Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Relationship Between Sporadic Renal Cysts and Renal Function Detected by Isotope Renography in Type 2 Diabetes
Authors Li Y, Lou Q, Wen S , Zhou M, Xu D, Wang C, Liu X, Zhou L
Received 2 May 2022
Accepted for publication 3 August 2022
Published 10 August 2022 Volume 2022:15 Pages 2443—2454
DOI https://doi.org/10.2147/DMSO.S373120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Yanyan Li,1,* Qingqing Lou,2,* Song Wen,1 Mingyue Zhou,3 Dongxiang Xu,1 Chaoxun Wang,1 Xingdang Liu,2 Ligang Zhou1,4
1Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China; 2Department of Nuclear Medicine, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China; 3Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, CA, USA; 4Shanghai Key Laboratory of Vascular Lesions Regulation and Remodeling, Shanghai, 201399, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ligang Zhou, Department of Endocrinology, Shanghai Pudong Hospital, Fudan University, Shanghai, 201399, People’s Republic of China, Tel +8613611927616, Email [email protected]
Purpose: This study aimed to reveal the relationship between the volume of sporadic renal cysts and renal function in patients with type 2 diabetes (T2D).
Materials and Methods: One hundred and seventy-one patients that underwent renal imaging and other routine examinations at the Shanghai Pudong Hospital were included in this study. The Gates’ method of glomerular filtration rate (GFR) was measured by 99mTc-DTPA renal dynamic imaging in addition to the eGFR, calculated by the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI).
Results: Our results showed that BMI, total iGFR, and eGFR showed significant differences between patients with T2D with or without SRC (p < 0.05). Spearman correlation analysis showed that cyst volume was positively correlated with Scr and gender but not iGFR (p > 0.05). The total iGFR positively correlated with eGFR (r = 0.83, p < 0.0001) and negatively with Scr (r = − 0.78, p < 0.0001), age (r = − 0.43, p < 0.0001), duration of T2D (r = − 0.25, p = 0.001), and BMI (r = − 0.21, p = 0.006) but not gender (r = − 0.03, p = 0.668). The multilinear regression model revealed that gender (β = 0.346, p < 0.001), iGFR (β = − 0.705, p < 0.001), and serum uric acid (β = 0.195, p = 0.032) were independent predictors of Scr. Moreover, we observed a significant increase in Scr in males (p < 0.05). Finally, we found that the split kidney function reflected by iGFR and related parameters such as time to peak (PTT) and half time of excretion (excrete t1/2) did not mutually distinguish from each other significantly whether they are measured in patients with renal cysts or in those without renal cysts (p > 0.05).
Conclusion: Our preliminary results suggest that in T2D, SRCs may be a renal complication of diabetic nephropathy. Although we found that the patients with renal cysts may display reduced iGFR, the volume of simple cysts seems not to exacerbate renal insufficiency. Isotope renography is a useful tool to evaluate the split kidney functions in diabetic patients who acquire single-side cysts.
Keywords: type 2 diabetes, SRCs, kidney function, isotope glomerular filtration rate, cysts volume, gender disparity
Introduction
Type 2 diabetes (T2DM) is well-established to be highly prevalent in most economically developed nations, with a reported prevalence rate exceeding 10.9% in 2017.1 Diabetic nephropathy (DN) is one of the most common microvascular complications of T2DM, resulting in chronic renal insufficiency. In some cases, progression to end-stage renal disease may occur, limiting the patient’s selection of hypoglycemic drugs, increasing the risk of cardiovascular diseases (CVD), and affecting the quality of life. Diabetic nephropathy (DN) has been established as the leading cause of CKD and renal failure in developed countries. Over the past two decades, the morbidity and mortality associated with DN have been rising rapidly worldwide.2 The therapeutic approach in the clinic is often based on kidney function reflected by glomerular filtration rate (GFR). Many methods and equations are currently available for estimating the GFR, including blood sample creatinine-based estimated GFR by CKD-EPI equation or isotope GFR (iGFR), which has the advantage of evaluating the split kidney function when there is a decline in eGFR. The most commonly used isotope for measurement of iGFR is 51Cr-EDTA and 99mTc-DTPA, with clearance rates comparable to non-radiopharmaceutical markers such as inulin, iohexol, and iothalamate.3 Importantly, this method enables the detection of unilateral renal blood flow and kidney function, with more details available from the time-uptake curve. The whole procedure is non-invasive and does not require collecting blood and urine, taking approximately 20 min, and is not subject to interference by the diet.4
