Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Relationship Between Family History and Quality of Life in Patients with Psoriasis: A Cross-Sectional Study from China
Authors Jiang F, Lu L, Wang S, Yuan F, Cao L, Xu S, Lin B
Received 3 December 2023
Accepted for publication 13 April 2024
Published 20 April 2024 Volume 2024:17 Pages 891—900
DOI https://doi.org/10.2147/CCID.S453078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Video abstract of “Family history and quality of life in patients with plaque psoriasis” [453078].
Views: 46
Fan Jiang, Lingyi Lu, Sihan Wang, Feng Yuan, Lu Cao, Suling Xu,* Bingjiang Lin*
Department of Dermatology, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang; National Clinical Medical Research Center for Skin and Immune Diseases, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Suling Xu; Bingjiang Lin, Department of Dermatology, The First Affiliated Hospital of Ningbo University, No. 59 Liuting Street, Ningbo, Zhejiang, 315000, People’s Republic of China, Email [email protected]
Purpose: The purpose of this study was to investigate the comprehensive impact of family history of psoriasis, lesion size, disease severity, and the possibility of joint involvement on patients’ quality of life(QoL).
Patients and Methods: Data from 5961 patients with psoriasis recruited from 440 hospitals throughout China were analyzed. The effects of family history of psoriasis, Body Surface Area(BSA), Psoriasis Area and Severity Index(PASI), and Psoriasis Epidemiology Screening Tool(PEST) on their Dermatology Life Quality Index(DLQI) were studied using a moderated chained mediated effects test.
Results: A total of 912 patients (15.30%) had a family history of psoriasis, and 5071 patients (85.10%) had plaque psoriasis. In patients with plaque psoriasis, the variables of family history, PASI, PEST, and DLQI were positively correlated with each other. Additionally, in patients with other types of psoriasis, PASI was positively correlated with PEST and DLQI. Age was positively correlated with PASI and PEST and negatively correlated with DLQI in patients with plaque psoriasis; their Body Mass Index(BMI) and disease duration were in positive correlation with PASI and PEST. The mediation effect of PASI and PEST between family history and DLQI was remarkable in patients with plaque psoriasis and not in those with other types of psoriasis. BSA moderated the association between family history and PASI in patients with plaque psoriasis.
Conclusion: PASI and PEST play a chain mediating role in the relationship between family history and DLQI in patients with plaque psoriasis, and high levels of BSA increase the ability of family history to positively predict PASI in plaque psoriasis, thereby affecting the patient’s QoL.
Keywords: plaque psoriasis, PASI, PEST, quality of life, family history
Introduction
Psoriasis is a chronic, immune-mediated skin disease that can be combined with other systemic abnormalities, such as visceral and joint damage.1 Plaque psoriasis is the most common variant, accounting for more than 80% of the psoriasis cases.2 Familial aggregation studies, epidemiological studies, and association studies with human leukocyte antigens support a strong genetic predisposition to psoriasis.3 In recent years scientists have focused on plaque psoriasis, and its rapid advances are reflected in pathogenesis, genetics, comorbidities, and biological treatments.4
Current measures that can effectively assess quantitative psoriatic disease activity are remarkably well established. The most basic assessment of psoriasis lesional burden is the BSA score.5 The PASI score is a quantitative measure of the extent and severity of psoriasis lesions.6 It takes into account the BSA affected, as well as the severity of erythema, infiltration, and desquamation. The PASI score is widely used as the gold standard for assessing extensive psoriasis.7 Validated PEST is designed to help identify undiagnosed psoriatic arthritis(PsA) in patients with psoriasis at an early stage.8 DLQI is the most widely used tool to measure the impact of psoriasis on QoL.9,10
Numerous studies have found some differences between familial and sporadic psoriasis cases in terms of disease characteristics, such as earlier age of onset, more severe disease, and lower QoL.11–13 However, few studies have used BSA, PASI, PEST, and DLQI as assessment indicators to investigate the combined effects of psoriasis family history, lesion area, disease seriousness, and the possibility of involving joints on patients’ QoL, to provide some theoretical guidance for the management of psoriasis patients and early physical and mental health interventions.
