Back to Journals » Journal of Inflammation Research » Volume 15
Relationship Between Prognostic Nutrition Index and New York Heart Association Classification in Patients with Coronary Heart Disease: A RCSCD-TCM Study
Authors Ma M, Liu Y, Liu F, Li Z, Cheng Q, Liu Z, Yang R, Yu C
Received 16 April 2022
Accepted for publication 1 July 2022
Published 28 July 2022 Volume 2022:15 Pages 4303—4314
DOI https://doi.org/10.2147/JIR.S371045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Mei Ma,1,* Yijia Liu,1,* Fanfan Liu,1 Zhu Li,1 Qi Cheng,1 Zhao Liu,2,* Rongrong Yang,1 Chunquan Yu1
1Department of Graduate Schools, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 2Department of Information Center, Second Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Rongrong Yang; Chunquan Yu, Department of Graduate Schools, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China, Email [email protected]; [email protected]
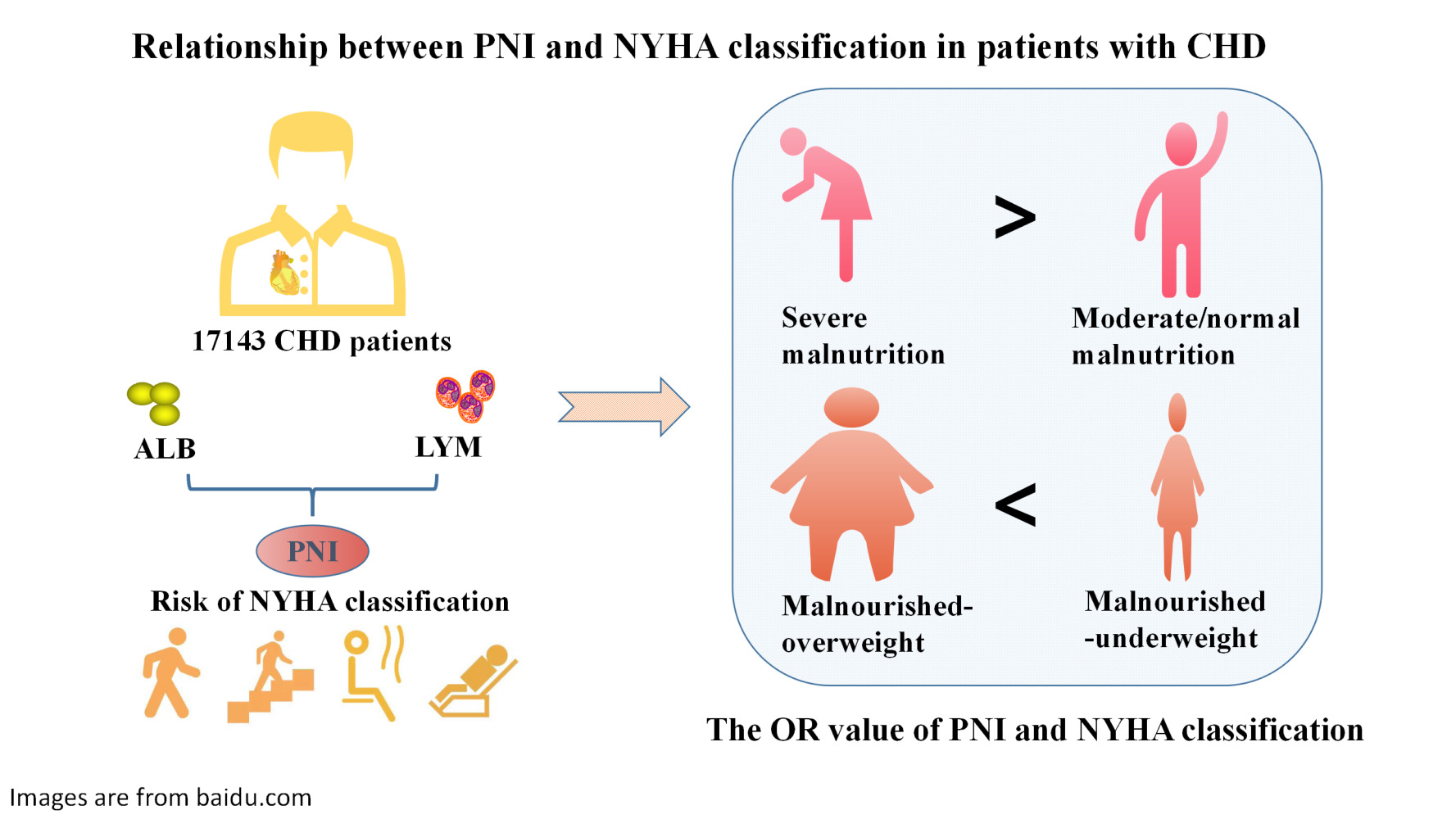
Aim: This study aimed to elucidate the relationship between the prognostic nutrition index (PNI) of patients with coronary heart disease (CHD) and the New York Heart Association (NYHA) classification and the complex relationship between PNI combined body mass index (BMI) and NYHA classification.