Sporadic renal cysts (SRCs) are the most common renal cystic diseases in adults. The prevalence of SRC varies by population, geographic region, and the imaging modality used. The prevalence of SRCs detected by ultrasound (US) in the general population was estimated as 5.0–20.8%, with advanced age as the main risk factor.5,6 Current evidence suggests that the prevalence of SRCs in Chinese is 7.2–10.5%, especially in males and the elderly.7–9 Most SRCs are asymptomatic, being incidental findings detected by abdominal ultrasonography or computed tomography during a medical check-up or when another medical condition is being evaluated. Simple renal cysts may not require treatment when asymptomatic. Nonetheless, some cysts tend to increase in size and can be sufficiently large to cause pain, hematuria, and/or urinary obstruction.10,11 Under such conditions, treatment such as surgery is necessary.12,13 It is widely thought that the size and the volume of SRCs influence renal function and determine the treatment plan. However, the relationship between the SRCs and renal function remains obscure. An increasing body of evidence suggests that SRC may be associated with deterioration of renal function.14 Herein, we employed isotope radiography, a well-established approach equivalent to the eGFR method, to explore split kidney function associated with SRCs.
Materials and Methods
Source of Inpatient Data
The information of patients with T2DM was collected from Shanghai Pudong Hospital’s inpatient information system. The subjects were inpatients with T2DM in the endocrinology department from June 2021 to December 2021 who underwent ultrasound examination prior to evaluation of renal function by eGFR and iGFR. Subjects with T1DM, polycystic kidney, and malignant renal cysts were excluded from the study. We also excluded the extremely conditions like severe cardiovascular and cerebral diseases, diabetic ketoacidosis, hyperglycemic hyperosmolar state, severe infection, etc. T2D diagnosis was established based on the World Health Organization (WHO) guidelines published in 1999.
Blood Sampling and Evaluation of Laboratory Data
We included laboratory data on diabetes status, kidney function, and thyroid function. After blood sampling, all parameters, including fasting blood glucose, c-peptide, HbA1C, eGFR, and creatinine, were evaluated.
99m Tc-DTPA Renal Dynamic Imaging
The patient demographics (age and gender) and their height and weight were documented. The patients were required to drink 300–500 mL of water and then empty their bladders 30 minutes before imaging. All patients underwent dynamic renal studies with 99mTc DTPA (with radiochemical purity of over 95%).
Using a large-field-of-view scintillation camera, dynamic imaging was performed in the supine position at a rate of acquisition of 1 frame/2 s for 1 minute and subsequently 1 frame/20 s for 19 minutes, with the kidneys and inferior border of the heart included in the imaged field. Using a GE Discovery NM/CT 670 ES SPECT/CT scanner equipped with a low-energy high-resolution collimator, peak energy of 140 keV, a 64×64 matrix, and a window width of 20%, dynamic scintigraphy was performed at the time of intravenous administration of 99mTc DTPA bolus, with a dose of activity 185 MBq of 99mTc-DTPA. Before and after injecting 99mTc-DTPA into patients, the radioactivity in the syringe was measured. The total injected dose was calculated by subtracting the post-count syringe activity from the pre-count syringe activity. The ROI (Region of Interest) over each kidney was manually assigned on the composite image 2 to 3 minutes after injection, and the net count for each kidney was calculated in the time frame 2 to 3 minutes after the tracer was injected. The Gates’ approach was used to automatically compute the GFR using commercially available software.
Statistical Analyses
Statistics analyses were performed in SPSS (IBM, version 26.0) and Prism (GraphPad, version 9.0). The independent sample t-test or two-way ANOVA was used to compare differences in levels of plasma C-peptide, HbA1c, total iGFR, split renal iGFR, and other parameters between different groups. Spearman correlation analyses were used to determine the relationships between gender disparity, duration, BMI, cyst volume, iGFR, time-to-peak (TTP), half time excretion (t1/2), and eGFR. Multilinear regression analyses were established to determine the predictors of iGFR and serum creatinine level change. For all analyses, a p-value <0.05 was statistically significant.