Materials and Methods
Study Population
The China Center for Standardized Psoriasis Treatment recruited 7037 patients with psoriasis from 440 hospitals nationwide between June 2020 and October 2022. Inclusion criteria were as follows: Diagnosis confirmed by at least 2 specialized dermatologists using a double-blind, independent method after a detailed history, thorough physical examination, and necessary ancillary tests (eg, dermoscopy); availability of complete data on age, weight, height, and calculated BMI, disease duration, family history, and BSA, PASI, PEST, and DLQI.
The Psoriasis Area and Severity Index (PASI) is a quantitative scale used by physicians to assess the severity of psoriasis lesions. It evaluates the area and severity of lesions in four zones: head, neck, trunk, upper extremities, and lower extremities. The sum of these scores is the PASI, with higher scores indicating a more severe form of the disease. Measure is available from the original article.9
The PEST comprises five straightforward yes/no questions. Each affirmative response is assigned a value of 1, with a score of 3 or higher indicating a potential risk of psoriatic arthritis and the need for referral to a rheumatologist. (Table S1)
The DLQI is a self-assessment questionnaire that evaluates the impact of a dermatologic condition on a patient’s QoL. It covers symptoms, treatment side effects, daily activities, work or school, relationships, leisure activities, and feelings of embarrassment. Higher scores on the DLQI indicate greater impairment of the patient’s QoL. Measure is available from the original article.10
Informed consent was signed before data collection. A total of 5961 patients (84.71%) were included after excluding invalid data. Ethical approval was obtained from the Ethics Committee of Ningbo First Hospital (2022–028RS-01), and all patients gave informed consent before data collection. This study strictly adhered to the Declaration of Helsinki.
Control Variables
As patients’ age and disease duration increase, their psoriasis lesions will further deteriorate, which may have obvious effects on variables such as BSA and PASI, which need to be controlled in model construction; in addition, there may also be remarkable variations in disease severity among psoriasis patients with different BMI, so BMI should also be included as a control variable.
Statistical Analysis
SPSS 23.0 was used to process the data for analysis; Data are presented as medians and quartiles; Spearman correlation analysis was used to identify potential relationships; A multivariate logistic regression model was used, and SPSS model 6 was used to test for chained mediated effects and SPSS model 83 was used to test for moderated chained mediated effects. A p<0.05 was considered statistically significant. To reduce common method bias due to the self-reported collection of data, the study was somewhat controlled by anonymous surveys and reverse scoring of some questions.
Results
Descriptive Statistics and Correlation Analysis of Each Variable
3853 (64.6%) males and 2108 (35.4%) females were studied; the mean age was 42.71±15.89 years with a range of 1–98 years; the mean BMI was 24.42±5.04 kg/m² with a range of 10.97–65.92 kg/m²; the duration of patients’ psoriasis ranged from 0–73 years with a mean of 11.09±10.08 years; in family history, 912 (15.30%) patients had relatives who also had psoriasis; in typology, 5071 (85.10%) of them had plaque psoriasis and 890 (14.90%) had non-plaque psoriasis.
To better understand which patient characteristics are associated with impaired QoL in patients with psoriasis, we assessed their correlation with disease parameters using heat maps. The findings indicate that among patients with plaque psoriasis, there is a correlation between the variables of psoriasis family history, PASI, PEST, and DLQI, albeit with relatively weak linear associations; PASI and BSA were significantly positively correlated with each other, and weakly correlated with PEST, DLQI and BSA (Figure 1). PASI and PEST were significantly associated with DLQI, and high psoriasis severity and high likelihood of comorbid arthritis were associated with increased QoL impairment. Family history was also found to be weakly associated with QoL (Table S2).
