phenotypes and insulin traits. J Clin Med Res. 2020;12(6):377–388. doi:10.14740/jocmr4165
35. Cervantes A, Singh RG, Kim JU, DeSouza SV, Petrov MS. Relationship of anthropometric indices to abdominal body composition: a multi-ethnic New Zealand magnetic resonance imaging study. J Clin Med Res. 2019;11(6):435–446. doi:10.14740/jocmr3820
36. Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–1212.
37. Lind L, Salihovic S, Risérus U, et al. The plasma metabolomic profile is differently associated with liver fat, visceral adipose tissue, and pancreatic fat. J Clin Endocrinol Metab. 2021;106(1):e118–e129. doi:10.1210/clinem/dgaa693
38. Wu ZE, Fraser K, Kruger MC, et al. Untargeted metabolomics reveals plasma metabolites predictive of ectopic fat in pancreas and liver as assessed by magnetic resonance imaging: the TOFI_Asia study. Int J Obes. 2021;45(8):1844–1854. doi:10.1038/s41366-021-00854-x
39. Al-Ani Z, Ko J, Petrov MS. Intra-pancreatic fat deposition across the pancreatitis spectrum and the influence of gut hormones. Dig Liver Dis. 2023;55(8):1081–1090. doi:10.1016/j.dld.2023.02.013
40. Abunahel BM, Pontre B, Ko J, Petrov MS. Towards developing a robust radiomics signature in diffuse diseases of the pancreas: accuracy and stability of features derived from T1-weighted magnetic resonance imaging. J Med Imaging Radiat Sci. 2022;53(3):420–428. doi:10.1016/j.jmir.2022.04.002
41. Ko J, Al-Ani Z, Long K, Tarrant C, Skudder-Hill L, Petrov MS. Intra-pancreatic, liver, and skeletal muscle fat depositions in first attack of acute pancreatitis versus health. Am J Gastroenterol. 2022;117(10):1693–1701. doi:10.14309/ajg.0000000000001951
42. Abunahel BM, Pontre B, Petrov MS. Effect of gray value discretization and image filtration on texture features of the pancreas derived from magnetic resonance imaging at 3T. J Imaging. 2022;8(8):220. doi:10.3390/jimaging8080220
43. Bharmal SH, Kimita W, Ko J, Petrov MS. Cytokine signature for predicting new-onset prediabetes after acute pancreatitis: a prospective longitudinal cohort study. Cytokine. 2022;150:155768. doi:10.1016/j.cyto.2021.155768
44. Bharmal SH, Kimita W, Ko J, Petrov MS. Pancreatic and gut hormones as predictors of new-onset prediabetes after non-necrotising acute pancreatitis: a prospective longitudinal cohort study. Endocr Connect. 2021;10(7):715–724. doi:10.1530/EC-21-0229
45. Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–820. doi:10.1053/j.gastro.2010.06.010
46. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–184. doi:10.1038/s41575-018-0087-5
47. Bharmal SH, Cho J, Ko J, Petrov MS. Glucose variability during the early course of acute pancreatitis predicts two-year probability of new-onset diabetes: a prospective longitudinal cohort study. United Eur Gastroenterol J. 2022;10(2):179–189. doi:10.1002/ueg2.12190
48. Bharmal SH, Cho J, Alarcon Ramos GC, et al. Trajectories of glycaemia following acute pancreatitis: a prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol. 2020;55(8):775–788. doi:10.1007/s00535-020-01682-y
49. Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9(1):140. doi:10.1186/s40168-021-01101-1
50. Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21(4):236–247. doi:10.1038/s41579-022-00805-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


