Back to Journals » International Journal of General Medicine » Volume 14
Relationship of Histopathological Representation of Excessive Proliferation of Trophoblast Cells with the Possibility of Malignant Events After Complete Hydatidiform Mole
Authors Tobing M, Arabia F, Hidayat Y, Mantilidewi K
Received 16 July 2020
Accepted for publication 23 March 2021
Published 18 May 2021 Volume 2021:14 Pages 1899—1904
DOI https://doi.org/10.2147/IJGM.S271635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Maringan Tobing,1,2 Futiha Arabia,1,2 Yudi Hidayat,1,2 Kemala Mantilidewi1,2
1Faculty of Medicine, Padjadjaran University, Bandung, West Java, Indonesia; 2Dr. Hasan Sadikin Central General Hospital, Bandung, West Java, Indonesia
Correspondence: Kemala Mantilidewi Tel +6281220828092
Email [email protected]
Introduction: Fifteen to twenty percent of the patients with complete hydatidiform mole transform malignancy into gestational trophoblastic tumors. The marked proliferation of trophoblastic cells is one of the characteristics that determines high risk for the occurrence of post-hydatidiform mole malignancy. The objective of the study was to analyze the histopathologic feature of the marked proliferation of trophoblastic cells as a role in post-hydatidiform mole malignancy that can be used as a determinant of the risk of malignancy post-hydatidiform mole.
Methods: The method of the study was analytical observational with a case–control study design. The data were taken retrospectively from medical records of patients with a post-complete hydatidiform mole malignancy and patients who do not develop post-complete hydatidiform mole malignancy (n = 34). The study took place in the Department of Anatomical Pathology Laboratorium at Dr. Hasan Sadikin Hospital, Bandung, Indonesia.
Results: The results showed a highly significant difference with the histopathologic characteristics of marked trophoblastic cell proliferation in post-complete hydatidiform mole malignancy, reaching up to 73.5%. In contrast, the difference between those who do not develop malignancy was 11.8%. The odds ratio (OR) was 20.83, with an interpretation that patients with a complete hydatidiform mole with the histopathological feature of marked trophoblastic cell proliferation had a risk of developing into malignancy 20.83 times higher compared to cases without marked trophoblastic cell proliferation.
Conclusion: The conclusion of the study was there is a significant correlation between marked trophoblastic cell proliferation with the incidence of post-complete hydatidiform mole malignancy.
Keywords: post-complete hydatidiform mole malignancy, histopathologic trophoblastic cells, cells proliferation
Introduction
Trophoblast disease is still commonly found in various parts of the world, and high incidence rates are found in developing countries, including Indonesia. The incidence of hydatidiform mole in Western countries occurs within the ranges of 1:1000 to 1:2500 pregnancies, while choriocarcinoma cases occur between 1:14,000 and 1:40,000 pregnancies.1
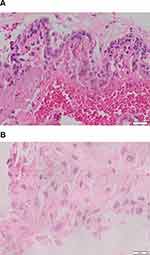 |
Figure 1 (A) Area of excessive proliferation (magnification 400×). (B) Syncytiotrophoblast proliferation (magnification 400×). |
The incidence of hydatidiform molar pregnancy in Japan (2/1000 pregnancies) is three times greater than the incidence reported in Europe or North America (0.6–1.1/1000 pregnancies). In Taiwan, 1/125 pregnancies are molar pregnancies, while in the United States, the incidence occurs in 1/1500 live births. Setyorini reported that within 181 patients with gestational tumors who were treated in 20 hospitals in Bandung in the period from January 1 to December 31, 1998, 122 were patients with cases of hydatidiform mole, and 59 were patients with cases of malignant trophoblastic tumors among 19,242 deliveries. The average incidence of hydatidiform mole in all hospitals in Bandung is 1:427 deliveries and the incidence of malignant trophoblastic tumors is 1:882 deliveries.2