 |
Figure 1 Correlation of variables in patients with plaque psoriasis. |
Patients with other types of psoriasis (Figure 2) showed a significant positive correlation between PASI and BSA and a weak correlation with PEST and DLQI. (Table S3)
 |
Figure 2 Correlation of variables in patients with other types of psoriasis. |
PASI and PEST Affect the Chain-Mediated Effect Test of the Relationship Between a Family History of Psoriasis and DLQI in Patients
We hypothesized that the low QoL of psoriasis patients was at least partially caused by their family history of psoriasis and assessed the cumulative effect of different clinical variables (disease duration, PASI, PEST) and sociodemographic factors (age, BMI, family history) on DLQI by multivariate logistic regression. To account for the heterogeneity of the variables, we adjusted for age, disease duration, and BMI as covariates, and used SPSS Process Model 6 combined with Bootstrap method to test for mediating effects. Regression analysis showed that the age of patients with plaque psoriasis (Table 1) was positively correlated with their PASI (β=0.003, P<0.001), PEST (β=0.004, P<0.001) and negatively correlated with DLQI (β=−0.002, P<0.05); their BMI was positively correlated with PASI (β=0.012, P<0.001) and PEST (β=0.008, P<0.001); their disease duration was correlated in positive terms with PASI (β=0.012, P<0.001) and PEST (β=0.005, P<0.01).
 |
Table 1 Results of Regression Analysis for Each Variable in Patients with Plaque Psoriasis (N=5071) |
Family history significantly and positively predicted patients’ PASI (β=0.15, P<0.001) and PEST (β=0.14, P<0.001); their PASI significantly and positively predicted PEST (β=0.09, P<0.001) and positively predicted patients’ DLQI (β=0.25, P<0.001); patients with plaque psoriasis’ PEST significantly and positively predicted their DLQI (β=0.26, P<0.001).
PASI in patients with other types of psoriasis (Table 2) was able to significantly and positively predict patients’ PEST (β=0.088, P<0.01) and positively predict patients’ DLQI (β=0.260, P<0.001); and patients’ PEST actively predicted their DLQI (β=0.239, P<0.001).
 |
Table 2 Results of Regression Analysis for Each Variable in Patients with Other Types of Psoriasis (N=890) |
From further Bootstrap test results, the total indirect effect value of family history on DLQI in patients with plaque psoriasis (Table 3) was 0.079 with a confidence interval not including 0. This indicates a significant mediating effect of PASI and PEST between family history and DLQI scores in patients with plaque psoriasis. The three mediated pathways of the patient’s family history of psoriasis → PASI → DLQI, patient’s family history of psoriasis → PEST → DLQI, and patient’s family history of psoriasis → PASI → PEST → DLQI were all significant (95% confidence interval did not contain 0) with effect values of 0.038, 0.037, and 0.004, respectively, accounting for 48.101%, 46.835%, and 5.063% of the total effect values. The results demonstrated one direct pathway and three mediating effect pathways between family history and DLQI in patients with plaque psoriasis; that is, patients with a family history of plaque psoriasis may have a higher PASI, which increases their PEST, which in turn leads to an increase in DLQI.
 |
Table 3 Analysis of PASI and PEST as a Chain Mediator Effect Test Between Family History and DLQI in Patients with Plaque Psoriasis (N=5071) |
The total indirect effect value for patients with other types of psoriasis (Table S4) was −0.021 with a confidence interval including 0, which reflects a non-significant mediating effect of PASI and PEST between family history and DLQI in patients with other types of psoriasis.
Moderating Effect Test
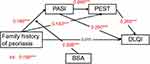
Using the family history of patients with plaque psoriasis as the independent variable, DLQI as the dependent variable, PASI and PEST as mediating variables, and BSA as the moderating variable, a moderated chain mediation model was constructed using the SPSS Process model 83. Regression analysis showed (Table 4) a positive effect of family history on PASI in patients with plaque psoriasis (β=0.166, P<0.001); the interaction term of their family history and BSA regressed markedly on PASI (β=0.159, P<0.001). It indicates that this chain mediated between patient familial history and PASI is moderated by BSA. In addition, none of the variance inflation factors of all predictor variables in this study were higher than 2, indicating that there was no multicollinearity problem and the moderated chain mediation model held (shown in Figure 3).