Results
Clinical Baseline Characteristics of T2D Patients with or without Renal Cysts
The characteristics of the study subjects are presented in Table 1, including the gender, age, diabetes duration, body mass index (BMI), glycosylated hemoglobin A1c (HbA1c), fasting C-peptide, and cyst volume.
 |
Table 1 Baseline Characteristics of the Study Participants |
The BMI, eGFR, and Total iGFR Significantly Differed Between T2D Patients with or without Renal Cysts
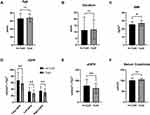
We first assessed the kidney function in both groups of patients based on iGFR and eGFR. Significant differences were found in total iGFR (no Cyst vs Cyst: 67.58± 24.86 mL/min*1.73m2 vs 57.84±20.76 mL/min*1.73m2, p=0.001), split kidney iGFR (left iGFR: no Cyst vs Cyst: 32.76±13.12 mL/min*1.73m2 vs 27.41±10.34 mL/min*1.73m2, p=0.001; right iGFR: no Cyst vs Cyst: 34.76±12.74 mL/min*1.73m2 vs 30.45±11.93 mL/min*1.73m2, p=0.001) (Table 2), and eGFR (no Cyst vs Cyst: 76.68± 29.21 mL/min*1.73m2 vs 65.02±22.71 mL/min*1.73m2, p=0.006), and BMI (no Cyst vs Cyst: 25.48±3.60 kg/m2 vs 27.01±3.54 kg/m2, p=0.011), between T2D patients with or without kidney cysts. However, no differences in age (no Cyst vs Cyst: 66.20±12.42 years vs 67.69±10.21 years, p=0.414), duration (no Cyst vs Cyst: 11.38±10.01 years vs 11.80±7.58 years, p=0.764), and serum creatinine levels (no Cyst vs Cyst: 96.42±100.27 μmol/L vs 105.53±41.48 μmol/L, p=0.795) were found (Figure 1).
 |
Table 2 The Isotope Glomerular Filtration Rate (iGFR) in T2D with or without Renal Cysts |
The Volume of Renal Cysts Was Positively Associated with the Serum Creatinine Level and Gender
We thereafter analyzed the relationships between the volume of renal cysts and kidney function expressed as iGFR and eGFR respectively, and also between the volume of renal cysts and general characteristics of the patients. The analytic results showed that the volume of both left and right kidney cysts were positively correlated with the serum creatinine level (left cysts volume: r=0.24, p=0.002; right cysts volume: r=0.15, p=0.046) and gender (left cysts volume: r=0.24, p=0.001; right cysts volume: r=0.20, p=0.008). Although volume of left renal cysts faintly correlated with left iGFR, others did not significantly correlate (total iGFR: left cysts volume: r=−0.15, p=0.052; right cysts volume: r=−0.09, p=0.237; left iGFR: left cysts volume: r=−0.18, p=0.022; right iGFR: right cysts volume: r=−0.10, p=0.21); However, only left cysts volume was weakly correlated with eGFR (left cysts volume: r=−0.19, p=0.013; right cysts volume: r=−0.11, p=0.156). The total iGFR was positively correlated with eGFR (r=0.83, p<0.0001), but negatively associated with serum creatinine level (r=−0.78, p<0.0001), age (r=−0.43, p<0.0001), duration of T2D (r=−0.25, p=0.001), and BMI (r=−0.21, p=0.006), but not gender (r=−0.03, p=0.668). The serum creatinine level was negatively correlated with iGFR (total iGFR: r=−0.78, p<0.0001; left iGFR: r=−0.72, p<0.0001; right iGFR: r=−0.76, p<0.0001) and eGFR (r=−0.91, p<0.0001), but positively correlated with gender (r=0.32, p<0.0001), age (r=0.26, p=0.001), duration (r=0.17, p=0.028), BMI (r=0.16, p=0.036), FPCP (r=0.38, p<0.0001), and serum uric acid (r=0.51, p<0.0001) (Figure 2).