Most hydatidiform mole patients will be cured after treatment. Still, approximately 15–20% will undergo a malignant transformation into gestational trophoblastic tumor (GTT).1,3,4 The risk factors that are thought to play a role in the occurrence of malignancy after hydatidiform mole are not known yet. Several epidemiological studies report that the risk of choriocarcinoma increases with age.5,6 Martaadisoebrata says that the occurrence of choriocarcinoma after hydatidiform mole at the age of 35 years is higher, which is approximately 23.1% compared to <35 years, which is around 17.9%.7 Similarly, Jayamasa et al found that patients aged >35 years old with hydatidiform mole have a risk of experiencing malignancy 2.1–3.8 times greater than patients aged <35 years.8 In addition to age, several other risk factors for malignancy were parity, uterine size, presence of lutein cysts, suspicious histopathological features, and high βhCG levels above 100,000 mIU/mL.1,2,5–8
Currently, the management of hydatidiform mole patients includes improving general conditions, evacuation of molar tissue, prevention of malignancy, and early detection of malignancy after mole evacuation.9,10 Gestational trophoblastic tumors are mostly preceded by hydatidiform mole, where 15% are preceded by complete hydatidiform mole and 1% by partial hydatidiform mole.11,12 There is a high-risk group for post-hydatidiform mole malignancies such as people aged 35 years, uterine size greater than 20 weeks, βhCG levels above 100,000 mIU/mL, presence of lutein cysts, history of second pregnancy with mole, post-evacuation bleeding, presence of lungs involvement, and complications of preeclampsia, histopathological features of excessive trophoblastic hyperplasia.11,12
Hydatidiform mole has been known as a benign trophoblastic disorder caused by the process of trophoblast cell proliferation, while choriocarcinoma is a malignant form with a depiction of metastasis. However, there is still little science that discusses the role of genes at the molecular level and regulates the process of proliferation or trophoblast differentiation in this disease.11
Trophoblast cell activity that does not disappear spontaneously after an evacuation can develop into gestational trophoblastic tumor. Although complete hydatidiform mole has a higher rate of occurrence to become a gestational trophoblastic tumor, this representation cannot be used as the same prediction for everyone, and it is not known yet what factors regulate such change.12,13
Based on this background, the central theme in this study is that 15–30% hydatidiform mole can develop into gestational trophoblastic tumors (GTT). The pathogenesis of post-hydatidiform malignancy is not known yet.12,13 The pathogenesis of post-hydatidiform malignancy is also influenced by clinical risk factors, including age ≥35 years, uterine size ≥20 weeks, the presence of unilateral/bilateral lutein cysts, βhCG levels ≥100,000 mIU/mL, and suspicious histopathological features have been used as variables of clinical risk factors related to the incidence of post-hydatidiform malignancies. The proliferation of trophoblast cells is influenced by several factors, including angiogenesis and cell apoptosis. βhCG levels are the predictor for the occurrence of hydatidiform mole malignancy that has been used to date. In this study, the researchers thought that this study should link βhCG levels with excessive proliferation of trophoblast cells. However, it is important to note that not all trophoblast cell types produce βhCG, and the cells that can produce βhCG are syncytiotrophoblastic cells, and it is difficult to study anatomical pathology. Therefore other help might be required to enforce it. In the case of post-hydatidiform malignancies almost all showed an increase in βhCG levels, but in cases where there was an increase in βhCG levels, not all showed trophoblast cell features with excess proliferation in the histopathological picture. Therefore in this study, the researchers analyzed the histopathological picture of the proliferation of trophoblast cells in complete hydatidiform mole as a risk factor for the occurrence of malignancy after a complete hydatidiform mole.
Methods
The subjects of this study were all patients with complete hydatidiform mole (CHM) and gestational trophoblastic tumors (GTT) from 2007 to 2011 who had been treated at the Dr. Hasan Sadikin Hospital. The inclusion criteria were complete hydatidiform mole patients who had undergone dilation and curettage at Dr. Hasan Sadikin Hospital and who had histopathological results.
This study was an observational analytic study with a case–control study design. Data were taken retrospectively from the medical record of complete hydatidiform mole patients who experienced malignancy and who did not experience malignancy. The sample size is determined by consecutive sampling until the minimum sample size is met.
