Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Relationship Between Gallstone Disease and Cardiometabolic Risk Factors in Elderly People with Non-Alcoholic Fatty Liver Disease
Authors Hung MC , Chen CF, Tsou MT , Lin HH , Hwang LC , Hsu CP
Received 12 June 2020
Accepted for publication 6 August 2020
Published 9 October 2020 Volume 2020:13 Pages 3579—3585
DOI https://doi.org/10.2147/DMSO.S266947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Man-Chen Hung,1 Chuen-Fei Chen,1 Meng-Ting Tsou,2 Hsin-Hui Lin,2 Lee-Ching Hwang,1,2 Ching-Ping Hsu2
1Department of Medicine, Mackay Medical College, New Taipei City, Taiwan; 2Department of Family Medicine, Mackay Memorial Hospital, Taipei, Taiwan
Correspondence: Ching-Ping Hsu
Department of Family Medicine, Mackay Memorial Hospital, No. 92, Sec. 2, Zhongshan N. Road, Zhongshan District, Taipei City 104, Taiwan
Tel +886-2-25433535
Fax +886-2-25213847
Email [email protected]
Background: The prevalence of gallstone disease (GSD) increases with age, and the elderly have a much higher mortality risk and incidence of surgical comorbidities. The aim of this study was to explore the relationship between GSD and cardiometabolic risk factors in elderly people with non-alcoholic fatty liver disease (NAFLD).
Methods: In this cross-sectional study, we analyzed the data of elderly people who underwent annual health check-ups at a Northern Taiwan health examination center. These data were collected from physical examination, blood tests, abdominal ultrasonography, and medical histories. We excluded those with hepatitis B or C infections, heavy alcohol consumption, or cholecystectomy.
Results: The analysis included 3,037 participants with a mean age of 73.6± 6.0 years. Over 70% were overweight or obese, and the overall prevalence of GSD was 17.7%. In our univariate analysis, GSD was positively correlated with age, body mass index, metabolic syndrome, diabetes mellitus (DM), hypertension (HTN), and various metabolic factors (fasting plasma glucose [FPG], triglyceride, uric acid, and high-density lipoprotein cholesterol [HDL-C] levels). After adjustment for age, gender, and body mass index, metabolic syndrome showed a positive association with GSD (odds ratio [OR] 1.31 [95% confidence interval [CI], 1.05– 1.64]; P=0.020). Specific components of metabolic syndrome that increased the risk for GSD in NAFLD elderly include lower levels of HDL-C (OR 1.35 [95% CI, 1.10– 1.66]; P< 0.001) and elevated FPG (OR 1.36 [95% CI, 1.10– 1.69]; P< 0.001).
Conclusion: Our study concluded that GSD is significantly associated with metabolic syndrome in elderly people with NAFLD. Reduced HDL-C and elevated FPG both heighten the risk of developing GSD. Therefore, to lower the risk of GSD in NAFLD patients, their FPG levels and HDL-C levels must be regularly followed-up, and these patients should be educated about the symptoms of GSD if they meet the criteria for metabolic syndrome.
