Back to Journals » International Journal of General Medicine » Volume 15
Relation Between Non-Alcoholic Fatty Pancreas and Clinical and Biochemical Parameters in Women with Polycystic Ovary Syndrome: A Multi-Centric Study
Authors Osman MAA, Alkhouly M, Elmohaseb GF, Nassef EM , Mohamed IGR , El mancy IM, Sabry S, Abdulrehim MM, Eliwa A , Eisa YH, Abdel-Ghany A, Abdelghani Y
Received 28 July 2022
Accepted for publication 28 September 2022
Published 19 November 2022 Volume 2022:15 Pages 8225—8233
DOI https://doi.org/10.2147/IJGM.S384073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mustafa AA Osman,1 Mohamed Alkhouly,1 Ghada F Elmohaseb,2 Eman Mostafa Nassef,2 Ibrahim Ghonim Ramadan Mohamed,2 Ismail Mohamed El mancy,2 Seham Sabry,2 Marwa M Abdulrehim,2 Ahmed Eliwa,2 Yasmine H Eisa,3 Ahmed Abdel-Ghany,4 Yasser Abdelghani5
1Gynecology and Obstetrics Department, Al-Azhar University, Cairo, Egypt; 2Internal Medicine Department, Al-Azhar University, Cairo, Egypt; 3Community Medicine Department, October 6 University, Giza, Egypt; 4Gynecology and Obstetrics Department, Minia University, Minia, Egypt; 5Tropical Medicine Department, Minia University, Minia, Egypt
Correspondence: Eman Mostafa Nassef, Internal Medicine Department, Al-Azhar University, Cairo, Egypt, Tel +20 1098002232, Email [email protected]
Background: Polycystic ovary syndrome (PCOS) is the most common endocrinological disease affecting women in the reproductive age. Non-alcoholic fatty pancreas disease (NAFPD) can promote many aspects of pancreatic dysfunction. The present study aimed to determine the prevalence of NAFPD and to identify its association with clinical and biochemical parameters in PCOS patients.
Methods: The present study included 150 patients with PCOS and 150 age-matched healthy controls. All patients were submitted to careful history taking and thorough clinical examination. Performed laboratory investigations included fasting and postprandial blood glucose, lipid profile, liver function tests, serum prolactin and total testosterone. Fatty pancreas was diagnosed using abdominal ultrasound.
Results: Among PCOS women, NAFPD was diagnosed in 57 women (38.0%) in contrast to 18 women (12.0%) in the control group (p < 0.001). Patients with NAFPD were significantly older [median (IQR): 38.0 (35.0– 43.0) versus 29.0 (25.5– 33.0) years, p = 0.001] with higher BMI [median (IQR): 31.5 (29.1– 34.7) versus 30.4 (28.6– 32.4) kg/m2, 0.042]. Moreover, they had significantly higher frequency of metabolic syndrome (84.2% versus 54.8%, p = 0.001), insulin resistance (68.4% versus 26.9%, p < 0.001) and severe NAFLD (22.8% versus 2.2%, p < 0.001). NAFPD patients had significantly lower sex hormone binding globulin (SHBG) [median (IQR): 36.0 (30.8– 40.7) versus 38.1 (35.15– 42.7), p = 0.002] and significantly higher free androgen index (FAI) [median (IQR): 4.08 (3.3– 4.92) versus 3.47 (3.12– 4.05), p < 0.001].
Conclusion: NAFPD is prevalent PCOS. It is related to metabolic syndrome, insulin resistance, dyslipidemia and hyperandrogenism.
Keywords: polycystic ovary, non-alcoholic fatty pancreas, free androgen index
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinological disease affecting women in the reproductive age. The condition is cardinally manifested by oligo/anovulation, hyperandrogenism and polycystic ovaries.1 PCOS is associated with a wide range of clinical manifestations affecting multiple body systems. The metabolic consequences related to PCOS range from insulin resistance to metabolic syndrome, diabetes mellitus and non-alcoholic fatty liver disease (NAFLD).2 Hyperandrogenism in particular plays an essential role in the development of metabolic derangement in those patients.3 These effects may be exacerbated by the associated obesity and chronic low-grade inflammation.4,5
The association between PCOS and NAFLD is well documented. It’s thought that development of NAFLD in PCOS patients is mediated through reduction of hepatic sex hormone-binding globulin and enhanced apoptotic processes.6 In contrast to the liver, fat accumulation in the pancreas is a less-studied condition. It entails a spectrum of pathological states ranging from fatty replacement to non-alcoholic fatty pancreas disease (NAFPD).7 NAFPD can promote many aspects of pancreatic dysfunction including insulin resistance, β-cell dysfunction, pancreatitis and pancreatic cancer in affected patients.8,9
Unfortunately, the clinical consequences and interrelations between NAFPD and different clinical conditions are not well studied.10 Interestingly, recent experimental research revealed that PCOS rat models had significant reduction of pancreatic β-cell mass.11 The present study aimed to determine the prevalence of NAFPD and to identify its association with the clinical and biochemical parameters in PCOS patients.