Methods: The PNI was applied to 17,413 consecutive patients with CHD. Patients were divided into three groups according to PNI: normal nutrition (PNI ≥ 38), moderate malnutrition (35 < PNI < 38), and severe malnutrition (PNI ≤ 35). A total of 2,052 CHD patients with BMI were selected and stratified by combined subgroups of nutritional status and BMI. Logistic regression analysis was used to evaluate the relationship between the PNI and NYHA classification and to adjust for confounding factors.
Results: The prevalence of malnutrition among the 17,413participants with CHD was 4.2%. Moderate and severe malnutrition were significantly related to NYHA class III and V, and the strongest relationship was observed in NYHA class V (odd ratio [OR]: 6.564; 95% confidence interval [CI]: 4.043– 10.658). Malnourished-underweight patients and malnourished-overweight patients were significantly associated with higher NYHA classification, and malnourished-underweight patients (OR: 8.038; 95% CI: 2.091– 30.892) were significantly more than malnourished-overweight patients (OR: 3.580; 95% CI: 1.286– 9.966).
Conclusion: There were differences in the NYHA classification of CHD patients with different nutritional statuses. The lower the PNI, the worse the NYHA classification of CHD patients. Malnourished-underweight patients had a worse NYHA classification than malnourished-overweight patients.
Keywords: coronary heart disease, prognostic nutrition index, malnourished-underweight, malnourished-overweight, New York Heart Association classification
Graphical Abstract:
Introduction
Cardiovascular diseases (CVD) are the leading causes of death and disease burden globally.1–3 The incidence rate increased from 271 million in 1990 to 523 million in 2019, and the death toll increased steadily from 12.1 million in 1990 to 18.6 million in 2019, making it an important public health challenge.4,5 Atherosclerosis (AS) is the major cause of coronary heart disease (CHD) and other CVD. In 2016, approximately 2.4 million people died of atherosclerotic cardiovascular disease (ASCVD), accounting for 61% of all CVD deaths.6,7 Malnutrition is usually caused by insufficient or unbalanced nutrient intake, leading to insufficient calories, protein, or other nutrients needed for tissue maintenance and repair. Currently, malnutrition affects every stage of life and is a global health problem affecting more than 1 billion people. However,8 to a large extent, clinicians understand the insufficiency and shortage of treatment.9 Malnutrition is common in patients with CVD, and various physiological changes and psychological pressures caused by CVD affect the nutritional status of individuals, especially in some acute diseases, which often lead to nutritional imbalance. Therefore, rapid assessment and improvement in nutritional status play a vital role in the treatment and prognosis of patients with CVD.10 Prognostic nutritional index (PNI) is a new nutritional immune inflammation index determined by analyzing the complex linear prediction model of serum albumin level, transferrin, delayed hypersensitivity, and triceps skinfold thickness.11 PNI has been used to evaluate the nutritional status of patients with various diseases and is easy to calculate. Recently,12,13 studies have shown that PNI is independently related to the long-term survival of hospitalized patients with severely decompensated acute heart failure,14,15 and it is an important independent predictor of coronary collateral development and clinical outcome in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention.16 Recent research has shown that PNI is inversely and significantly associated with the development of contrast-associated acute kidney injury in acute STEMI. Assessing PNI at admission may be useful for the early risk stratification of patients with STEMI.17 Another study showed that a lower PNI value is independently related to the in-hospital mortality of acute Stanford type A aortic dissection (ATAAD) and may be a useful tool for predicting the early mortality of ATAAD patients after surgical repair.18,19 However, malnutrition assessed by PNI is manifested as being underweight or overweight. PNI combined with body mass index (BMI) may better reflect the nutritional status of the patients. However, few studies have explored the relationship between PNI and New York Heart Association (NYHA) classification, and data are particularly scarce for people with CHD. Therefore, we aimed to elucidate the relationship between the PNI of patients with CHD and NYHA classification and the complex relationship between the PNI combined with the BMI and NYHA classification.