 |
Table 4 Results of Moderated Chain-Mediated Regression Analysis in Patients with Plaque Psoriasis (N=5071) |
To further explain the modifying effect of BSA in patients, they were grouped into low, medium, and high groups by M±SD (26.230±28.885%), and ANOVA was performed to plot the modifying effect analysis shown in Figure 4. The results revealed that when BSA was at both moderate and high levels, it modulated the family history and PASI of this chain mediator in patients with plaque psoriasis, ie, patients with a family background had an elevated positive predictive effect on both their PASI. And under a high level of BSA moderation (β=0.301, t=8.964, P<0.001), patients with family histories had a higher role in positively forecasting their PASI than under a moderate level of BSA moderation (β=0.102, t=3.769, P<0.001), suggesting that when plaque psoriasis patients have a combined genetic background, the higher their BSA, the more likely they are to have more advanced PASI and more severe disease. Whereas, at low BSA levels, it was unable to regulate the patient’s family history with PASI (β=0.038, t=1.168, P>0.05).
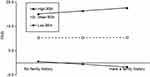 |
Figure 4 Moderating effect of Body Surface Area(BSA) between family history and Psoriasis Area and Severity Index(PASI) in patients with plaque psoriasis. |
Discussion
How can we predict in advance the possibility of transformation to PsA and subsequent changes in QoL level based on the basic information of psoriasis patients such as family history and severity of lesions, to take early measures such as aggressive treatment to prevent further progression of the disease? Our results show for the first time that a family history of psoriasis contributes to the severity of the disease in patients and consequently increases the likelihood of comorbid arthritis, which in turn leads to a reduced QoL in severely affected patients.
The findings indicate that family history positively predicts QoL in patients with plaque psoriasis compared to patients with other types of psoriasis, ie, patients with familial aggregation have lower subjective well-being. This is consistent with the results of a previous study in which Jose Luis López-Estebaranz recognized that the presence of a family history of psoriasis seems to disrupt their QoL regardless of disease severity.13 For familial patients, they have earlier exposure to the disease and may have witnessed the social rejection and psychological distress experienced by close relatives, and have a deeper awareness and concern about the disease; when they develop the disease themselves, it will inevitably have a wide range of harmful effects on all aspects of their daily life, body, and mind. Several studies have shown that psoriasis has a major negative impact on the overall well-being of not only the sufferers themselves, but also their cohabitants such as parents and partners, who experience higher levels of anxiety and depression and have a severely impaired QoL.14,15 Of course, QOL is influenced by many factors, including socioeconomic factors, understanding of the disease and coping strategies, and expectations and acceptance of treatment. T. Hawro believes that psoriasis has a negative impact on patients’ financial status, which in turn is a mediating factor in QoL impairment.16 Here, we test the hypothesis that the impact of family history on QoL in patients with plaque psoriasis is partly indirectly mediated by severe skin lesions and underlying joint involvement.