Keywords: gallstone disease, cardiometabolic risk factors, metabolic syndrome
Introduction
The prevalence of gallstone disease (GSD) in Taiwan varies from 4.3% to 10.7% and rapidly increases in individuals aged over 40 years.1–5 Although GSD is asymptomatic in two-thirds of cases, the medical burden related to GSD should not be neglected. Previous studies suggested that the cumulative probability of developing biliary colic among asymptomatic patients after 10 years ranges between 15% and 25%, and the mortality rate increases once severe complications occur.6,7
Taiwan is predicted to become a super-aged society in 2026. Older patients with GSD are expected to require surgical treatment for GSD-related conditions.8,9 In addition, elderly patients had a higher risk of mortality after a cholecystectomy and a higher incidence of peri-operative complications than younger ones.9–11
Previous studies demonstrated that GSD is strongly associated with female gender, advanced age, race, obesity, hyperlipidemia, alcohol consumption, liver cirrhosis, diabetes mellitus (DM), and metabolic syndrome (MetS).1,4,12–16 In particular, obesity, hyperlipidemia, DM, and MetS are notorious cardiometabolic risk factors. The risk factors of non-alcoholic fatty liver disease (NAFLD) and GSD are highly overlapped but the sequential relationship of NAFLD and GSD remains controversial.17 As predisposing factors are metabolic abnormalities, NAFLD is also regarded as the liver manifestation of the metabolic syndrome.18–20
NAFLD in the elderly is more severe and carries worse prognosis and more comorbidities such as cirrhosis and hepatocellular carcinoma than younger age groups.21 Age-related alterations in hepatic cholesterol homeostasis cannot be defined yet; however, some studies support that a decline of liver function by aging may result in abnormal cholesterol metabolism, including a decrease in low-density lipoprotein cholesterol (LDL-C) turnover and an increase in biliary cholesterol output. This is possibly associated with a reduction in cholesterol degradation to bile acid which creates a suitable environment for cholesterol gallstone to generate.22
It is expected that Taiwan will become a super-aged society in 2026 and the increase in geriatric population raises concern for even more NAFLD elderly at risk for MetS and GSD. Therefore, our current study aims to explore the relationship between GSD and cardiometabolic risk factors in this high-risk population.
Materials and Methods
Study Population
This study examined 3,233 people aged ≥65 who underwent annual health checkups at a health examination center in Northern Taiwan. Fatty liver was diagnosed by abdominal ultrasound. We collected data including physical examination, blood tests, abdominal ultrasonography, health-related behavior, and medical histories. After we excluded those who had a history of hepatitis B or C infection, consumed alcohol regularly, or had received a cholecystectomy, the final sample for analyses consisted of 3,037 elderly people.
Study Design and Variables
This cross-sectional study collected data regarding age, sex, height, weight, waist circumference, body mass index (BMI), blood pressure, and medical history of DM and hypertension (HTN). Blood biochemical data included fasting plasma glucose (FPG), lipid profile (total cholesterol, high-density lipoprotein cholesterol [HDL-C], LDL-C, and triglyceride), uric acid, and liver function tests (aspartate transaminase [AST] and alanine transaminase [ALT]).
Diagnostic Criteria
According to the Taiwan National Health Department, those who met at least three of the following criteria were considered to have MetS: 1) abdominal obesity (waist circumference ≥90 cm in men and ≥80 cm in women); 2) elevated triglyceride levels (≥150 mg/dL); 3) reduced serum HDL-C levels (<40 mg/dL in men and <50 mg/dL in women); 4) elevated blood pressure (systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or a history of HTN based on self-reports or medical records); and 5) elevated FPG levels (≥100 mg/dL) or a history of DM (based on self-reports or medical records).
Although the gold standard for the diagnosis of NAFLD is percutaneous liver biopsy, our study chose to use abdominal sonography as the diagnostic method for the following reasons. First, there are certain complications of liver biopsy, including haemorrhage and pneumothorax.23 Second, none of our subjects met the indications for a biopsy, which includes 1) to diagnose patients a high risk for nonalcoholic steatohepatitis (NASH) or advanced fibrosis; 2) to determine the extent of liver damage for treatment and prognosis.24,25 Lastly, using sonography as the first imaging procedure by a well-trained gastrologist is a reliable way for diagnosing fatty liver.26
Diagnosis of NAFLD by ultrasonography was according to the following criteria: 1) increased echogenicity of liver parenchyma in contrast to the kidney; 2) decreased clarity of the borders of intrahepatic vessels; and 3) decreased penetration of ultrasound signal and visibility of the diaphragm.26
GSD was diagnosed based on at least one of the following criteria: 1) visible gallbladder lumen presenting one or more echogenic movable structures with an acoustic shadow. 2) non-visualization of the gallbladder lumen and high-density echogenic material with acoustic shadow in the gallbladder fossa.27,28
Statistical Analysis
All statistical analyses were performed using SPSS version 26.0 software (IBM Corp.). The participants were classified into two groups based on the presence or absence of GSD. Continuous variables are expressed as means with standard deviations while categorical variables are expressed in the form of frequencies and percentages.