Methods
Participants
A total of 107,301 patients with CHD diagnosed in six tertiary hospitals in Tianjin, China, from January 1, 2014, to September 30, 2020, were selected as the study participants. Patients with CHD were identified with at least one or more of the International Classification of Disease codes obtained from the hospital’s diagnosis and treatment system.
Patients below 35 or above 75 years, patients with severe liver and renal failure, infection, tumor, and cancer, and patients without clear NYHA classification and lack of PNI were included in this study. Finally, 17,413 patients were enrolled in the study. A flow chart of case screening is shown in Figure 1. This study was conducted in accordance with the principles of the Declaration of Helsinki. The registration number of the China Clinical Trials Register is ChiCTR1900024535, and the registration number of American Clinical Trials.gov is NCT04026724. This study was approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine. The approval number for the reviewed institutions is TJUTCM-EC2019008.
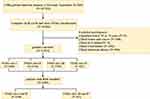 |
Figure 1 The flow chart of case screening. |
Data Collection
We collected the clinical data of patients’ hospitalization, including demographics, such as age, sex, ethnicity, smoking, and drinking history, from the hospital diagnosis and treatment system. Basic demographic data were collected and recorded by the hospital doctors through standard structured questionnaires 4 h after admission. The NYHA classification was evaluated by a doctor within 24 h after admission. Fasting blood samples were collected from the study center in the morning.20 Lymphocytes (LYM) were measured using an automatic hematology analyzer. Albumin (ALB), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), glycated hemoglobin A1c (HbA1c), and lactate dehydrogenase (LDH) levels were measured using an automatic biochemical analyzer. Fibrinogen (FIB) and D-dimer levels were measured using an automatic coagulation analyzer. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in the right arm artery of the subject using a standard electronic sphygmomanometer after the patient rested in a supine position for 5 min. The accuracy and reproducibility of the results were ensured by consistent training and systematic analytical processes of hospital doctors.21,22 Hypertension was defined as SBP ≥140 mmHg and DBP ≥90 mmHg.23,24 Hyperlipidemia was defined as TC ≥6.2 mmol/L (240 mg/dL), TG ≥2.3 mmol/L (200 mg/dL) or LDL-C ≥4.1 mmol/L (160 mg/dL), HDL-C≤1.0 mmol/L (40 mg/dL), or the use of oral lipid-lowering drugs or a self-reported doctor’s diagnosis.25 Type 2 diabetes was defined as a FBG level ≥7.0 mmol/L, the use of oral hypoglycemic agents or insulin, or a self-reported doctor’s diagnosis.26,27
The PNI was calculated as follows: albumin (ALB, g/L)+ 5×lymphocytes (LYM, 109/L). According to the PNI, patients were divided into three groups: normal nutrition group (PNI≥38), moderate malnutrition (35<PNI<38), and severe malnutrition (PNI≤35).28 According to the NYHA classification, cardiac function can be divided into four grades: NYHA class I: physical activity is unrestricted, and daily activities do not cause excessive fatigue, dyspnea, or palpitation; NYHA class II: slightly limited physical activity, no symptoms at rest, daily activities can cause fatigue, palpitation, dyspnea, or angina pectoris; NYHA class III: physical activity is considerably limited, and there are no symptoms at rest, which can be caused by activities lighter than daily activities; NYHA class V: cannot engage in any physical activity, and also have symptoms of congestive HF or angina pectoris at rest, which is aggravated after any physical activity.
A total of 2,052 patients with BMI data were screened from 17,413 participants. BMI was calculated for all patients and defined as the body weight (in kilograms) divided by the square of the body height (in meters).29 According to the World Health Organization (WHO) Asian critical value, BMI was divided into underweight (BMI≤18.5), normal weight (18.5<BMI<25), and overweight (BMI≥25). Patients were stratified by combined subgroups of nutritional status and BMI: well-nourished-normal weight group (PNI≥38, 18.5<BMI<25), well-nourished-overweight group (PNI≥38, BMI≥25), well-nourished-underweight group (PNI≥38, BMI≤18.5), malnourished-normal weight group (PNI<38, 18.5<BMI<25), malnourished-overweight group (PNI<38, BMI≥25), and malnourished-underweight group (PNI<38, BMI≤18.5).
