Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Relationship Between Serum Fibrinogen Level and Depressive Symptoms in an Adult Population with Spinal Cord Injury: A Cross-Sectional Study
Authors Xie Z , Li C, Xing Z, Zhou W, Xie S, Li M, Zhou Y
Received 17 March 2021
Accepted for publication 11 June 2021
Published 6 July 2021 Volume 2021:17 Pages 2191—2198
DOI https://doi.org/10.2147/NDT.S311473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Zhiping Xie,1 Chengcai Li,1 Zelong Xing,1 Wu Zhou,1 Shenke Xie,1 MeiHua Li,1 Yujuan Zhou2
1Department of Neurosurgery, The First Affiliated Hospital of Nanchang University, Nanchang, 330006, Jiangxi, People’s Republic of China; 2Department of Orthopaedics, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, 330006, People’s Republic of China
Correspondence: MeiHua Li
Department of Neurosurgery, The First Affiliated Hospital of Nanchang University, 17 Yongwai Street, Nanchang, 330006, Jiangxi, People’s Republic of China
Email [email protected]
Yujuan Zhou
Department of Orthopaedics, The First Affiliated Hospital of Nanchang University, 17 Yongwai Street, Nanchang, 330006, Jiangxi, People’s Republic of China
Email [email protected]
Purpose: Depression is associated with an inflammatory immune response. There are minimal data regarding the association of inflammatory markers with depression in patients with spinal cord injury (SCI). We aimed to investigate the association of inflammatory markers with depression in middle-aged and elderly SCI patients.
Methods: Data were obtained from the Midlife in the United States (MIDUS) study, a longitudinal study of a representative sample of the adult population. We analyzed the associations of serum levels of fibrinogen, interleukin-6, tumor necrosis factor-ɑ, and C-reactive protein with depressive symptoms.
Results: The median participant age was 52.5 years; 44.9% of participants were men. Multivariate linear regression analyses showed that an increased serum fibrinogen level (Sβ = 0.114, p = 0.005) was associated with higher Centre for Epidemiological Studies-Depression (CES-D) scores after adjustment for age, sex, body mass index (BMI), ethnicity, education, marital status, smoking, alcohol use, exercise, perceived stress score, and cardiovascular disease (CVD). Multivariate logistic regression analysis showed that an increased serum fibrinogen level was independently associated with a history of depression (odds ratio [OR] = 1.240, 95% confidence interval [CI] = 1.103– 1.997, p = 0.012) and depressive symptoms (OR = 1.884, 95% CI = 1.165– 2.499, p < 0.001; CES-D score ≥ 16) after adjustment for confounding factors. Stratified analysis revealed that the association between serum fibrinogen level and depressive symptoms was affected by antidepressant use.
Conclusion: Serum fibrinogen level had a significantly positive association with depressive symptoms in middle-aged and elderly patients with SCI. Future longitudinal cohort studies should evaluate the possible use of serum fibrinogen for diagnosis of depression in SCI patients.
Keywords: fibrinogen, depression, spinal cord injury, cross-sectional study
Introduction
Spinal cord injury (SCI) adversely affects all aspects of life in affected patients. SCI increases the risks of mental disorders such as depression. Depression represents an inflammatory immune response to the injury; it increases the risk of adverse consequences such as pain, substance abuse, and medical complications.1–4 One-third of acute SCI patients are depressed.5–7 A Canadian cohort study showed that 30% of patients were depressed at 6 years after SCI; more than 6.5% of the patients in that study had severe depression.8 More than 10% of SCI patients in a cohort study from the United States had major depression.9 Depression interferes with SCI rehabilitation therapy because of negative expectations, reduced energy, and social withdrawal. Determining the risk factors for depression in SCI patients will allow early identification and treatment, thereby improving the outcomes of rehabilitation therapy.
The increased risk of depression in SCI patients has been linked to biological responses (eg, immune-mediated and inflammatory) and immune dysregulation with consequent neuroendocrine changes.10 Depression and inflammation have a bidirectional relationship. Inflammation increases the risk of depression, and depression increases the inflammatory response, leading to a vicious circle.11,12 Many studies have implicated chronic inflammation as the cause of adverse health outcomes in depressed patients.13–15, Elevated levels of inflammatory markers, such as C-reactive protein (CRP) and cytokines, increase the risk of depression.16,17 The inflammatory response involves secretion of pro-inflammatory cytokines and activation of immune cells, which cause changes in brain structure18,19 and function.17 In addition, exposure to pro-inflammatory cytokines produces depressive symptoms, which are attenuated by antidepressant use.20,21
Levels of inflammatory markers (eg, interleukin [IL]-1, tumor necrosis factor-ɑ [TNF-ɑ], IL-6, and fibrinogen) are elevated in SCI patients, which may explain the link between SCI and depression. Similar to CRP, inflammatory markers (eg, fibrinogen, IL-6, and TNF-ɑ) also increase the risk of depression. Previous studies have measured the levels of inflammatory markers in severely depressed patients with SCI, but not in SCI patients with mild or moderate depression.