The Volume of Renal Cysts Did Not Account for Increased Serum Creatinine and Decreased iGFR
Furthermore, we examined whether the renal cyst volume in these patients played a critical role in increased serum creatinine by establishing a multilinear regression model. Gender, serum uric acid and iGFR were independent predictors of serum creatinine level but not renal cyst volume (Table 3). This analysis revealed that total iGFR, gender and serum uric acid (UA) were independent driving factors of the change in serum creatinine: total iGFR was negatively correlated with serum creatinine, while male gender and UA were positively correlated. The model could explain 69% (r square was 0.69) of the observed serum creatinine change in the current study.
 |
Table 3 A Multilinear Regression Model of Serum Creatinine Level (Represented as Log SCr) in Patients with Renal Cysts |
We also analyzed a multilinear regression model of iGFR and found that neither the volume of left renal cysts, nor the volume of right renal cysts is a significant determinant of total iGF (Table 4). The model revealed that serum creatinine, gender, free triiodothyronine (FT3), and fasting plasma glucose (FPG) were independent predictors of the change in total iGFR. Specifically, creatinine was negatively related to iGFR, while gender, FT3, and FPG were positively related to iGFR, indicating that male patients with higher FT3 and FPG levels were associated with higher iGFR. These parameters could explain 67.8% (R2=0.678) of the observed iGFR in the current study.
 |
Table 4 A Multilinear Regression Model of iGFR in Patients with Renal Cysts |
Gender Disparity May Significantly Influence the Serum Creatinine Levels in Patients with Renal Cysts
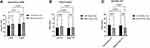
We compared the kidney function (expressed as iGFR and eGFR respectively) and general patients’ characteristics between male and female T2D patients with renal cyst. We noticed a significant higher serum creatinine level in male patients (male vs female: 112.13±43.60μmol/L vs 85.74±26.94μmol/L, p=0.014), whereas the age (male vs female: 66.38±10.01years vs 71.62±10.18 years, p=0.122), duration of T2D (male vs female: 11.17± 7.75 years vs 13.69±6.98 years, p=0.091), BMI (male vs female: 28.87±3.48kg/m2 vs 27.44±3.85kg/m2, p=0.639), cyst volume (left cysts: male vs female: 10.13±30.82cm3 vs 2.77±6.27cm3, p=0.913; right cysts: male vs female: 11.66±31.83cm3 vs 49.20±155.73cm3, p=0.108), iGFR (total iGFR: male vs female: 59.63±20.07mL/min*1.73m2 vs 52.47±22.69 mL/min*1.73m2, p=0.363; left iGFR: male vs female: 28.31±10.13 mL/min*1.73m2 vs 24.69±10.93 mL/min*1.73m2, p=0.837; right iGFR: male vs female: 31.34±10.94 mL/min*1.73m2 vs 27.78±14.68 mL/min*1.73m2, p=0.844), eGFR (male vs female: 65.57±23.43 mL/min*1.73m2 vs 63.36±21.21 mL/min*1.73m2, p=0.754), FPG (male vs female: 8.80 ± 4.54mmol/L vs 6.97 ± 2.84mmol/L, p=0.097), HbA1c (male vs female: 9.84 ± 2.33% vs 8.42 ± 1.76%, p=0.0501), and FPCP (male vs female: 0.62 ± 0.49 nmol/L vs 0.62 ± 0.53 nmol/L, p=0.982) did not reach statistical significance between males and females (Figure 3) (Table 5).
 |
Table 5 The Gender Disparity in General Characteristics, Renal Cyst Volume, Kidney Function, Glucose Metabolism, and Serum Creatinine Levels |
The Split Kidney Function Reflected by iGFR and Related Parameters Did Not Show Significant Bilateral Disparity, and with Those of Different Side of Cysts
Finally, we intended to determine whether the ipsilateral renal cyst had impacts upon contralateral kidney iGFR or related parameters, and was significantly different from the bilateral iGFR or related parameters of the opposite renal cyst from other patients. The analyses of the comparison showed that no special disparity in iGFR whether cysts were in the different sides of the kidney (iGFR: left renal cysts: left iGFR: 25.91±10.99 mL/min*1.73m2; right iGFR: 29.78±12.24 mL/min*1.73m2; right renal cysts: left iGFR: 29.30±10.32 mL/min*1.73m2; right iGFR:32.12±12.74 mL/min*1.73m2; TTP: left renal cysts: left TTP: 3.845±1.913 min; right TTP: 3.674±2.058 min; right renal cysts: left TTP: 4.414±3.441 min; right TTP:3.993±3.168 min, p>0.05; half time excretion (t1/2): left renal cysts: left half time excretion (t1/2): 25.256±14.581 min; right half time excretion (t1/2): 23.985±17.549 min; right renal cysts: left half time excretion (t1/2): 17.859±9.222min; right half time excretion (t1/2): 18.529±10.322min, p>0.05) (Figure 4) (Table 6).