All results of the curettage of the patient with complete hydatidiform mole were made into block paraffin preparations. Then, these paraffin blocks are examined in the trophoblast cells to determine whether the cells undergo excessive proliferation or not.
After that, trophoblast cells that either proliferate excessively or not are compared with the incidence of malignancy post-complete hydatidiform mole.
Operational definition of complete hydatidiform post-mole malignancy/gestational trophoblastic tumor is a malignant form of trophoblast. Gestational trophoblastic tumor can include invasive mole, choriocarcinoma, placental site trophoblastic tumors, or epithelioid trophoblastic tumors determined by an anatomical pathologist.
Complete hydatidiform mole tissue taken from paraffin preparation blocks was examined histopathologically on trophoblast cells. The tissue is determined whether it runs into excessive proliferation or not. Proliferative groups of trophoblast cells outside the chorionic villi with four or more layers of thickness and mitosis of cells found in number more than 4 in 10 visual fields are classified as excessive proliferation. While categorized as a no excessive proliferation groups if no trophoblast cells were found outside the corialis villi with four or more layers of thickness and no mitotic cells were found in more than 4 per 10 visual fields.
The study was approved by the Research Ethics Committee, Faculty of Medicine Padjadjaran University/Dr. Hasan Sadikin Hospital, Bandung, Indonesia, No. 173/UN6.C2.1.2/KEPK/PN/2014 all study participants gave informed consent, patients consent to participate was written. All authors hereby declare that all patients have been examined in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Results
Research on histopathological analysis of trophoblast cells with excess cell proliferation in complete hydatidiform mole patients was performed in 34 cases of hydatidiform mole malignancy (GTT) and 34 cases that did not become malignant (hydatidiform mole) (Figure 1A and B).
Characteristics and histopathological analyses of the subjects were registered in the Department of Anatomical Pathology at Dr. Hasan Sadikin General Hospital, Bandung, Indonesia. The complete research results are presented in Table 1.
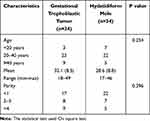 |
Table 1 Characteristics of Patients with Complete Hydatidiform Mole Transforming into Malignancy |
The characteristics of the study subjects based on age showed that the average age of patients who undergo malignancy (gestational trophoblastic tumor) was 32.1 years and the average age of those who did not undergo malignancy (hydatidiform mole) was 28.6 years. The number of patients under the age of 20 who underwent malignancy was 3; between the ages of 20 and 40, 22; and over the age of 40, 9. The number of patients who did not have a malignancy (hydatidiform mole) under 20 years of age was seven people; between the ages of 20 and 40, 22; and over the age of 40, 9 people.
The characteristics of the study subjects based on parity showed that there were 17 patients with malignancy (gestational trophoblastic tumor) below 1 parity, 8 patients with 2–3 parity, and 4 patients with 4 parity. The characteristics of the study subjects based on parity showed that 22 patients did not undergo malignancy (hydatidiform mole) below one parity, 7 patients with 2–3 parity, and 5 patients with 4 parity. The characteristics of age and parity in the two study groups were not statistically significant (p>0.05).
Table 2 presents the correlation between the histopathologic features of trophoblast cells with post-hydatidiform mole malignancy with very significant results. In the case of malignancy after complete hydatidiform mole (GTT), the percentage of histopathological characteristics of the over-proliferation of trophoblast cells is 73.5%. In contrast, the percentage of the non-malignancy group is 11.8. The risk (Odds Ratio) is calculated, and the result is OR = 20.83. This means that patients with complete hydatidiform mole with histopathological characteristics of excessive trophoblast cell proliferation are at risk of developing gestational trophoblastic tumor after complete hydatidiform mole 20.83 times more compared with cases that have histopathological features of non-excessive trophoblast cells proliferation.
 |
Table 2 Correlation Between Histopathological Features of Trophoblastic Cells and the Occurrence of Malignancy Post-Complete Hydatidiform Mole |
Discussion
As mentioned in the ‘Íntroduction’ section, the purpose of this study is to analyze the histopathological feature of the excessive trophoblast cells proliferation that plays a role in the incidence of malignancy following complete hydatidiform mole of the period 2007–2011.