We analyzed data from the Midlife in the United States (MIDUS) study, a longitudinal study of a representative sample of the United States adult population aged 34–84 years. We adjusted the data for covariates (demographic characteristics, lifestyle behaviors, and comorbidities) to determine the relationship between chronic inflammation and depression in SCI patients. We aimed to evaluate the relationships of serum levels of fibrinogen, IL-6, TNF-ɑ, and CRP with depression in SCI patients.
Methods
Participants and Study Design
The MIDUS study investigated the relationships of psychological and behavioral factors with adverse health outcomes. The Biomarkers project, a sub-study of the MIDUS study, involved comprehensive biological assessments of 1255 participants, and collected sufficient data to evaluate the relationships of behavioral and psychosocial factors with biological factors.22 The Biomarkers project included 225 SCI patients (mean age, 52.4 years; range, 43.7–60.4 years). SCI patients with history of any trauma were included, and patients with active inflammatory diseases were excluded from the study. We selected the variables described below because they are most closely related to our factors of interest.
The MIDUS study Biomarkers project protocol has been published previously.22,23 The data and specific codebooks from this study are available at http://www.midus.wisc.edu/. In brief, participants in the MIDUS study were selected from a national sample by using a random-digit dialing procedure. All participants in the first MIDUS survey who could safely travel to the clinic were included in the Biomarkers project. The participants were subsequently followed up by means of e-mails and phone calls. Survey data were collected between 2004 and 2009 at one of the three MIDUS study research centers (University of California-Los Angeles; Georgetown University; and University of Wisconsin-Madison). During the 2-day visit, participants were asked to complete self-administered questionnaires and participate in medical interviews. Participant blood specimens were also collected in accordance with a standardized protocol that was consistent across the three sites. The travel expenses of participants were reimbursed, and each participant was paid $200 for their participation. This study complied with the Declaration of Helsinki. Approval was obtained from the ethics committees of the three research centers and written informed consent was obtained from all participants.
Fasting blood samples were collected from SCI patients during their 2-day visits in the study period (2004–2009). We measured the following serum inflammatory markers: fibrinogen, IL-6, TNF-ɑ, and CRP. The measurement methods have been described in detail elsewhere.22–24
Depression Score
The Centre for Epidemiological Studies-Depression (CES-D) scale was used to evaluate depression.25 The CES-D scale is widely used in epidemiological studies, and a cutoff score of 16 indicates depression.26 In addition, the participants were considered to have depression if they had a history of using antidepressant medications. The CES-D is a reliable scale for identifying depressive symptoms and was easily administered by the well-trained research nurses in our study.
Covariates
Self-administered questionnaires were used to collect data concerning sociodemographic characteristics (age, sex, body mass index [BMI], ethnicity, education, and marital status), lifestyle factors (tobacco use, alcohol use, and exercise), and history of cardiovascular diseases (CVD; heart disease, hypertension, transient ischemic attack, stroke, or diabetes). Marital status was recorded as “currently married” or “not currently married.” Education was recorded as “bachelor’s degree or higher” or “high school education or lower.” Smoking status was recorded as “current smoker” or “not a current smoker.” Drinking status was recorded as “current alcohol consumption” or “no current alcohol consumption.” Exercise was recorded as present in participants with a frequency of exercise “≥ 3/week.” History of depression or antidepressant use was dichotomized into “yes” or “no.” The Perceived Stress Scale is a 10-item tool for assessing the degree of stress in participants. Each item (eg, “In the past month, how often have you been upset because of something that happened unexpectedly?”) was rated on a 5-point scale ranging from 1 (never) to 5 (very often), and the responses were reverse-coded such that higher scores were indicative of greater perceived stress [ɑ = 0.84].