Statistical Analyses
Statistical analyses were performed using IBM SPSS Statistics 19.0 (IBM Corp, New York, NY, USA). Descriptive statistics were presented as median (interquartile range (IQR)) for continuous variables and number (%) for categorical variables. Baseline characteristics were compared using the Kruskal–Wallis H analysis of variance test and χ2 test for continuous and categorical variables, respectively. Ordered logistic regression analysis was used to evaluate the relationship between the PNI of patients with CHD and the NYHA classification. However, ordered logistic regression analysis violated the test proportional odds assumption. Accordingly, multivariate logistic regression analysis was used to evaluate the relationship between the PNI of patients with CHD and the NYHA classification. Binary logistic regression analysis of the relationship between PNI combined with BMI and the NYHA classification was performed. Three logistic regression models were constructed: Model a: unadjusted; Model b: adjusted for age, sex, SBP, DBP, FBG, and HbA1c; Model c was based on model 2 with TC, TG, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensive medication, and use of antilipidemic medication. Collinearity between independent variables was evaluated to ensure that it was appropriate to include them in the same model. Missing values were imputed using the multiple imputation method, calculated by the chain equation, and five complete datasets were created. Statistical significance was set at P<0.05.
Result
Baseline Demographics of the Study Participants
The baseline demographics of the study participants are shown in Table 1. The mean age of the patients was 64 (59–69) years, males constituted 55.5%. The highest number of patients with NYHA class II was 9,739 (55.9%), 7,071 (40.6%) patients had hypertension, and 5,739 (33.0%) patients had diabetes. FBG, LDH, FIB, D-dimer, diabetes, current antihypertensive medication, and hospitalization time were positively correlated with malnutrition, whereas HDL-C was negatively correlated with malnutrition.
 |
Table 1 Baseline Demographics of Study Participants |
Relationship Between Univariate and NYHA Classification
As shown in Table 2, sex, age, SBP, FBG, HbA1c, TC, TG, HDL-C, LDL-C, FIB, D-dimer, LDH, drinking history, current antilipidemic medication, and current antihypertensive medication were all related to NYHA classes II, III, and IV; smoking history was related to NYHA classes II and III. Hypertension history was related to NYHA classes II and IV. DBP and diabetes history were related to NYHA class III and IV.
 |
Table 2 Relationship Between Univariate and NYHA Classification |
Relationship Between PNI Index and NYHA Classification
Three logistic regression models were constructed to evaluate the relationship between the PNI and NYHA classification (Table 3). In the multivariate logistic regression analysis, when the PNI was a continuous variable, it was significantly related to NYHA class III and IV. The strongest association was observed for NYHA class IV (odd ratio [OR]: 0.863; 95% confidence interval [CI]: 0.850–0.877). After PNI was used as a classification variable, moderate and severe malnutrition were significantly related to NYHA class III and IV, and the highest association was observed in NYHA class IV (OR: 8.613; 95% CI: 5.526–13.424). In the further adjusted Model 3 (Figure 2), moderate and severe malnutrition were still significantly related to NYHA class III and IV, and the highest association was observed in NYHA class IV (OR: 6.564; 95% CI: 4.043–10.658).
 |
Table 3 Relationship Between PNI and Classification of NYHA |
 |
Figure 2 Relationship between PNI and NYHA classification, **:P<0.01. Abbreviations: PNI, prognostic nutrition index; NYHA, New York Heart Association classification. |
Relationship Between PNI Combined with BMI and NYHA Classification
Three logistic regression models were constructed to evaluate the association between PNI, BMI, and NYHA classification (Table 4). In the multivariate logistic regression analysis, we observed that malnourished-underweight patients and malnourished-overweight patients were significantly associated with poor NYHA classification, and malnourished-underweight patients (OR: 10.132; 95% CI: 2.712–37.852) were more than malnourished-overweight patients (OR: 4.825; 95% CI: 1.809–12.870). In the further adjusted Model c (Figure 3), the number of malnourished-underweight patients (OR: 8.038; 95% CI: 2.091–30.892) was still higher than that of malnourished-overweight patients (OR: 3.580; 95% CI: 1.286–9.966).