The two groups were compared using the Student’s t-test (continuous variables) and the Χ2 test (categorical variables). Variables in the univariate analyses that showed significant association with GSD were included in a binary logistic regression model. In model 1, we controlled potential confounders including age, gender, BMI, DM, and HTN to compare if MetS increased the risk of GSD. In model 2, we added the five components of MetS to determine the specific factors associated with risk of GSD. The odds ratios (ORs) and 95% confidence intervals (CIs) were presented, and any P-value <0.05 was considered statistically significant.
Results
The final sample for analyses included 3,037 eligible participants, including 886 (29.2%) men and 2,151 (70.8%) women, with a mean age of 73.6±6.0 years. The overall prevalence of participants with GSD was 17.7% (18.4% in men vs 17.4% in women).
Over 70% of all participants were either overweight or obese (40.7% having a BMI of 24.0–26.9 kg/m2 and 32.4% having a BMI ≥27 kg/m2) with a mean FPG of 110.3±23.7 mg/dL. Participants with MetS, DM, and HTN accounted for 63.2%, 18.2%, and 56.9% of the total population, respectively.
The differences in age, gender, and individual metabolic components between the GSD and non-GSD groups are shown in Table 1. Compared to the non-GSD group, the GSD group were of older age, had a higher BMI, and a larger waist circumference. In addition, they had a higher prevalence of MetS, DM, and HTN (P<0.05). In respect to the metabolic parameters, the GSD group had higher levels of FPG, triglyceride, uric acid, but lower levels of HDL-C than the non-GSD group (P<0.05).
 |
Table 1 Elderly People’s Demographic Characteristics, Biochemical Variables and Metabolic Components, by the Presence of Gallstone Disease |
The binary logistic regression models in Table 2 show the associations between clinical factors and the risk of GSD. After adjustment for age, gender, and BMI, MetS was positively associated with GSD (OR 1.31 [95% CI, 1.05–1.64]; P=0.02). Among the components of MetS, reduced HDL-C (OR 1.35 [95% CI, 1.10–1.66]; P<0.001) and elevated FPG (OR 1.36 [95% CI, 1.10–1.69]; P<0.001) were associated with a higher risk for GBD.
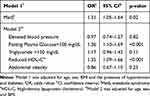 |
Table 2 Binary Logistic Regression Models for Predicting Factors Associated with the Development of Gallstone Disease |
Discussion
Gallstones can be distinguished into cholesterol stone, black pigment stones, and brown pigment stones.29 Cholesterol stones result from supersaturated cholesterol in the bile, which associated with abnormal cholesterol metabolism. On the other hand, black pigment stones form in sterile bile and are primarily related to chronic hemolysis or cirrhosis while brown pigment stones are relevant to bacterial infection.30,31
One Taiwanese study revealed that the percentage of cholesterol and pigment stones had been roughly 40% and 60%, respectively.32 Due to the improvement of living standards and Westernization of diet, the proportion of cholesterol stones is increasing. Metabolic risk factors are considered determinants of cholesterol gallstone formation.
GSD and Sex
In our study, prevalence of GSD in elderly men with NAFLD was 18.4%, compared to 17.4% in their female counterparts, indicating no significant association between GSD and sex. However, the role of sex in the development of GSD is inconsistent across studies. Unlike studies in Asian countries, most of those conducted in Western countries showed that female sex is a risk factor for GSD.4,5,33 Besides, the composition of gallstones varies across races. Cholesterol gallstones are more prevalent in Western populations (70~80%) while pigment gallstones, which are related to bacterial infection, liver disease, or other medical conditions, are predominant in Asian populations.34,35 Female sex is reported to be highly associated with GSD in Western studies possibly because estrogen increases the concentration of cholesterol in bile, leading to the formation of cholesterol gallstones.36,37 Like some Taiwanese studies, our study demonstrated that sex distribution was not an influential factor for GSD development in Taiwanese people.1,16 Some studies suggest that GSD was closely associated with the use of oral contraceptives but not with female sex, which indicates that estrogen may be more predominant than sex alone.16 However, as our study only focused on elderly participants with NAFLD, this hormone played a relatively minor role in their metabolic systems. Thus, sex distribution was not a crucial contributor to the development of GSD in our study population.