Statistical Analysis
The Kolmogorov–Smirnov test and Q-Q plots were used to analyze the normality of data. A p-value ≤ 0.05 was considered statistically significant. Data with non-normal distribution were expressed as medians and interquartile ranges. Normally distributed data were expressed as means and standard deviations. First, Pearson correlation was performed to analyze the associations of serum levels of fibrinogen, IL-6, TNF-ɑ, and CRP with CES-D scores. All continuous variables were standardized by calculation of z-scores. Multivariate linear regression analysis was conducted to assess the relationships of serum inflammatory markers with depression scores. Multivariate logistic regression was used to evaluate the associations of serum fibrinogen with depressive symptoms. The associations between serum fibrinogen level and depressive symptoms were also assessed using stratified analysis with antidepressant use as a covariate. All analyses were performed using SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of Participants with SCI
The sociodemographic characteristics of 225 participants with SCI are presented in Table 1. The median participant age was 52.5 years. There were 101 (44.9%) male and 212 (94.2%) white participants. The participants were divided into two groups based on depressive symptoms (CES-D score ≥ 16). In comparison with participants who lacked depressive symptoms (CES-D score< 16), participants with depressive symptoms were older, had a higher BMI, used alcohol more frequently, and exercised more frequently. Participants with depressive symptoms were also more likely to be married and educated, compared with participants who lacked depressive symptoms. Participants with history of depression or CVDs had a higher frequency of depressive symptoms than did participants without depression or CVDs. Participants with depressive symptoms had higher perceived stress scores and levels of fibrinogen, IL-6, TNF-ɑ, and CRP than did participants without depressive symptoms. Pearson correlation showed significant associations of serum levels of fibrinogen (r = 0.170; p = 0.001; Figure 1), IL-6 (r = 184; p = 0.006; Figure 2), TNF-ɑ (r = 0.169, p = 0.012; Figure 3), and CRP (r = 0.155, p = 0.041, Figure 4) with depression. Multiple linear regression analysis showed that increased serum levels of fibrinogen (β = 0.011, 95% confidence interval [CI] = 0.006–0.015, p = 0.005; model 2) and CRP (β = 0.010, 95% CI = 0.003–0.035, p = 0.009; model 2) were associated with higher CES-D scores, after adjustment for age, sex, ethnicity, education, BMI, current smoking, current alcohol consumption, exercise, perceived stress score, and CVDs (Table 2).
 |
Table 1 Characteristics of Participants with Spinal Cord Injury |
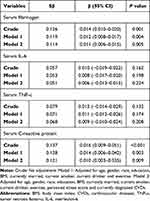 |
Table 2 Multiple Linear Regression Analysis for Relationship Between Inflammatory Markers and Depression Score |
 |
Figure 1 The relationship between serum fibrinogen and depression score (CES-D scale) in all subjects. |
 |
Figure 2 The relationship between serum IL-6 and depression score (CES-D scale) in all subjects. |
 |
Figure 3 The relationship between serum TNF-ɑ and depression score (CES-D scale) in all subjects. |
 |
Figure 4 The relationship between serum C-reactive protein and depression score (CES-D scale) in all subjects. |
Multiple Logistic Regression Analysis of the Association Between Serum Fibrinogen Level and History of depression
As shown in Table 3, an increased serum fibrinogen level was positively associated with history of depression, both before adjustment (odds ratio [OR] = 1.374, 95% CI = 1.235–2.469, p < 0.001) and after adjustment for age, sex, ethnicity, education, BMI, marital status, smoking, alcohol use, and exercise (OR = 1.311, 95% CI = 1.162–2.084, p = 0.005; model 1). This association remained statistically significant after adjustment for other confounding factors, including the perceived stress score and current CVDs (OR = 1.240, 95% CI = 1.103–1.997, p = 0.012, model 2). However, there was no significant association between CRP and history of depression (data not shown).
 |
Table 3 Multiple Logistic Regression Analysis for Relationship Between Serum Fibrinogen and History of Depressive Symptoms |
Multiple Logistic Regression Analysis of the Association Between Serum Fibrinogen Level and Depressive Symptoms (CES-D Score ≥ 16)
As shown in Table 4, an increased serum fibrinogen level was positively associated with any depressive symptoms, both before adjustment (OR = 1.970, 95% CI = 1.248–2.733, p < 0.001) and after adjustment for age, sex, ethnicity, education, BMI, current marital status, smoking, alcohol use, and exercise (OR = 1.923, 95% CI = 1.224–2.681, p < 0.001; model 1). This association remained statistically significant after adjustment for other confounding factors, including the perceived stress score and current CVDs (OR = 1.884, 95% CI = 1.165–2.499, p < 0.001; model 2).