 |
Table 4 Relationship Between PNI Combined BMI and NYHA Classification |
 |
Figure 3 Relationship between PNI combined BMI and NYHA classification, **:P<0.01. |
Discussion
This study demonstrated that PNI, a new index of immune nutrition and inflammation, was significantly related to the NYHA classification of patients with CHD.18,30 Previous studies have shown that malnutrition is highly prevalent among patients with congestive heart failure (CHF) and is strongly associated with NYHA classification, disease severity, and increased mortality.6 One study that examined the acute coronary syndrome (ACS) effect of malnutrition showed that malnutrition may be the trigger of ACS risk,31 leading to an increase in AS burden and the risk of plaque rupture, and is closely related to an increase in mortality and cardiovascular events.32,33 Another study showed that PNI was significantly related to ventricular remodeling and diastolic function. An increase in PNI may improve cardiac function and reduce the incidence of cardiovascular events, thus reducing mortality. These results are consistent with our results.34–36
Generally, obesity is a recognized and important predictor of morbidity and mortality of patients with CVD and other diseases (including chronic nephropathy and chronic obstructive pulmonary disease).37,38 However, some studies have reported that obesity is related to reduced mortality in patients with HF.39–41 A meta-analysis using BMI to relate nutritional status with HF has shown that obese patients with HF lived longer than non-obese patients; this finding was called the “obesity paradox”. Importantly, we observed that malnourished-underweight patients had a higher risk of worse NYHA classification than malnourished-overweight patients in this study, which further verified the “obesity paradox”, which is highly debated. Interestingly,32 recent studies have shown that malnourished-overweight individuals have the highest burden of complications, unsuitable cardiac remodeling, and the worst cardiac prognosis compared to malnourished-underweight individuals. We believe this inconsistency may be because BMI, waist-hip ratio, and body fat percentage were used as the definitions for being overweight and underweight in this study. In contrast, BMI was only used as the definition of weight loss and obesity in our study.42,43 Studies have shown that the obesity paradox is observed only when BMI is used to define obesity, further verifying that our hypothesis may be reasonable.
This was a large-scale, multicenter clinical study in China, with a relatively large amount of data and more convincing results. However, this study had some limitations. First, this was a cross-sectional study, which could not explain the causal relationship between the PNI and NYHA classification in patients with CHD. In addition, we used only the NYHA classification to evaluate CHD severity. Other examination results, such as the coronary angiography Gensini score, may be more sensitive; however, these examination results were relatively few in our study. Hence, this relationship needs to be verified in future prospective studies combined with more objective indicators such as the Gensini score and coronary artery lesion index. Finally, due to the missing data on BMI in this study, the number of malnourished-overweight and malnourished-underweight patients was relatively small; thus, the results may be biased.
Nevertheless, our study was novel in comprehensively evaluating the relationship between the PNI of patients with CHD and NYHA classification and the complex relationship between the PNI combined with BMI and NYHA classification.
Conclusion
Overall, PNI was significantly related to the NYHA classification. Clinicians can highlight PNI to evaluate the nutrition of patients with CHD and perform early interventions, especially for malnourished underweight patients.
Abbreviations
RCSCD-TCM, Retrospective Cohort Study on Adjuvant Treatment of Coronary Heart Disease Angina Pectoris With Chinese Patent Medicine; PNI, Prognostic Nutrition Index; NYHA, New York Heart Association; BMI, body mass index; CHD, coronary heart disease; AS, atherosclerosis; CVD, cardiovascular diseases; ASCVD, atherosclerotic cardiovascular disease; STEMI, ST-segment elevation myocardial infarction; ATAAD, Stanford type A aortic dissection; WHO, World Health Organization; CHF, congestive heart failure; ACS, acute coronary syndrome; HF, heart failure; LYM, Lymphocytes; ALB, Albumin; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FBG, Fasting blood glucose; HbA1c, Glycosylated hemoglobin A1c; TC, Total cholesterol; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; LDH, lactate dehydrogenase; FIB, Fibrinogen; IQR, Interquartile range; OR, odds ratio; CI, confidence interval; β, regression coefficient.