Regarding the severity of psoriatic lesions, Qing Min studied 208 children with psoriasis vulgaris and found that patients with a family history of psoriasis had a higher PASI score.17 The aggravation of skin and joint symptoms in familial psoriasis is mostly due to the genetic predisposition of the disease, which is highly hereditary and has a high risk of recurrence in families.3,18 Interestingly, in our study, whether plaque psoriasis or other types of psoriasis, the more severe the skin lesions in patients with a family history of psoriasis, the greater the negative impact on their QoL. This is consistent with previous findings that QoL as assessed by the DLQI is positively associated with disease severity in patients with psoriasis.19–21 Such results are not surprising; when patients with a family history of exacerbated lesions or even psoriasis comorbidities, the stigmatizing nature of the disease and subsequent long-term behavioral changes can lead to reduced life opportunities, lower economic status, and a lower QoL related to their work, school, and interpersonal relationships.16,22 Although statistically significant, PASI and DLQI were weakly correlated in this study (plaque psoriasis: r = 0.260; other types: r = 0.352), which may be due to the large sample size of the population in this study. This finding is consistent with a recent study by Jing Yang, who reported inconsistent scores on the PASI and DLQI in 4125 patients with psoriasis, and stratified analyses showing that lesion location (feet) was a significant risk factor for disease burden.23 A Danish study also found a weak correlation between the PASI and the DLQI, which they attributed to bias in the contribution of individual questions in the DLQI.24
Concerning PEST, there are no previous in-depth studies on its relationship with genetic factors and DLQI. As reported elsewhere, PEST was significantly correlated with the DLQI.25 The research results suggest that family history can predict DLQI by positively predicting PEST and thus DLQI, and can positively predict DLQI through the chain mediating effect of PASI and PEST. Although patients were not followed up to confirm the presence or absence of comorbid psoriatic arthritis, the PEST can be used as a scoring facet to provide early indications that psoriatic patients may have arthritis.26 According to population-based epidemiological studies, approximately 40% of patients with psoriasis or PsA have a family history of these two diseases in first-degree relatives.27 Familial cases are more likely to progress to articular skeletal symptoms compared to sporadic cases, and patients with high PEST scores on a self-administered PsA screening tool are more likely to have a family history of psoriasis.25 Shirley Braga Lima Gamonal found that among patients with plaque psoriasis, those with arthritis had a higher rate of PASI than those without arthritis.28 Patients who screen positive for PEST have a much greater impact on QoL.29 When the disease manifests not only as skin lesions but also involves joints with severe pain or even physical disability, their lives are severely affected and their psychological burden, including various psychiatric disorders such as depression, anxiety, and suicidal ideation, will rise.30 This idea coincides with Deepan S. Dalal, who found that despite aggressive treatment, patients with PsA had a poorer QoL compared to those with psoriasis.31 Screening and early management treatment of PsA is therefore essential for patients diagnosed with psoriasis, and screening for early signs of mental illness is also needed to reduce the physical as well as psychological burden.
This survey also revealed that the area of body surface involvement moderated the relationship between family history and PASI, a chained mediator variable, in patients with plaque psoriasis. The performance characteristics of PASI are attenuated in patients with low injury load, so it is not usually applied in patients with psoriatic injury with <3% BSA involvement,9 hence low levels of BSA in this study did not moderate the family history of patients with PASI. In contrast, when familial psoriasis cases are at moderate or high levels of BSA, their PASI levels are elevated, ie, BSA influences the trend of response between the two, while at high BSA levels, family history is a stronger positive predictor of PASI in patients. The literature supports that family history has a critical impact on both clinical phenotype and disease activity in patients with psoriasis,32 with lesion areas at moderately high levels indicating severe and persistent progression. The differences in the presence or absence of family history and overall lesion area levels among patients with plaque psoriasis suggest that controlling the BSA of lesions is an important factor to consider in cases of familial psoriasis and also helps clinicians to screen at-risk patients early for appropriate interventions to maximize patient benefit.
The older the patient with plaque psoriasis, the longer the duration of the disease, the greater the BMI, the larger the size and severity of the lesions, and the higher the incidence of joint damage, results that have been well described in the literature.11,31,32 In addition, we found that PASI and PEST significantly mediated between family history and DLQI in patients with type of plaque psoriasis, while other types such as guttate, pustular, and erythrodermic were not significant. Dilek Solmaz suggests that plaque psoriasis is more common in families with a history of psoriasis and suggests that the association between a family history of psoriasis/psoriatic arthritis and the pustular/plaque phenotype may indicate different genetic backgrounds and causative mechanisms in these subgroups.3 Therefore, we hypothesized that genetic history and PASI would be more important for chronic plaque psoriasis than for other phenotypes, and that the correlation with subsequent arthropathy in patients would be stronger. This correlation may also reflect the psychological state and QoL of the patients. We recommend that patients realize the importance of early treatment of the disease, cooperate with doctors to take sequential and combined treatment to eliminate skin lesions and slow down the progression of the disease as soon as possible, while relatives and friends assist in psychological and behavioral interventions to achieve early physical and mental health.33
This study has several limitations. First, the means of PEST and BSA for patients with plaque psoriasis and other types of psoriasis were less than the standard deviation and far from the median, suggesting that the data were volatile and unevenly distributed and that it may be more accurate to stratify the sample according to the type of psoriasis, disease duration, and other factors before data analysis. Second, family history may be biased, and the lack of genetic analysis of patients prevents us from drawing firm conclusions.3 Finally, data were collected at 440 centers in China, and despite precautionary measures to improve homogeneity, data collection may have varied among centers.