Patients and Methods
The present cross-sectional study was conducted at Al-Azhar, October 6 and Minia University Hospitals, Egypt. The study protocol was approved by the local ethical committee and informed consent was obtained from all participants. The study included 150 patients with PCOS. Diagnosis was established if at least two of the three 2003 Rotterdam criteria were fulfilled. These criteria included: 1) Chronic ovulatory dysfunction 2) Biochemical and/or clinical evidence of hyperandrogenism and 3) Ultrasound evidence of polycystic ovaries.12 In addition, there were 150 age-matched healthy controls.
Chronic ovulatory dysfunction was diagnosed in the presence oligomenorrhea (menstrual cycles of 35–90 days) or amenorrhea (absence of menses >90 days). In patients with regular cycles, anovulation was documented by ultrasound and/or mid-luteal phase serum progesterone in two consecutive cycles. Biochemical hyperandrogenism was identified in the presence of total testosterone ≥ 0.73 ng/mL and/or a free androgen index (FAI) ≥ 4.513 while clinical hyperandrogenism included hirsutism diagnosed based on a standardized scoring system,14 acne, or androgenic alopecia.15 Patients were excluded from the study if they had associated malignancies, liver cirrhosis or autoimmune diseases eg, systemic lupus erythematosus.
All patients were submitted to careful history taking and thorough clinical examination. Performed laboratory investigations included assessment of fasting and postprandial blood glucose, lipid profile, liver function tests, serum prolactin, follicle stimulating hormone (FSH), luteinizing hormone (LH), sex hormone binding globulin (SHBG) and total testosterone.
In the present study, we defined metabolic syndrome (MetS) according to the Joint Interim Statement criteria.16 These criteria included elevated waist circumference, elevated triglycerides, reduced high-density lipoprotein cholesterol, elevated blood pressure and elevated fasting glucose. Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the equation: HOMA-IR= fasting glycemia (mg/dL) × fasting insulinemia (µUI/mL)/405.17 Fatty pancreas was diagnosed using abdominal ultrasound if there is increased echogenicity of the pancreas over the liver or renal cortex.18 Depending on the echogenicity of the hepatic parenchyma, non-alcoholic fatty liver disease (NAFLD) was classified into three grades: mild, moderate, and severe.19
Data obtained from the present study were presented as median and interquartile range (IQR) or number and percent. Numerical data were compared using Mann–Whitney U-test while categorical data were compared using chi-square test. Correlation analysis was achieved using Spearman correlation coefficient. Binary logistic regression analysis was used to identify predictors of outcome. All statistical computations were processed using SPSS 25.0 with p value < 0.05 considered statistically significant.
Results
The present study included 150 women with PCOS and 150 age-matched healthy controls. Among PCOS women, NAFPD was diagnosed in 57 women (38.0%) in contrast to 18 women (12.0%) in the control group (p < 0.001). Comparison between patients NAFPD and patients without NAFPD revealed that the former group are significantly older [median (IQR): 38.0 years (35.0–43.0) versus 29.0 years (25.5–33.0), p = 0.001] with higher BMI [median (IQR): 31.5 kg/m2 (29.1–34.7) versus 30.4 kg/m2 (28.6–32.4), 0.042], higher fasting blood glucose (FBG) levels [median (IQR): 94.0 mg/dl (85.0–104.5) versus 90.0 mg/dl (82.0–95.0), p = 0.012] and higher total cholesterol levels [median (IQR): 193.0 mg/dl (172.8–233.0) versus 172.0 mg/dl (163.0–224.0), p = 0.013]. Also, patients with NAFPD had significantly higher frequency of metabolic syndrome (84.2% versus 54.8%, p = 0.001), insulin resistance (68.4% versus 26.9%, p < 0.001) and severe NAFLD (10.5% versus 2.2%, p < 0.001) (Table 1).