Ethics Approval and Informed Consent
All research data were obtained from the hospital’s medical record system, and informed consent was not obtained. To protect patients’ privacy, the information included in our research was anonymous, and all traceable personal identifiers were deleted from the analysis dataset; hence, the need for patients’ informed consent was waived. All researchers involved in this study have the responsibility to protect patient data. All research data of this study should be kept strictly confidential, and the leaker should bear any consequences caused by leakage. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Tianjin University of Traditional Chinese Medicine (TJUTCM-EC2019008). It has been registered in the Clinical Trials.gov (registration number: NCT04026724), and the Chinese Clinical Trial Registration Centre (registration number: ChiCTR1900024535).
Data Sharing Statement
The data supporting the research results can be obtained from the corresponding author.
Acknowledgments
We would like to thank the six hospitals in Tianjin that provided data, including the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Second Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin Chest Hospital, Tianjin Nankai Hospital, Tianjin Medical University General Hospital, and Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital. Mei Ma, Yijia Liu, Zhao Liu are co-first authors.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, and took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Basic Research Program of China [973 project, grant number: 2014CB542902]
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
2. Okui T. Age-period-cohort analysis of cardiovascular disease mortality in Japan, 1995–2018. J Prev Med Public Health. 2020;53(3):198–204. doi:10.3961/jpmph.20.037
3. Xing DM, Zhu MJ, Liu CX, Wang H. Outcome measures in clinical trials of traditional Chinese medicine for stable angina pectoris. Acupunct Herb Med. 2021;1(2):99–106. doi:10.1097/HM9.0000000000000014
4. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi:10.1161/CIR.0000000000001052
5. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16(4):203–212. doi:10.1038/s41569-018-0119-4
6. Freeman AM, Aggarwal M. Malnutrition in the obese: commonly overlooked but with serious consequences. J Am Coll Cardiol. 2020;76(7):841–843. doi:10.1016/j.jacc.2020.06.059
7. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: Academy of Nutrition and Dietetics and American Society for parenteral and enteral nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36(3):275–283. doi:10.1177/0148607112440285
8. Bellanti F, Lo Buglio A, Quiete S, Vendemiale G. Malnutrition in hospitalized old patients: screening and diagnosis, clinical outcomes, and management. Nutrients. 2022;14(4):910. doi:10.3390/nu14040910
9. Kinugawa S, Fukushima A. Malnutrition in heart failure: important but undervalued issue. JACC Heart Fail. 2018;6(6):487–488. doi:10.1016/j.jchf.2018.03.014
10. House M, Gwaltney C. Malnutrition screening and diagnosis tools: implications for practice. Nutr Clin Pract. 2022;37(1):12–22. doi:10.1002/ncp.10801
11. Ando T, Yoshihisa A, Kimishima Y, et al. Prognostic impacts of nutritional status on long-term outcome in patients with acute myocardial infarction. Eur J Prev Cardiol. 2020;27(19):2229–2231. doi:10.1177/2047487319883723
12. Cheng YL, Sung SH, Cheng HM, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6(6):e004876. doi:10.1161/JAHA.116.004876
13. Shirakabe A, Hata N, Kobayashi N, et al. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessels. 2018;33(2):134–144. doi:10.1007/s00380-017-1034-z
14. Akbuğa K, Ferik ÖK, Yayla KG, et al. Prognostic nutritional index as a new prediction tool for coronary collateral development. Acta Cardiol Sin. 2022;38(1):21–26. doi:10.6515/ACS.202201_38(1).20210906A
15. Chen QJ, Qu HJ, Li DZ, et al. Prognostic nutritional index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Sci Rep. 2017;7(1):3285. doi:10.1038/s41598-017-03364-x
16. Kurtul A, Gok M, Esenboga K. Prognostic nutritional index predicts contrast-associated acute kidney injury in patients with ST-Segment elevation myocardial infarction. Acta Cardiol Sin. 2021;37(5):496–503. doi:10.6515/ACS.202109_37(5).20210413A
17. Keskin HA, Kurtul A, Esenboğa K, Çiçek MC, Katırcıoğlu SF. Prognostic nutritional index predicts in-hospital mortality in patients with acute Stanford type A aortic dissection. Perfusion. 2021;36(7):710–716. doi:10.1177/0267659120961937