Compared to the total population of Indonesia in 2006, of 222,192,000 people, the number of women aged 15–64 years in reproductive age was 65,414,370 (49,86%), while the number of women who died of cancer was 4.3% of all causes of death due to the disease.14 The incidence ratio of hydatidiform mole is 1:150 childbirth in Southeast Asia.15,16 At Dr. Cipto Mangunkusumo Hospital in Jakarta, Indonesia, the ratio is quite high (1 in 77 pregnancies) especially for young women and old women, and approximately 15–20% experience malignancy in becoming gestational trophoblastic tumor, while the factors that cause changes in hydatidiform mole to gestational trophoblastic tumor are still under study.1,3,4
The Bracken MB study proclaimed that maternal age affects molar pregnancies. Women over 40 years of age have 5–10 times greater risks of suffering from complete hydatidiform mole.17 This is confirmed by the studies conducted by Acaia B, Parazzini F, and Vecchia which conclude that eggs in older women are more susceptible to abnormal fertilization that can result in complete hydatidiform moles.18 On the other hand, the studies of Graham IH, Fajardo AM, and Richard RL stated that they occur mostly at the age above 20–24 years. This is consistent with the largest population of pregnancies at reproductive age (20–35 years).19
The analysis results of our study in Table 1 for parity in the group of patients who undergo malignancy (GTT) show that 17 patients have 0–1 parity, 8 have 2–3 parity, and 8 have more than 4 parity. The result of parity in groups without malignancy shows that as many as 22 patients have 0–1 parity, 7 have 2–3 parity, and 5 have more than 4 parity. For parity mostly below ≤1; for gestational trophoblastic disease case 50% and hydatidiform mole 65%. This is not consistent with previous studies which stated that the incidence increased simultaneously with parity. The characteristics of age and parity in the two study groups were not statistically significant (p>0.05)
Gestational trophoblastic disease is a group of diseases originating from fetal khorion characterized by abnormal trophoblastic tissue proliferation.20 Martaadisoebrata found that 36.6% of mola patients who had become choriocarcinomas had an excessive proliferation of trophoblastic cells compared to 10% of those without malignancy.7 While Mukawi TJ, based on the results of studies on hydatidiform moles, suggests that complete hydatidiform moles with excessive hyperplasia tend to turn malignant in the subsequent course of the disease.21
Malignancy is characterized by the loss of growth control. The marker for cancer is the uncontrolled proliferation of cells in the cellular cycle and tumor cells undergo typical abnormalities in the genes that directly regulate these cell cycles.22 Normally, there are three classes of regulatory genes (regulatory genes), namely: growth-enhancing genes (proto-oncogenes), growth-inhibiting cancer suppressor genes (antioncogenes) and programmed cell death (apoptosis). Several genes that regulate cell function in the pathophysiology of hydatidiform mole are not clearly known yet.22
Oncoproteins can trigger cell growth and stimulate tumor suppressor genes that will function to block cell proliferation. Damage to this suppressor gene means the ability to restrain cell growth is disrupted. The increase in the number of cells is likely due to increased proliferation and increased cells caused by decreased apoptosis or decreased differentiation processes.23
Table 2 presents the relationship between histopathological features of trophoblast cells with malignancy post-hydatidiform mole which show very significant results, where the percentage in the case of malignancy post-complete hydatidiform mole with histopathological features of excessive trophoblastic cell proliferation is 73.5%; while in cases that did not experience a malignancy is 11.8%. The risk (odds ratio) is calculated, and the result is OR = 20.83. This means that patients with complete hydatidiform mole with histopathological characteristics of excessive trophoblast cell proliferation are at risk of developing gestational trophoblastic tumor after complete hydatidiform mole 20.83 times more compared with cases that have histopathological features of non-excessive trophoblast cells proliferation. This is in accordance with previous studies acknowledging that the excessive proliferation of trophoblast cell proliferation in complete hydatidiform mole is more likely to turn malignant in the subsequent course of the disease.