 |
Table 6 The Split Kidney Function Reflected by iGFR and Related Parameters |
Discussion
Sporadic or simple renal cysts are usually incidental findings during imaging examination and can be solitary or multiple, unilateral or bilateral. Because they are often symptomatic, clinicians may ignore their consequence on renal function. But till now, the emerged distinct studies results reported on whether SRCs are associated with impaired renal functions are confusing due to multiple reasons. Some studies affirmed that SRCs could exacerbate the renal eGFR and other complications,15 while others proposed that these cysts do not need further treatment when they are asymptomatic while others proposed that these cysts do not need further treatment when they are asymptomatic.13,16 In the present study, we investigated whether SRCs significantly impact kidney function in patients with type 2 diabetes (T2D) examined by isotope glomerular filtration rate (iGFR). Our analyses suggested that in patients with T2D, SRCs may be observed in patients with decrease renal function due to multiple causes or agents, but the kidney function was not influenced by the volume of renal cysts.
Herein, we found that SRCs affected 30% of T2D patients, especially in male patients, consistent with the literature.17 We found that age, duration, HbA1c level and basal C-peptide were comparable in both groups of patients. The mean volume of right cysts was larger than the left, which could be attributed to the limited sample size. However, whether there exist anatomic and physiological kidney disparities that predispose to this discrepancy remains to be determined. Thereafter, we evaluated the general characteristics and kidney function between these two groups and observed significant differences in the BMI, eGFR, and iGFR, consistent with the literature.15,18,19 This higher BMI in T2D with cysts could be attributed to insulin resistance, hypertension, hyperuricemia induced by adipose tissue inflammation or oxidative stress, as previously reviewed in multiple studies.20 A previous study reported that obesity prevalence is significantly higher in patients with renal cysts than in those without.21 Although we found a significant decrease in total iGFR and eGFR, we did not find a statistically different iGFR during split kidney function analysis, which may be due to the limited sample size. As well, we did not expect the increased serum creatinine level, suggesting although the kidney lesion may co-exist, and our groups were in relative early stage of chronic kidney disease (CKD), but it did not adequately to induce remarkably creatinine increasing, and renal insufficiency in these patients.
Subsequently, we analyzed the correlation between cyst volume and renal function. Intriguingly, we did not find a significant correlation with iGFR/eGFR, while a significant association was found with serum creatinine level and gender disparity. We speculate that cyst volume is not the sole critical factor associated with iGFR levels. Consistently, some studies revealed that the number, location, and type of cysts might influence kidney function.16,22–24 Given that this is a correlational study, we only observed that serum creatinine was correlated with cyst volume, indicating that the volume and the level change of creatinine may be the consequences of reduced renal function. On the other hand, our results suggested that iGFR is associated with age, duration of T2D, BMI, eGFR and creatinine, consistent with the literature.
Given that creatinine was significantly associated with cyst volume, we established a multilinear regression model of creatinine and iGFR. No significant relationship was found between cyst volume and serum creatinine. We found that UA level was an independent predictor of changes in serum creatinine; FT3 and FPG were predictors of iGFR, consistent with the literature.25–30 This is further confirmed our assumption that SRCs may be a manifestation of declined renal function in T2D (mean eGFR: 65.02±22.71 mL/min*1.73m2), but the cyst volume parameter in the current study (as shown in Table 1) did not significantly alter the kidney function. We substantiate that besides the number, location and type of renal cysts, the volume of renal cysts may not be the ideal or exclusive indicator to predict the effect of renal cysts on kidney filtration function, unlike previous reports.7,31 Besides, we found that iGFR is consistent with the eGFR, which is in accordance with a previous study,32 suggesting that iGFR is a useful and precise tool for evaluating the split kidney function in diabetic patients.
To exclude the effects of gender disparity on cyst volume, we compared the data of males and females with renal cysts by stratifying by age, duration, BMI, cyst volume, iGFR, eGFR, and serum creatinine level. As expected, the gender disparity did not influence the renal cyst volume, suggesting although differences in incidence of SRCs may prevail, the renal cyst volume is not a crucial factor. However, we found that the serum creatinine level was especially higher in male groups with renal cysts; this could be attributed to muscle and hormone-associated comorbidities.