 |
Table 1 Clinical and Metabolic Findings in the Studied Patients (n = 150) |
Regarding the gynecological parameters, it was shown that NAFPD patients had significantly higher testosterone levels [median (IQR): 1.44 ng/mL (1.28–1.65) versus 1.39 ng/mL (1.19–1.53), p = 0.018], lower LH levels [median (IQR): 9.61 U/L (8.34–10.74) versus 10.53 U/L (9.56–11.52), p < 0.001], lower SHBG [median (IQR): 36.0 nmol/L (30.8–40.7) versus 38.1 nmol/L (35.15–42.7), p = 0.002] and higher FAI [median (IQR): 4.08 (3.3–4.92) versus 3.47 (3.12–4.05), p < 0.001] (Table 2, Figure 1).
 |
Table 2 Gynecological Parameters in the Studied Patients |
 |
Figure 1 FAI in patients with and without NAFPD. |
Correlation analysis showed a significant direct correlation between FAI and patients age (r = 0.24, p = 0.003) (Table 3). It was also noted that patient with MetS had significantly higher FAI that patients without MetS [median (IQR): 3.68 (3.22–4.5) versus 3.47 (3.07–3.98), p = 0.023] (Figure 2) and patients with IR had significantly higher FAI when compared with patients without IR [median (IQR): 3.97 (3.22–4.58) versus 3.49 (3.15–4.13), p = 0.037] (Figure 3).
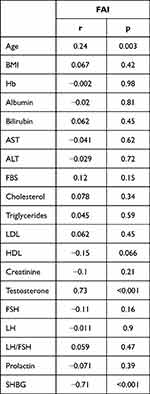 |
Table 3 Correlation Between FAI and Clinical and Laboratory Data |
 |
Figure 2 FAI in patients with and without metabolic syndrome. |
 |
Figure 3 FAI in patients with and without insulin resistance. |
Multivariate logistic regression analysis identified patients’ age [OR (95% CI): 1.72 (1.4–2.13), p < 0.001], FAI [OR (95% CI): 2.23 (1.12–4.46), p = 0.023] and IR [OR (95% CI): 4.68 (1.25–17.55), p = 0.022] as significant predictors of NAFPD in the studied patients (Table 4).
 |
Table 4 Predictors of NAFPD in the Studied Patients |
Discussion
The recent years had witnessed increased clinical awareness of the relatively newly introduced entity of NAFPD. However, knowledge about the condition and its consequences remain limited and the methods of diagnosis are still non-standardized.9,20–22 Reportedly, NAFPD has been linked to pancreatic endocrine impairment,23 increased epicardial adipose tissue and aortic intima-media thickness,24 incident diabetes mellitus25,26 pancreatic adenocarcinoma27 and acute pancreatitis.28
In the present study, we identified NAFPD in 38.0% of PCOS patients which is significantly than the 12.0% of NAFPD cases detected in apparently healthy controls. It is remarkable that one study could diagnose NAFPD in 25.9% of patients referred to endosonography.29 This discrepancy is probably attributed to the variable standards used for diagnosis of NAFPD in different studies.
In contrast to our results, a recent study conducted on adolescents found no relation between pancreatic fat and PCOS.30 Conclusions of the latter study are very interesting if taken together with the present study finding that patients’ age was independently associated with development of NAFPD in PCOS patients. Probably, the metabolic interactions between PCOS and NAFPD need longer time to be evident as seen in adult patients included in the current study.
In our work, PCOS patients with NAFPD has significantly higher frequency of MetS. This finding is supported by previous studies that documented a significant relation between NAFPD and MetS or its components. For example, Jaghutriz et al31 who assessed the metabolomic characteristics of fatty pancreas in prediabetics identified as association between NAFPD and obesity. Also, Sotoudehmanesh et al29 and Yu et al32 found an association between NAFPD and dyslipidemia. Furthermore, Chiyanika et al34 reported a significant association between NAFPD and MetS in obese adolescents.
Moreover, the current study noted that most patients with NAFPD had radiologically documented severe form of NAFLD. The association between NAFPD and NAFLD was previously reported.33 Notably, a computed tomographic study on patients with biopsy-proven NAFLD found a significant correlation between radiological hepatic and pancreatic fat masses.35 It is also exciting to learn that IR is associated with the synergistic effects of both hepatic and pancreatic fat accumulation as shown by one study.36
In the present study, patients with NAFPD had significantly higher FAI when compared with patients without and FAI was associated with NAFPD in multivariate regression analysis. The association between NAFPD and biochemical hyperandrogenism in PCOS women is a novel finding. This association may be explained by the documented link between hyperandrogenism and the metabolic consequences of NAFPD namely metabolic syndrome, insulin resistance and dyslipidemia as shown by the present study. The relation between metabolic syndrome and hyperandrogenism in PCOS women was previously reported.37 Also, Rasool et al38 reported an association between insulin resistance and hyperandrogenism in PCOS women.