Conclusion
In conclusion, our study examined the effects of family history, PASI, PEST, and BSA on DLQI in patients with plaque psoriasis, enriching related research in the areas of genetics, skin phenotype, articular skeletal characteristics, disease severity, and QoL in patients with psoriasis, and helping clinicians to better understand the disease and patients, which translates into better disease management and patient adherence.
Author Contributions
Fan Jiang designed the direction of the article and drafted the paper. Sihan Wang and Feng Yuan participated in the data collection and analysis work. Lingyi Lu and Lu Cao provided professional revision and guidance on the writing of the paper. Suling Xu and Bingjiang Lin approved the final version of the paper to be published. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding or sponsorship was received for this study or publication of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/S0140-6736(20)32549-6
2. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi:10.1111/jdv.13854
3. Solmaz D, Bakirci S, Kimyon G, et al. Impact of having family history of psoriasis or psoriatic arthritis on psoriatic disease. Arthr Care Res. 2020;72(1):63–68. doi:10.1002/acr.23836
4. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
5. Long CC, Finlay AY. The finger-tip unit--A new practical measure. Clin Exp Dermatol. 1991;16(6):444–447. doi:10.1111/j.1365-2230.1991.tb01232.x
6. Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi:10.1159/000250839
7. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheumatic Dis. 2005;64(Suppl 2):ii65–68; discussion ii69–73. doi:10.1136/ard.2004.031237
8. Mease PJ, Palmer JB, Hur P, et al. Utilization of the validated psoriasis epidemiology screening tool to identify signs and symptoms of psoriatic arthritis among those with psoriasis: a cross-sectional analysis from the US-based corrona psoriasis registry. J Eur Acad Dermatol Venereol. 2019;33(5):886–892. doi:10.1111/jdv.15443
9. Mease PJ. Measures of psoriatic arthritis: tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthr Care Res. 2011;63(Suppl 11):S64–85.
10. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--A simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
11. Ohata C, Anezaki H, Kaneko S, et al. Clinical characteristics of patients with psoriasis with family history: a multicenter observational study. J Dermatol. 2023;50(6):746–752. doi:10.1111/1346-8138.16733
12. Liu H, Lu C, Yang F, et al. Associations between family history of psoriatic disease and clinical characteristics on patients with psoriatic arthritis: a nationwide study from the Chinese Registry of Psoriatic Arthritis (CREPAR II). Clin Exper Rheumatol. 2023;41(9):1901–1907. doi:10.55563/clinexprheumatol/gbg5i5
13. López-Estebaranz JL, Sánchez-Carazo JL, Sulleiro S. Effect of a family history of psoriasis and age on comorbidities and quality of life in patients with moderate to severe psoriasis: results from the Arizona study. J Dermatol. 2016;43(4):395–401. doi:10.1111/1346-8138.13157
14. Basra MK, Sue-Ho R, Finlay AY. The family dermatology life quality index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156(3):528–538. doi:10.1111/j.1365-2133.2006.07617.x
15. Martínez-García E, Arias-Santiago S, Valenzuela-Salas I, et al. Quality of life in persons living with psoriasis patients. J Am Acad Dermatol. 2014;71(2):302–307. doi:10.1016/j.jaad.2014.03.039
16. Hawro T, Zalewska A, Hawro M, et al. Impact of psoriasis severity on family income and quality of life. J Eur Acad Dermatol Venereol. 2015;29(3):438–443. doi:10.1111/jdv.12572