Given that we established that patients with renal cysts exhibited reduced renal function, but renal insufficiency was not exacerbated, we sought to compare the split kidney function between the lesioned (with cysts) and healthy side (without cysts) after excluding the cyst volume. As the split kidney function was reduced revealed by iGFR and related parameters, there were no significant differences between: 1) the left iGFR and related parameters whether there were left renal cysts; 2) the right iGFR and related parameters whether there were right renal cysts; 3) left and right iGFR and related parameters whether there were left renal cysts or right renal cysts. This furtherly supports our findings, and concurrently indicates: the respective iGFR of split kidney with SRCs, did not distinguish its coexisted side from its contralateral side. The TTP and half time excretion t1/2 parameters were used to observe the isotope dynamics during split kidney function analysis represented as absorption, distribution, and excretion. We also found no distinct changes in the presence of SRCs, which supports our assumption that SRC did not influence the split renal function.
SRCs originate from the distal convoluted or collecting duct and are thought to arise from renal tubular diverticula or tubular cell hyperplasia secondary to nephron loss.33 It is thought that renal cysts originate from weakening the tubular basement membrane of the distal convoluted or collecting duct cells. As a result, diverticula formed can subsequently develop into a simple renal cyst.34 Overwhelming evidence substantiates that SRCs are associated with ageing, gender, prehypertension, hypertension, diabetes, arterial stiffness, albuminuria, and increased serum creatinine, which are well-established risk factors for cardiovascular diseases.35–37 However, the relationship between the T2D and SRCs remains unclear and may be subject to differences due to the study population, sample size, stage of hypertension, and techniques for detecting renal cysts.19,38,39 In a recent study, SRCs were associated with worse renal function in patients with T2D, age, gout, proteinuria, cerebrovascular disease (CVD), and increased serum phosphorus levels.15 Consistently, another study demonstrated that hyperuricemia was the critical risk factor for SRCs in T2D.28 In our study, we also observed a decreased iGFR and eGFR in T2D patients with SRCs, but our analyses showed that the volume of SRCs was not associated with the eGFR or iGFR level, and it did not significantly affect the split kidney function. We hypothesize that the number, location, and type of the cysts may be closely related to renal function. In our future investigations, we will include more precise parameters associated with isotope analysis to further investigate the implication of SRCs on renal function.
Conclusion
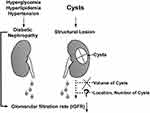
Our preliminary results suggest that SRCs may be associated with decreased renal function in patients with T2D. In the current investigation, we used isotope renography to assess kidney function and discovered that, while patients with renal cysts had a decreased iGFR, the volume of simple cysts did not increase risk of renal insufficiency (Figure 5). Isotope renography is a valuable method for assessing split kidney function in patients with unilateral nephropathy. Further research on the effects of SRCs on diabetic renal disease is warranted to identify more precise biomarkers.
Ethical Statement
The study, including sampling, examinations, and access or utilization of the raw data for this study, obtained ethical approval from the Shanghai Pudong Hospital. Study participants provided informed consent before the study. The guidelines outlined and whole procedures were conducted in accordance with the Declaration of Helsinki. All the data used in this study were anonymized before their use.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Talents Training Program of Shanghai Pudong Hospital (YQ202101), The Pudong New Area Clinical Characteristic Discipline Project (No.PWYts2021-01), Pudong Hospital, Fudan University, College Level Project (No.YJYJRC202108, No.YJYJRC202101), National Key Research and Development Program of China (No.2017YFC0113300), Fudan Zhangjiang Clinical Medicine Innovation Fund Project (KP0202118), the Project of Key Medical Discipline of Pudong Hospital of Fudan University (Zdxk2020-11), Project of Key Medical Specialty and Treatment Center of Pudong Hospital of Fudan University (Zdzk2020-24), Integrative Medicine special fund of Shanghai Municipal Health Planning Committee (ZHYY- ZXYJHZX-2-201712), Special Department Fund of the Pudong New Area Health Planning Commission (PWZzk2017-03), Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (PWR12014-06), Pudong New Area Clinical Plateau Discipline Project (PWYgy-2021-03), the Natural Science Foundation of China (21675034), National Natural Science Foundation of China (81370932), Shanghai Natural Science Foundation (19ZR1447500).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi:10.1001/jama.2017.7596
2. Bell S, Fletcher EH, Brady I, et al. End-stage renal disease and survival in people with diabetes: a national database linkage study. QJM. 2015;108(2):127–134. doi:10.1093/qjmed/hcu170
3. Warwick J, Holness J. Measurement of glomerular filtration rate. Semin Nucl Med. 2022;52(4):453–466. doi:10.1053/j.semnuclmed.2021.12.005
4. Xie P, Huang JM, Liu XM, Wu WJ, Pan LP, Lin HY. (99m)Tc-DTPA renal dynamic imaging method may be unsuitable to be used as the reference method in investigating the validity of CDK-EPI equation for determining glomerular filtration rate. PLoS One. 2013;8(5):e62328. doi:10.1371/journal.pone.0062328