Conclusions
There is a significant relationship between histopathological features of excessive trophoblast proliferation and the occurrence of malignancy after a complete hydatidiform mole. The histopathological feature of the over-proliferation of trophoblast cells is a risk factor for the occurrence of malignancy after a complete hydatidiform mole.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Gestational trophoblastic diseases: report of a WHO scientific group [meeting held in Geneva from 6 to 10 December 1982]. World Health Organization; 1983.
2. Irianti S, Martaadisoebrata D, Anwar A. Studi epidemiologi penyakit trofoblas gestasional di kotamadya Bandung dan sekitarnya Denpasar: KOGI; 2000.
3. Goldstein DP. Worldwide controversies in gestational trophoblastic neoplasms. Int J Gynaecol Obstet. 1977;15(3):207–215. doi:10.1002/j.1879-3479.1977.tb00676.x
4. Curry SL, Hammond CB, Tyrey L, Creasman WT, Parker R. Hydatidiform mole: diagnosis, management, and long-term followup of 347 patients. Obstet Gynecol. 1975;45(1):1–8.
5. Ober WB. Choriocarcinoma: historical Notes. In: Gestational Trophoblastic Disease. New York: Springer-Verlag; 1987:1–7.
6. Hernandez E, Huh WK. Gestational trophoblastic neoplasia. Inglés) Obstetrics and Gynecology eMedicine com Consultado el. 2013;7.
7. Martaadisoebrata D. Problematik Penyakit Trofoblas Ditinjau Dari Segi Epidemiologi Serta Pengelolaan. Bandung: Universitas Padjadjaran; 1980.
8. Jayamasa KA, Dasuki D. Hubungan antara umur dan paritas terhadap degenerasi maligna mola hidatidosa. Bandung: PIT VIII POGI; 1992.
9. Martaadisoebrata D. Protokol Pengelolaan Penyakit Trofoblas Gestasional.
10. Andrijono. Penyakit trofoblas gestasional. Jakarta: Divisi Onkologi Departemen Obstetri Ginekologi FKUI; 2007.
11. Hancock BW, Berkowitz RS, Cole LA. Gestational Trophoblastic Disease: Clinical Features of Molar Pregnancies and Gestational Trophoblastic Neoplasia.
12. Martaadisoebrata D. Buku Pedoman Pengelolaan Penyakit Trofoblas Gestasional.
13. Steigrad SJ. Epidemiology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol. 2003;17(6):837–847. doi:10.1016/S1521-6934(03)00049-X
14. Aziz MF. Gynecological cancer in Indonesia. J Gynecol Oncol. 2009;20(1):8–10. doi:10.3802/jgo.2009.20.1.8
15. Cunningham FG, Leveno KG, Bloom SL, Hauth JC, Gilstrap LC, Wenstrom KD. Williams Obstetrics.
16. Moore LE. Hydatidiform mole; 2007.
17. Bracken MB. Incidence and aetiology of hydatidiform mole: an epidemiological review. BJOG. 1987;94(12):1123–1135. doi:10.1111/j.1471-0528.1987.tb02311.x
18. Acaia B, Parazzini F, La Vecchia C, Ricciardiello O, Fedele L, Candiani GB. Increased frequency of complete hydatidiform mole in women with repeated abortion. Gynecol Oncol. 1988;31(2):310–314. doi:10.1016/S0090-8258(88)80009-X
19. Graham I, Fajardo A, Richards R. Epidemiological study of complete and partial hydatidiform mole in Abu Dhabi: influence age and ethnic group. J Clin Pathol. 1990;43(8):661–664. doi:10.1136/jcp.43.8.661
20. Berkowitz RS, Goldstein DP. Gestational Trophoblastic Disease.
21. Ty M. Histogram DNA pada mola hidatidosa dengan hiperplasia berlebih sel-sel trofoblas. Jakarta: KONAS IAPI IX; 1987.
22. Fisher R, Hodges M. Genomic imprinting in gestational trophoblastic disease—a review. Placenta. 2003;24:S111–S8. doi:10.1053/plac.2002.0939
23. Kumar V, Fausto N, Mitchell R. Robbins Basic Pathology.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