In conclusion, the present study found that NAFPD is prevalent in PCOS women as compared to healthy counterparts. It is related to older age, metabolic syndrome, insulin resistance and hyperandrogenism in those patients.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethical and Consent
The study protocol was approved by the local ethical committee of October 6 University and informed consent was obtained from all participants in accordance with Helsinki Declaration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was not funded by any organization.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Carvalho LML, Dos Reis FM, Candido AL, Nunes FFC, Ferreira CN, Gomes KB. Polycystic ovary syndrome as a systemic disease with multiple molecular pathways: a narrative review. Endocr Regul. 2018;52(4):208–221. doi:10.2478/enr-2018-0026
2. Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2020;11:2042018820938305. doi:10.1177/2042018820938305
3. Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. doi:10.1016/j.molmet.2020.01.001
4. Shaaban Z, Khoradmehr A, Amiri-Yekta A, Jafarzadeh Shirazi MR, Tamadon A. Pathophysiologic mechanisms of obesity- and chronic inflammation-related genes in etiology of polycystic ovary syndrome. Iran J Basic Med Sci. 2019;22(12):1378–1386.
5. Nasser HA, Ezz NZA, Abdel-Mageed HM, Radwan RA. Body mass index and C-reactive protein are potential predictors of asthma development in Egyptian polycystic ovary syndrome patients. J Med Biochem. 2019;38(4):427–436.
6. Salva-Pastor N, Chávez-Tapia NC, Uribe M, Nuño-Lámbarri N. Understanding the association of polycystic ovary syndrome and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2019;194:105445.
7. Romana BS, Chela H, Dailey FE, Nassir F, Tahan V. Non-Alcoholic Fatty Pancreas Disease (NAFPD): a silent spectator or the fifth component of metabolic syndrome? A literature review. Endocr Metab Immune Disord Drug Targets. 2018;18(6):547–554.
8. Yu TY, Wang CY. Impact of non-alcoholic fatty pancreas disease on glucose metabolism. J Diabetes Investig. 2017;8(6):735–747.
9. Lesmana CRA, Gani RA, Lesmana LA. Non-alcoholic fatty pancreas disease as a risk factor for pancreatic cancer based on endoscopic ultrasound examination among pancreatic cancer patients: a single-center experience. JGH Open. 2017;2(1):4–7.
10. Pinte L, Balaban DV, Băicuş C, Jinga M. Non-alcoholic fatty pancreas disease - practices for clinicians. Rom J Intern Med. 2019;57(3):209–219. doi:10.2478/rjim-2019-0005
11. Abuelezz NZ, Shabana EM, Rashed L, Nb Morcos G. Nanocurcumin modulates miR-223-3p and NF-κB levels in the pancreas of rat model of polycystic ovary syndrome to attenuate autophagy flare, insulin resistance and improve ß cell mass. J Exp Pharmacol. 2021;13:873–888. doi:10.2147/JEP.S323962
12. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. doi:10.1093/humrep/deh098
13. Amiri M, Ramezani Tehrani F, Nahidi F, Bidhendi Yarandi R, Behboudi-Gandevani S, Azizi F. Association between biochemical hyperandrogenism parameters and Ferriman-Gallwey score in patients with polycystic ovary syndrome: a systematic review and meta-regression analysis. Clin Endocrinol (Oxf). 2017;87(3):217–230. doi:10.1111/cen.13389
14. Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140(7):815–830. doi:10.1016/0002-9378(81)90746-8
15. Tan AU, Schlosser BJ, Paller AS. A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol. 2017;4(2):56–71. doi:10.1016/j.ijwd.2017.10.006