5. Pal DK, Kundu AK, Das S. Simple renal cyst: an observation. J Indian Med Assoc. 1997;95(10):555, 558.
6. Ekart R, Hojs R, Krajnc I. [Simple renal cysts and hypertension]. Wien Klin Wochenschr. 2001;113(Suppl 3):43–46. German.
7. Kong X, Ma X, Zhang C, Su H, Gong X, Xu D. Increased risk of kidney damage among Chinese adults with simple renal cyst. Int Urol Nephrol. 2018;50(9):1687–1694. doi:10.1007/s11255-018-1880-3
8. Ozveren B, Onganer E, Türkeri LN. Simple renal cysts: prevalence, associated risk factors and follow-up in a health screening cohort. Urol J. 2016;13(1):2569–2575.
9. Yang B, Qiu C, Wan S, et al. Long-term follow-up study of the malignant transformation potential of the simple renal cysts. Transl Androl Urol. 2020;9(2):684–689. doi:10.21037/tau.2020.03.29
10. Stonebrook E, Hoff M, Spencer JD. Congenital anomalies of the kidney and urinary tract: a clinical review. Curr Treat Options Pediatr. 2019;5(3):223–235. doi:10.1007/s40746-019-00166-3
11. Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. Urology. 2008;71(1):
12. Eissa A, El Sherbiny A, Martorana E, et al. Non-conservative management of simple renal cysts in adults: a comprehensive review of literature. Minerva Urol Nefrol. 2018;70(2):179–192. doi:10.23736/S0393-2249.17.02985-X
13. Skolarikos A, Laguna MP, de la Rosette JJ. Conservative and radiological management of simple renal cysts: a comprehensive review. BJU Int. 2012;110(2):170–178. doi:10.1111/j.1464-410X.2011.10847.x
14. Kwon T, Lim B, You D, et al. Simple renal cyst and renal dysfunction: a pilot study using dimercaptosuccinic acid renal Scan. Nephrology. 2016;21(8):687–692. doi:10.1111/nep.12654
15. Wei L, Xiao Y, Xiong X, et al. The relationship between simple renal cysts and renal function in patients with type 2 diabetes. Front Physiol. 2020;11:616167. doi:10.3389/fphys.2020.616167
16. Chin HJ, Ro H, Lee HJ, Na KY, Chae DW. The clinical significances of simple renal cyst: is it related to hypertension or renal dysfunction? Kidney Int. 2006;70(8):1468–1473. doi:10.1038/sj.ki.5001784
17. Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002;167(1):21–23. doi:10.1016/S0022-5347(05)65373-6
18. Chen J, Ma X, Xu D, Cao W, Kong X. Association between simple renal cyst and kidney damage in a Chinese cohort study. Ren Fail. 2019;41(1):600–606. doi:10.1080/0886022X.2019.1632718
19. Choi JD. Clinical characteristics and long-term observation of simple renal cysts in a healthy Korean population. Int Urol Nephrol. 2016;48(3):319–324. doi:10.1007/s11255-015-1186-7
20. Gong M, Wen S, Nguyen T, Wang C, Jin J, Zhou L. Converging relationships of obesity and hyperuricemia with special reference to metabolic disorders and plausible therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:
21. Suher M, Koc E, Bayrak G. Simple renal cyst prevalence in internal medicine department and concomitant diseases. Ren Fail. 2006;28(2):149–152. doi:10.1080/08860220500530841
22. Karoli R, Bhat S, Fatima J, et al. Simple renal cysts: can they be overlooked? J Assoc Physicians India. 2016;64(3):14–17.