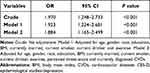 |
Table 4 Multiple Logistic Regression Analysis for Relationship Between Serum Fibrinogen and Depressive Symptoms (CES-D Score≥ 16) |
We repeated the analysis with antidepressant use as a covariate and found a strongly positive association between serum fibrinogen level and risk of depressive symptoms in SCI participants without antidepressant use (OR = 1.892, 95% CI = 1.461–2.420, p < 0.001; model 2; Table 5). However, there was no significant association in participants with antidepressant use (OR = 1.134, 95% CI = −0.577 to −1.448, p = 0.079; model 2).
 |
Table 5 Multiple Logistic Regression Analysis for Relationship Between Serum Fibrinogen and Depressive Symptoms (CES-D Score≥ 16) Stratified by Antidepressants Use |
Discussion
We investigated the associations between depressive symptoms and inflammatory markers (fibrinogen, IL-6, TNF-ɑ, and CRP) in middle-aged and elderly patients with SCI. Serum fibrinogen, IL-6, and TNF-ɑ levels had strong and independent associations with depression scores, according to multivariate linear regression analysis. An increased serum fibrinogen level was associated with an increased risk of depressive symptoms after adjustment for confounding factors in the multivariate logistic regression analysis. However, this association between serum fibrinogen and depressive symptoms was affected by antidepressant use in the stratified analysis. Our results are consistent with previous findings that antidepressants may influence inflammation.27,28 SCI often leads to long-term disability because of reduced mobility and loss of physical function.29–31 Notably, inflammation after SCI may have a major impact on physical activity.31,32 However, there are minimal data concerning the effects of inflammation on neuropsychiatric disorders, especially depression, in SCI patients.33–35 Depression interferes with SCI rehabilitation because of negative expectations, reduced energy, and social withdrawal. More than 20% of SCI patients are depressed,36–38 which is 4–5-fold greater than the worldwide prevalence of depression.39
Our results add to the emerging evidence concerning associations between serum levels of inflammatory markers and depressive symptoms.5,8,9,18,36–39 Few studies have analyzed the associations of fibrinogen, IL-6, and TNF-ɑ with depression in SCI patients. Recent sex-specific analyses support a role for inflammation in the onset of depression in SCI patients. A previous study concerning the roles of inflammatory markers in depression in female patients with suspected coronary ischemia found a robust association between CRP and depression, but inflammatory markers minimally contributed to the association between depression and CVD.40,41 Similarly, a prospective study found evidence of a positive association between depressive symptoms and risk of incident stroke; however, inflammation (represented by baseline CRP) did not mediate this association.42 Chronic inflammation in depressed patients may be a result of oxidative reactions. In our study, serum fibrinogen and CRP levels were positively associated with the depression score, consistent with findings in previous studies. In contrast to our results, a previous study found reduced serum fibrinogen levels in depressed patients. This discrepancy may have resulted from differences in study populations and analysis methods. We also found elevated serum IL-6, TNF-ɑ, and CRP levels in the present investigation, similar to the results in previous studies;43,44 however, some previous studies have reported normal or reduced inflammatory marker levels in depressed patients.45 The normal serum IL-6, TNF-ɑ, and CRP levels observed in this study may have resulted from the inclusion of patients with a history of SCI and mild to moderate depression, rather than patients with severe depression alone.
Limitations
First, this was a cross-sectional study with a small sample size. Second, depressive symptoms were assessed using a self-administered questionnaire, instead of a clinical interview. However, the scales used have good predictive value and discriminatory validity in both normative and clinical populations, as well as disabled people. Third, we included all SCI patients and did not categorize them based on their severity, classification, and treatment, which may have led to bias in the study results. Fourth, the results of the self-administered questionnaire may have been biased because many patients overstate or understate their lifestyle habits (eg, smoking and drinking alcohol).
Conclusions
An elevated serum fibrinogen level was significantly positively associated with an increased risk of depressive symptoms among middle-aged and elderly SCI patients. Future longitudinal cohort studies should assess the predictive value of serum fibrinogen for diagnosis of depression in SCI patients.