GSD and Cardiometabolic Risk Factors
Cardiometabolic risk factors consist of obesity, insulin resistance, and hyperlipidemia. The relationship between GSD with these risk factors will be explained separately in the following paragraphs.
Obesity
The overall GSD prevalence in the elderly people with NAFLD in our current study was 17.7%, higher than that in other Asian studies (3–15%).38 This high prevalence may result from the characteristics of our study participants aged ≥65 years who were also diagnosed with NAFLD.1,16 Besides, obesity is characteristic of our study population because over 70% of the elderly people in our study were overweight or obese (40.7% and 32.4% having a BMI of 24.0–26.9 kg/m2 and ≥27 kg/m2, respectively). Obesity is an important risk factor for GSD possibly attributed to hepatic lipid accumulation, leading to the dysfunction of gallbladder contraction.19,28,39,40
Insulin Resistance
One pathophysiological hypothesis of metabolic syndrome is insulin resistance.41 Some studies supported that an increase in insulin concentration was associated with an increased relative risk of developing GSD.42
The mechanism is that hyperinsulinemia stimulates the activity of hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and increases bile cholesterol saturation.34,39,43 In addition, according to an animal study, hepatic insulin resistance markedly prompted cholesterol gallstone formation. This may result from the changes in specific transcription factors that cause biliary cholesterol secretion and bile acid reduction.44 On the other hand, in the elderly, decreased liver function and blood perfusion result in abnormal cholesterol metabolism. Moreover, dyslipidemia is another precipitating factor for gallstone generation.21
Our study showed that in elderly people with NAFLD, reduced serum HDL-C levels and elevated FPG levels (≥100 mg/dL) increased the risk of GSD by 35% and 36%, respectively. Hyperglycemia has been recognized as an important risk factor for GSD, which is possibly attributed to insulin resistance.34,43 These results were consistent with some previous studies.39
Hyperlipidemia
High triglycerides and low HDL-C have strong association with GSD.45,46 Hyperlipidemia results in supersaturated cholesterol in bile, which may stimulate the formation of gallstones. For NAFLD patients, both serum and hepatic cholesterol levels are higher. Thus, HDL-C, which enhances cholesterol excretion, may play a decisive role for the development of GSD in this high-risk group.
Limitations
Our study had several limitations that need to be addressed in the future research. First, the participants in our study lived in the urban area of the capital city of Taiwan, affecting the generalization of the study results to other areas nationwide. Furthermore, because this is a cross-sectional study, we did not document their current medication when conducting elderly annual health check-ups. As a result, subjects taking lipid-lowering drugs and medications for the treatment of cardiovascular diseases may interfere with the result for patient selection. Last but not the least, due to the lack of data on the continuous change in GSD, we could not further clarify the role of MetS in the development of GSD. However, to our best knowledge, this study is the first to elaborate the relationship between MetS and GSD in elderly NAFLD patients.
Conclusion
Our study concluded that GSD is significantly associated with MetS in elderly patients with NAFLD. In this population, reduced HDL-C and elevated FPG levels increased the risk of developing GSD. Therefore, to lower the risk of GSD in these patients, their FPG levels and HDL-C levels must be regularly followed-up. If they meet the criteria for MetS, patient education about symptoms of GSD is essential, and possible treatment options for GSD-related conditions should also be discussed in advance with these patients.
Abbreviations
ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; FPG, fasting plasma glucose; GSD, gallstone disease; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio.
Data Sharing Statement
The data supporting the results are from the Health Evaluation Center of MacKay Memorial Hospital.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki, and that verbal informed consent from the patients was approved by the Mackay Memorial Hospital, Taipei, Taiwan (approval ID: 18MMHIS137). Consent for publication: Verbal consent was witnessed and formally recorded.