23. Ozdemir AA, Kapucu K. The relationship between simple renal cysts and glomerular filtration rate in the elderly. Int Urol Nephrol. 2017;49(2):313–317. doi:10.1007/s11255-016-1414-9
24. Waldram MM, Thomas AG, Yu Y, et al. Long-term renal function in living kidney donors with simple renal cysts: a retrospective cohort study. Clin Transplant. 2020;34(9):e13905. doi:10.1111/ctr.13905
25. Han QX, Zhang D, Zhao YL, et al. Analysis of chronic kidney disease staging with different estimated glomerular filtration rate equations in Chinese centenarians. Chin Med J. 2019;132(5):512–518. doi:10.1097/CM9.0000000000000079
26. Koratala A, Singhania G, Alquadan KF, Shimada M, Johnson RJ, Ejaz AA. Serum uric acid exhibits inverse relationship with estimated glomerular filtration rate. Nephron. 2016;134(4):231–237. doi:10.1159/000448629
27. Hu Y, Li Q, Min R, Deng Y, Xu Y, Gao L. The association between serum uric acid and diabetic complications in patients with type 2 diabetes mellitus by gender: a cross-sectional study. PeerJ. 2021;9:e10691. doi:10.7717/peerj.10691
28. Han Y, Zhang M, Lu J, et al. Hyperuricemia and overexcretion of uric acid increase the risk of simple renal cysts in type 2 diabetes. Sci Rep. 2017;7(1):3802. doi:10.1038/s41598-017-04036-6
29. Hasegawa EM, Fuller R, Chammas MC, de Mello FM, Goldenstein-Schainberg C. Increased prevalence of simple renal cysts in patients with gout. Rheumatol Int. 2013;33(2):413–416. doi:10.1007/s00296-012-2380-x
30. Anderson JLC, Gruppen EG, van Tienhoven-Wind L, et al. Glomerular filtration rate is associated with free triiodothyronine in euthyroid subjects: comparison between various equations to estimate renal function and creatinine clearance. Eur J Intern Med. 2018;48:
31. Al-Said J, O’Neill WC. Reduced kidney size in patients with simple renal cysts. Kidney Int. 2003;64(3):1059–1064. doi:10.1046/j.1523-1755.2003.00193.x
32. Sugawara S, Ishii S, Kojima Y, Ito H, Suzuki Y, Oriuchi N. Feasibility of gamma camera-based GFR measurement using renal depth evaluated by lateral scan of (99m)Tc-DTPA renography. Ann Nucl Med. 2020;34(5):349–357. doi:10.1007/s12149-020-01455-w
33. Baert L, Steg A. Is the diverticulum of the distal and collecting tubules a preliminary stage of the simple cyst in the adult? J Urol. 1977;118(5):707–710. doi:10.1016/S0022-5347(17)58167-7
34. Darmady EM, Offer J, Woodhouse MA. The parameters of the ageing kidney. J Pathol. 1973;109(3):195–207. doi:10.1002/path.1711090304
35. Wu HY, Chang YF, Wu IH, et al. Simple renal cysts are associated with increased arterial stiffness in a Taiwanese population. Hypertens Res. 2019;42(7):1068–1073. doi:10.1038/s41440-018-0202-6
36. Afsar B, Afsar RE, Sen ST, et al. Simple renal cysts and circadian blood pressure: are they related to each other in patients with hypertension? Int Urol Nephrol. 2011;43(1):157–165. doi:10.1007/s11255-010-9734-7
37. Boo HJ, Lee JE, Chung SM, et al. The presence of simple renal cysts is associated with an increased risk of albuminuria in young adults. Korean J Intern Med. 2022;37(2):425–433. doi:10.3904/kjim.2020.576
38. Hong S, Lim JH, Jeong IG, Choe J, Kim CS, Hong JH. What association exists between hypertension and simple renal cyst in a screened population? J Hum Hypertens. 2013;27(9):539–544. doi:10.1038/jhh.2013.12
39. Mensel B, Kühn JP, Kracht F, et al. Prevalence of renal cysts and association with risk factors in a general population: an MRI-based study. Abdom Radiol. 2018;43(11):3068–3074. doi:10.1007/s00261-018-1565-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.