Acknowledgements
This work was supported by the National Natural Science Foundation fo China (NSFC; grant. no. 81860225).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Norrbrink BC, Hultling C, Lundeberg T. Quality of sleep in individuals with spinal cord injury: a comparison between patients with and without pain. Spinal Cord. 2005;43(2):85–95. doi:10.1038/sj.sc.3101680
2. Ronca E, Scheel-Sailer A, Koch HG, Gemperli A. Health care utilization in persons with spinal cord injury: part 2-determinants, geographic variation and comparison with the general population. Spinal Cord. 2017;55(9):828–833. doi:10.1038/sc.2017.38
3. Jacobson MH, Norman C, Nguyen A, Brackbill RM. Longitudinal determinants of depression among World Trade Center Health Registry enrollees, 14–15 years after the 9/11 attacks. J Affect Disord. 2018;229:483–490. doi:10.1016/j.jad.2017.12.105
4. Lee A, Wen B, Walter M, et al. Prevalence of postpartum depression and anxiety among women with spinal cord injury. J Spinal Cord Med. 2021;44(2):247–252. doi:10.1080/10790268.2019.1666239
5. Kennedy P, Rogers BA. Anxiety and depression after spinal cord injury: a longitudinal analysis. Arch Phys Med Rehabil. 2000;81(7):932–937. doi:10.1053/apmr.2000.5580
6. Wilson CS, Forchheimer M, Heinemann AW, Warren AM, McCullumsmith C. Assessment of the relationship of spiritual well-being to depression and quality of life for persons with spinal cord injury. Disabil Rehabil. 2017;39(5):491–496. doi:10.3109/09638288.2016.1152600
7. Hitzig SL, Tonack M, Campbell KA, et al. Secondary health complications in an aging Canadian spinal cord injury sample. Am J Phys Med Rehabil. 2008;87(7):545–555. doi:10.1097/PHM.0b013e31817c16d6
8. Dryden DM, Saunders LD, Rowe BH, et al. Depression following traumatic spinal cord injury. Neuroepidemiology. 2005;25(2):55–61. doi:10.1159/000086284
9. Bombardier CH, Richards JS, Krause JS, Tulsky D, Tate DG. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85(11):1749–1756. doi:10.1016/j.apmr.2004.07.348
10. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55(7):580–592. doi:10.1001/archpsyc.55.7.580
11. Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65(3):347–356. doi:10.1097/01.psy.0000041542.29808.01
12. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi:10.1016/j.biopsych.2009.09.033
13. Danner, M, Kasl, S V, Abramson, J L, & Vaccarino, V. (2003). Association between depression and elevated C-reactive protein. Psychosom Med, 65(3),347–356. doi:10.1097/01.psy.0000041542.29808.01
14. Ghiadoni L, Donald AE, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi:10.1161/01.cir.102.20.2473
15. Pizzi C, Manzoli L, Mancini S, Costa GM. Analysis of potential predictors of depression among coronary heart disease risk factors including heart rate variability, markers of inflammation, and endothelial function. Eur Heart J. 2008;29(9):1110–1117. doi:10.1093/eurheartj/ehn137
16. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi:10.1016/j.biopsych.2008.11.029
17. Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306. doi:10.1002/da.22084
18. Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21(10):1358–1365. doi:10.1038/mp.2015.168
19. Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23(2):149–158. doi:10.1016/j.bbi.2008.08.006
20. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi:10.1038/nri.2015.5
21. Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643–652. doi:10.1016/S0893-133X(01)00407-9
22. Dienberg LG, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2010;22(8):1059–1080. doi:10.1177/0898264310374355
23. Gruenewald TL, Karlamangla AS, Hu P, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74(1):75–83. doi:10.1016/j.socscimed.2011.09.037
24. Zainal NH, Newman MG. Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021;1–11. doi:10.1017/S0033291721000398
25. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;3(1):385–401. doi:10.1177/014662167700100306
26. Karim JWRB, Weisz R, Bibi Z, Ur Rehman S. Validation of the eight-item center for epidemiologic studies depression scale (CES-D) among older adults. Current Psychol. 2015;4(34):681–692. doi:10.1007/s12144-014-9281-y
27. Vogelzangs N, Duivis HE, Beekman AT, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. doi:10.1038/tp.2012.8
28. Crnkovic D, Buljan D, Karlovic D, Krmek M. Connection between inflammatory markers, antidepressants and depression. Acta Clin Croat. 2012;51(1):25–33.