Acknowledgment
The authors thank all members of the Department of Family Medicine, MacKay Memorial Hospital for help rendered for this study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen CY, Lu CL, Huang YS, et al. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Ageing. 1998;27(4):437–441. doi:10.1093/ageing/27.4.437
2. Liu CM, Tung TH, Chou P, et al. Clinical correlation of gallstone disease in a Chinese population in Taiwan: experience at Cheng Hsin general hospital. World J Gastroenterol. 2006;12(8):1281–1286. doi:10.3748/wjg.v12.i8.1281
3. Chen YC, Chiou C, Lin MN, Lin CL. The prevalence and risk factors for gallstone disease in taiwanese vegetarians. PLoS One. 2014;9(12):e115145. doi:10.1371/journal.pone.0115145
4. Lai SW, Ng KC. Risk factors for gallstone disease in a hospital-based study. South Med J. 2002;95(12):1419–1423. doi:10.1097/00007611-200295120-00013
5. Lu SN, Chang WY, Wang LY, et al. Risk factors for gallstones among Chinese in Taiwan. A community sonographic survey. J Clin Gastroenterol. 1990;12(5):542–546. doi:10.1097/00004836-199010000-00011
6. Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6(2):172–187. doi:10.5009/gnl.2012.6.2.172
7. Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011;140(2):508–516. doi:10.1053/j.gastro.2010.10.060
8. He W, Goodkind D, Kowal PR. An aging world: 2015. 2016.
9. Nassar Y, Richter S. Management of complicated gallstones in the elderly: comparing surgical and non-surgical treatment options. Gastroenterol Rep. 2019;7(3):205–211. doi:10.1093/gastro/goy046
10. Piccirillo JF, Vlahiotis A, Barrett LB, Flood KL, Spitznagel EL, Steyerberg EW. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol. 2008;67(2):124–132. doi:10.1016/j.critrevonc.2008.01.013
11. Sandblom G, Videhult P, Crona Guterstam Y, Svenner A, Sadr-Azodi O. Mortality after a cholecystectomy: a population-based study. HPB (Oxford). 2015;17(3):239–243. doi:10.1111/hpb.12356
12. Buchner AM, Sonnenberg A. Factors influencing the prevalence of gallstones in liver disease: the beneficial and harmful influences of alcohol. Am J Gastroenterol. 2002;97(4):905–909. doi:10.1111/j.1572-0241.2002.05607.x
13. Moro PL, Checkley W, Gilman RH, et al. Gallstone disease in high-altitude Peruvian rural populations. Am J Gastroenterol. 1999;94(1):153–158. doi:10.1111/j.1572-0241.1999.00787.x
14. Kratzer W, Kächele V, Mason RA, et al. Gallstone prevalence in relation to smoking, alcohol, coffee consumption, and nutrition. The Ulm gallstone study. Scand J Gastroenterol. 1997;32(9):953–958. doi:10.3109/00365529709011208
15. Ata N, Kucukazman M, Yavuz B, et al. The metabolic syndrome is associated with complicated gallstone disease. Can J Gastroenterol. 2011;25(5):274–276. doi:10.1155/2011/356761
16. Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol. 2006;21(11):1737–1743. doi:10.1111/j.1440-1746.2006.04381.x
17. Chang Y, Noh YH, Suh BS, et al. Bidirectional association between nonalcoholic fatty liver disease and gallstone disease: a cohort study. J Clin Med. 2018;7(11):458. doi:10.3390/jcm7110458
18. Koller T, Kollerova J, Hlavaty T, Huorka M, Payer J. Cholelithiasis and markers of nonalcoholic fatty liver disease in patients with metabolic risk factors. Scand J Gastroenterol. 2012;47(2):197–203. doi:10.3109/00365521.2011.643481
19. Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48(3):97–113. doi:10.3109/10408363.2011.596521
20. de Alwis NMW, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48:S104–S112. doi:10.1016/j.jhep.2008.01.009
21. Bertolotti M, Lonardo A, Mussi C, et al. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20(39):14185–14204. doi:10.3748/wjg.v20.i39.14185
22. Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing. 2009;38(1):11–18. doi:10.1093/ageing/afn242
23. Thampanitchawong P, Piratvisuth T. Liver biopsy: complications and risk factors. World J Gastroenterol. 1999;5(4):301–304. doi:10.3748/wjg.v5.i4.301
24. Nalbantoglu IL, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(27):9026–9037. doi:10.3748/wjg.v20.i27.9026
25. Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc. 2015;90(9):1233–1246. doi:10.1016/j.mayocp.2015.06.013
26. Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21(9):1023–1032 (). doi:10.7863/jum.2002.21.9.1023
27. Jørgensen T. Gall stones in a Danish population. Relation to weight, physical activity, smoking, coffee consumption, and diabetes mellitus. Gut. 1989;30(4):528–534. doi:10.1136/gut.30.4.528
28. Lee YC, Wu JS, Yang YC, Chang CS, Lu FH, Chang CJ. Moderate to severe, but not mild, nonalcoholic fatty liver disease associated with increased risk of gallstone disease. Scand J Gastroenterol. 2014;49(8):1001–1006. doi:10.3109/00365521.2014.920912
29. Cariati A. Gallstone classification in western countries. Indian J Surg. 2015;77(Suppl 2):376–380. doi:10.1007/s12262-013-0847-y