17. Qing M, Liu P, Zhu W, et al. Analysis for 208 children with psoriasis vulgaris. Zhong Nan da Xue Xue Bao Yi Xue Ban. 2020;45(7):804–811. doi:10.11817/j.issn.1672-7347.2020.190129
18. Di Lernia V, Ficarelli E, Lallas A, et al. Familial aggregation of moderate to severe plaque psoriasis. Clin Exp Dermatol. 2014;39(7):801–805. doi:10.1111/ced.12401
19. Chen Y, Wei L, Song Y, et al. Life quality among psoriasis patients based on dermatology life quality index evaluation and its association with psoriasis severity in China: a cross-sectional study. Ann Med. 2023;55(1):2231847. doi:10.1080/07853890.2023.2231847
20. Herédi E, Rencz F, Balogh O, et al. Exploring the relationship between EQ-5D, DLQI and PASI, and mapping EQ-5D utilities: a cross-sectional study in psoriasis from Hungary. Eur J Health Econ. 2014;15(Suppl 1):S111–119. doi:10.1007/s10198-014-0600-x
21. Wade AG, Crawford GM, Young D, et al. Severity and management of psoriasis within primary care. BMC Fam Pract. 2016;17(1):145. doi:10.1186/s12875-016-0544-6
22. Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheumatic Dis. 2005;64(Suppl 2):ii14–17. doi:10.1136/ard.2004.032482
23. Yang J, Hu K, Li X, et al. Psoriatic foot involvement is the most significant contributor to the inconsistency between PASI and DLQI: a Retrospective Study from China. Clin Cosmet Invest Dermatol. 2023;16:443–451. doi:10.2147/CCID.S396997
24. Loft ND, Egeberg A, Rasmussen MK, et al. Patient-reported outcomes during treatment in patients with moderate-to-severe psoriasis: a Danish nationwide study. Acta Dermato Venereologica. 2019;99(13):1224–1230. doi:10.2340/00015555-3331
25. Xia P, Chen J, Yang M, et al. Clinical features of Chinese psoriatic patients for early referral of arthritis using psoriasis epidemiology screening tool: a cross-sectional analysis from the registry database of Chinese psoriasis standardized diagnosis and treatment center. Chinese Med J. 2023;136(16):1999–2001. doi:10.1097/CM9.0000000000002748
26. Ogdie A, Harrison RW, McLean RR, et al. Prospective cohort study of psoriatic arthritis risk in patients with psoriasis in a real-world psoriasis registry. J Am Acad Dermatol. 2022;87(6):1303–1311. doi:10.1016/j.jaad.2022.07.060
27. Rahman P, Schentag CT, Beaton M, et al. Comparison of clinical and immunogenetic features in familial versus sporadic psoriatic arthritis. Clin Exper Rheumatol. 2000;18(1):7–12.
28. Gamonal SBL, Gamonal ACC, Brandão MAF, et al. Prevalence of psoriatic arthritis among patients with plaque psoriasis: a Brazilian retrospective study. São Paulo Med J. 2021;139(5):476–480. doi:10.1590/1516-3180.2020.0629.16032021
29. Armstrong AW, Bohannan B, Mburu S, et al. Patient Perspectives on Psoriatic Disease Burden: Results from the Global Psoriasis and Beyond Survey. Dermatology. 2023. Vol. 239(4):621–634.
30. Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2012;51(3):571–576. doi:10.1093/rheumatology/ker365
31. Dalal DS, Lin YC, Brennan DM, et al. Quantifying harmful effects of psoriatic diseases on quality of life: cardio-metabolic outcomes in psoriatic arthritis study (COMPASS). Semin Arthritis Rheumatism. 2015;44(6):641–645. doi:10.1016/j.semarthrit.2015.01.003
32. Naldi L, Parazzini F, Brevi A, et al. Family history, smoking habits, alcohol consumption and risk of psoriasis. Br J Dermatol. 1992;127(3):212–217. doi:10.1111/j.1365-2133.1992.tb00116.x
33. Lim DS, Bewley A, Oon HH. Psychological Profile of Patients with Psoriasis. Ann Acad Med Singapore. 2018;47(12):516–522. doi:10.47102/annals-acadmedsg.V47N12p516
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.