29. Song HY, Nam KA. Coping strategies, physical function, and social adjustment in people with spinal cord injury. Rehabil Nurs. 2010;35(1):8–15. doi:10.1002/j.2048-7940.2010.tb00025.x
30. Stone WJ, Stevens SL, Fuller DK, Caputo JL. Ambulation and physical function after eccentric resistance training in adults with incomplete spinal cord injury: a feasibility study. J Spinal Cord Med. 2019;42(4):526–533. doi:10.1080/10790268.2017.1417804
31. Franz M, Richner L, Wirz M, et al. Physical therapy is targeted and adjusted over time for the rehabilitation of locomotor function in acute spinal cord injury interventions in physical and sports therapy. Spinal Cord. 2018;56(2):158–167. doi:10.1038/s41393-017-0007-5
32. Paterniti I, Campolo M, Cordaro M, et al. PPAR-alpha modulates the anti-inflammatory effect of melatonin in the secondary events of spinal cord injury. Mol Neurobiol. 2017;54(8):5973–5987. doi:10.1007/s12035-016-0131-9
33. Zhou Y, Li N, Zhu L, Lin Y, Cheng H. The microglial activation profile and associated factors after experimental spinal cord injury in rats. Neuropsychiatr Dis Treat. 2018;14:2401–2413. doi:10.2147/NDT.S169940
34. Wang L, Jiang Y, Jiang Z, Han L. Effect of low-energy extracorporeal shock wave on vascular regeneration after spinal cord injury and the recovery of motor function. Neuropsychiatr Dis Treat. 2016;12:2189–2198. doi:10.2147/NDT.S82864
35. Bassi A, Bozzali M. Potential interactions between the autonomic nervous system and higher level functions in neurological and neuropsychiatric conditions. Front Neurol. 2015;6:182. doi:10.3389/fneur.2015.00182
36. Huang Y, Jiang Y, Zhu M. The relationship between global sleep score and inflammatory markers in obese adults from The United States. Nat Sci Sleep. 2019;11:317–324. doi:10.2147/NSS.S220436
37. Huang CY, Chen WK, Lu CY, et al. Mediating effects of social support and self-concept on depressive symptoms in adults with spinal cord injury. Spinal Cord. 2015;53(5):413–416. doi:10.1038/sc.2014.158
38. Cook KF, Kallen MA, Bombardier C, et al. Do measures of depressive symptoms function differently in people with spinal cord injury versus primary care patients: the CES-D, PHQ-9, and PROMIS((R))-D. Qual Life Res. 2017;26(1):139–148. doi:10.1007/s11136-016-1363-x
39. Mehta S, Janzen S, McIntyre A, Iruthayarajah J, Loh E, Teasell R. Are comorbid pain and depressive symptoms associated with rehabilitation of individuals with spinal cord injury? Top Spinal Cord Inj Rehabil. 2018;24(1):37–43. doi:10.1310/sci16-00027
40. Huang Y, Su Y, Jiang Y, Zhu M. Sex differences in the associations between blood pressure and anxiety and depression scores in a middle-aged and elderly population: the Irish Longitudinal Study on Ageing (TILDA). J Affect Disord. 2020;274:118–125. doi:10.1016/j.jad.2020.05.133
41. Itrat AGSD. The Association of Clinical Factors and Highly-Sensitive C-Reactive Protein (hsCRP) with Depression in Stroke Patients (P7.162). Neurology. 2014;6(70):1020–1028.
42. Cheng LS, Tu WJ, Shen Y, Zhang LJ, Ji K. Combination of high-sensitivity C-reactive protein and homocysteine predicts the post-stroke depression in patients with ischemic Stroke. Mol Neurobiol. 2018;55(4):2952–2958. doi:10.1007/s12035-017-0549-8
43. Duivis HE, Kupper N, Penninx BW, Na B, de Jonge P, Whooley MA. Depressive symptoms and white blood cell count in coronary heart disease patients: prospective findings from the Heart and Soul Study. Psychoneuroendocrinology. 2013;38(4):479–487. doi:10.1016/j.psyneuen.2012.07.006
44. Cepeda MS, Stang P, Makadia R. Depression is associated with high levels of c-reactive protein and low levels of fractional exhaled nitric oxide: results from the 2007–2012 National Health and Nutrition Examination Surveys. J Clin Psychiatry. 2016;77(12):1666–1671. doi:10.4088/JCP.15m10267
45. Schmidt FM, Schroder T, Kirkby KC, et al. Pro- and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Res. 2016;239:85–91. doi:10.1016/j.psychres.2016.02.052
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