30. Trotman BW. Pigment gallstone disease. Gastroenterol Clin North Am. 1991;20(1):111–126.
31. Acalovschi M. Cholesterol gallstones: from epidemiology to prevention. Postgrad Med J. 2001;77(906):221–229. doi:10.1136/pmj.77.906.221
32. Ho KJ, Lin XZ, Yu SC, Chen JS, Wu CZ. Cholelithiasis in Taiwan. Gallstone characteristics, surgical incidence, bile lipid composition, and role of beta-glucuronidase. Dig Dis Sci. 1995;40(9):1963–1973. doi:10.1007/BF02208665
33. Jørgensen T. Prevalence of gallstones in a Danish population. Am J Epidemiol. 1987;126(5):912–921. doi:10.1093/oxfordjournals.aje.a114728
34. Atamanalp SS, Keles MS, Atamanalp RS, Acemoglu H, Laloglu E. The effects of serum cholesterol, LDL, and HDL levels on gallstone cholesterol concentration. Pak J Med Sci. 2013;29(1):187–190. doi:10.12669/pjms.291.2798
35. Stringer MD, Fraser S, Gordon KC, Sharples K, Windsor JA. Gallstones in New Zealand: composition, risk factors and ethnic differences. ANZ J Surg. 2013;83(7–8):575–580. doi:10.1111/j.1445-2197.2012.06234.x
36. Novacek G. Gender and gallstone disease. Wien Med Wochenschr. 2006;156(19–20):527–533. doi:10.1007/s10354-006-0346-x
37. Jensen KH, Jørgensen T. Incidence of gallstones in a Danish population. Gastroenterology. 1991;100(3):790–794. doi:10.1016/0016-5085(91)80027-7
38. Yoo E-H, Lee S-Y. The prevalence and risk factors for gallstone disease. Clin Chem Lab Med. 2009;47:795–807. doi:10.1515/CCLM.2009.194
39. Méndez-Sánchez N, Chavez-Tapia NC, Motola-Kuba D, et al. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11(11):1653–1657. doi:10.3748/wjg.v11.i11.1653
40. Hsu HY, Huang CY, Hwang LC. Sex difference of the predictive value of BMI, waist circumference and percentage body fat mass for gallstone disease. Br J Nutr. 2019;121(8):955–960. doi:10.1017/S000711451900028X
41. Aganović I, Dušek T. Pathophysiology of metabolic syndrome. Ejifcc. 2007;18(1):3–6.
42. Scragg RK, Calvert GD, Oliver JR. Plasma lipids and insulin in gall stone disease: a case-control study. Br Med J. 1984;289(6444):521–525. doi:10.1136/bmj.289.6444.521
43. de Leon MP, Ferenderes R, Carulli N. Bile lipid composition and bile acid pool size in diabetes. Am J Dig Dis. 1978;23(8):710–716. doi:10.1007/BF01072357
44. Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14(7):778–782. doi:10.1038/nm1785
45. Andreotti G, Chen J, Gao YT, et al. Serum lipid levels and the risk of biliary tract cancers and biliary stones: a population-based study in China. Int J Cancer. 2008;122(10):2322–2329. doi:10.1002/ijc.23307
46. Thijs C, Knipschild P, Brombacher P. Serum lipids and gallstones: a case-control study. Gastroenterology. 1990;99(3):843–849. doi:10.1016/0016-5085(90)90978-A
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