18. Sze S, Pellicori P, Kazmi S, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. 2018;6(6):476–486.
19. Webb P, Stordalen GA, Singh S, Wijesinha-Bettoni R, Shetty P, Lartey A. Hunger and malnutrition in the 21st century. BMJ. 2018;361:k2238. doi:10.1136/bmj.k2238
20. Li Z, He Y, Wang S, et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):38. doi:10.1186/s12933-022-01470-3
21. Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324(12):1190–1200. doi:10.1001/jama.2020.14545
22. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138(17):e426–e483. doi:10.1161/CIR.0000000000000597
23. Opoku S, Gan Y, Yobo EA, et al. Awareness, treatment, control, and determinants of dyslipidemia among adults in China. Sci Rep. 2021;11(1):10056. doi:10.1038/s41598-021-89401-2
24. Yang L, Li Z, Song Y, et al. Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin Chim Acta. 2019;495:365–373. doi:10.1016/j.cca.2019.05.001
25. American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8–S16. doi:10.2337/dc15-S005
26. Cadwell JB, Afonso AM, Shahrokni A. Prognostic nutritional index (PNI), independent of frailty is associated with six-month postoperative mortality. J Geriatr Oncol. 2020;11(5):880–884. doi:10.1016/j.jgo.2020.03.013
27. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi:10.1016/0002-9610(80)90246-9
28. White PD, Myers MM. The classification of cardiac diagnosis. JAMA. 1921;77:1414–1415.
29. Weir CB, Jan A. BMI classification percentile and cut off points. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
30. Duarte RRP, Gonzalez MC, Oliveira JF, Goulart MR, Castro I. Is there an association between the nutritional and functional parameters and congestive heart failure severity? Clin Nutr. 2021;40(5):3354–3359. doi:10.1016/j.clnu.2020.11.008
31. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. 2020;76(7):828–840. doi:10.1016/j.jacc.2020.06.058
32. Chien SC, Chandramouli C, Lo CI, et al. Associations of obesity and malnutrition with cardiac remodeling and cardiovascular outcomes in Asian adults: a cohort study. PLoSMed. 2021;18(6):e1003661.
33. Yamada S, Yoshihisa A, Hijioka N, et al. Associations of the prognostic nutritional index with the cardiac function and survival after cardiac resynchronization therapy. Intern Med. 2021;60(7):985–991. doi:10.2169/internalmedicine.5961-20
34. Angadi SS, Gaesser GA. Body-mass index and all-cause mortality. Lancet. 2017;389(10086):2285. doi:10.1016/S0140-6736(17)31438-1
35. Ladhani M, Craig JC, Irving M, Clayton PA, Wong G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32(3):439–449. doi:10.1093/ndt/gfw075
36. Valenzuela PL, Santos-Lozano A, Barrán AT, et al. Joint association of physical activity and body mass index with cardiovascular risk: a nationwide population-based cross-sectional study. Eur J Prev Cardiol. 2022;29(2):e50–e52. doi:10.1093/eurjpc/zwaa151
37. Khan A, Van Iterson EH, Laffin LJ. The obesity paradox in heart failure: what is the role of cardiorespiratory fitness? Cleve Clin J Med. 2021;88(8):449–458. doi:10.3949/ccjm.88a.20098
38. Wakabayashi H, Maeda K, Nishioka S, Shamoto H, Momosaki R. Impact of body mass index on activities of daily living in inpatients with acute heart failure. J Nutr Health Aging. 2019;23(2):151–156. doi:10.1007/s12603-018-1111-8
39. Wawrzeńczyk A, Anaszewicz M, Wawrzeńczyk A, Budzyński J. Clinical significance of nutritional status in patients with chronic heart failure-A systematic review. Heart Fail Rev. 2019;24(5):671–700. doi:10.1007/s10741-019-09793-2
40. Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61(2):151–156. doi:10.1016/j.pcad.2018.05.005
41. Donataccio MP, Vanzo A, Bosello O. Obesity paradox and heart failure. Eat Weight Disord. 2021;26(6):1697–1707. doi:10.1007/s40519-020-00982-9
42. Chandramouli C, Tay WT, Bamadhaj NS, et al. Association of obesity with heart failure outcomes in 11 Asian regions: a cohort study. PLoS Med. 2019;16(9):e1002916. doi:10.1371/journal.pmed.1002916
43. Czapla M, Juárez-Vela R, Łokieć K, Karniej P. The association between nutritional status and in-hospital mortality among patients with heart failure-A result of the retrospective nutritional status heart study 2 (NSHS2). Nutrients. 2021;13(5):1669. doi:10.3390/nu13051669
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