16. Alberti KG, Eckel RH, Grundy SM, et al.; Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644.
17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi:10.1007/BF00280883
18. Lesmana CR, Pakasi LS, Inggriani S, Aidawati ML, Lesmana LA. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: a large cross sectional study. BMC Gastroenterol. 2015;15(1):174. doi:10.1186/s12876-015-0404-1
19. Xu J, Dai L, Zhang Y, et al. Severity of nonalcoholic fatty liver disease and risk of future ischemic stroke events. Stroke. 2021;52(1):103–110. doi:10.1161/STROKEAHA.120.030433
20. Shah N, Rocha JP, Bhutiani N, Endashaw O. Nonalcoholic fatty pancreas disease. Nutr Clin Pract. 2019;34(Suppl 1):S49–S56. doi:10.1002/ncp.10397
21. Dite P, Blaho M, Bojkova M, Jabandziev P, Kunovsky L. Nonalcoholic fatty pancreas disease: clinical consequences. Dig Dis. 2020;38(2):143–149. doi:10.1159/000505366
22. Filippatos TD, Alexakis K, Mavrikaki V, Mikhailidis DP. Nonalcoholic fatty pancreas disease: role in metabolic syndrome, “Prediabetes”, diabetes and atherosclerosis. Dig Dis Sci. 2022;67(1):26–41. doi:10.1007/s10620-021-06824-7
23. Miyake H, Sakagami J, Yasuda H, et al. Association of fatty pancreas with pancreatic endocrine and exocrine function. PLoS One. 2018;13(12):e0209448. doi:10.1371/journal.pone.0209448
24. Kul S, Karadeniz A, Dursun İ, et al. Non-alcoholic fatty pancreas disease is associated with increased epicardial adipose tissue and aortic intima-media thickness. Acta Cardiol Sin. 2019;35(2):118–125. doi:10.6515/ACS.201903_35(2).20181009A
25. Yamazaki H, Tauchi S, Wang J, et al. Longitudinal association of fatty pancreas with the incidence of type-2 diabetes in lean individuals: a 6-year computed tomography-based cohort study. J Gastroenterol. 2020;55(7):712–721. doi:10.1007/s00535-020-01683-x
26. Chan TT, Tse YK, Lui RN, et al. Fatty pancreas is independently associated with subsequent diabetes mellitus development: a 10-year prospective cohort study. Clin Gastroenterol Hepatol. 2021;2021:23.
27. Swislocki A. Fatty pancreas: an underappreciated intersection of the metabolic profile and pancreatic adenocarcinoma. Metab Syndr Relat Disord. 2021;19(6):317–324. doi:10.1089/met.2020.0070
28. Sbeit W, Khoury T. Fatty pancreas represents a risk factor for acute pancreatitis: a pilot study. Pancreas. 2021;50(7):990–993. doi:10.1097/MPA.0000000000001867
29. Sotoudehmanesh R, Tahmasbi A, Sadeghi A, Hosseini H, Mohamadnejad M. The prevalence of nonalcoholic fatty pancreas by endoscopic ultrasonography. Pancreas. 2019;48(9):1220–1224. doi:10.1097/MPA.0000000000001396
30. Ware MA, Kaar JL, Diniz Behn C, et al. Pancreatic fat relates to fasting insulin and postprandial lipids but not polycystic ovary syndrome in adolescents with obesity. Obesity. 2022;30(1):191–200. doi:10.1002/oby.23317
31. Jaghutriz BA, Wagner R, Heni M, et al. Metabolomic characteristics of fatty pancreas. Exp Clin Endocrinol Diabetes. 2020;128(12):804–810. doi:10.1055/a-0896-8671
32. Yu X, Wang D, Xiao W, et al. Relationship between fatty pancreas and hypertriglyceridemic waist phenotype: a cross-sectional study. Sci Rep. 2020;10(1):21937.
33. Milovanovic T, Dragasevic S, Stojkovic Lalosevic M, et al. Ultrasonographic evaluation of fatty pancreas in Serbian patients with non alcoholic fatty liver disease-a cross sectional study. Medicina. 2019;55(10):697.
34. Chiyanika C, Chan DFY, Hui SCN, et al. The relationship between pancreas steatosis and the risk of metabolic syndrome and insulin resistance in Chinese adolescents with concurrent obesity and non-alcoholic fatty liver disease. Pediatr Obes. 2020;15(9):e12653.
35. Koyuncu Sokmen B, Sahin T, Oral A, Kocak E, Inan N. The comparison of pancreatic and hepatic steatosis in healthy liver donor candidates. Sci Rep. 2021;11(1):4507.
36. Patel NS, Peterson MR, Lin GY, et al. Insulin resistance increases MRI-estimated pancreatic fat in nonalcoholic fatty liver disease and normal controls. Gastroenterol Res Pract. 2013;2013:498296.
37. Lee I, Vresilovic J, Irfan M, Gallop R, Dokras A. Higher incidence of metabolic syndrome in black women with polycystic ovary syndrome: a longitudinal study. J Clin Endocrinol Metab. 2022;107(4):e1558–e1567.
38. Rasool SUA, Ashraf S, Nabi M, Rashid F, Fazili KM, Amin S. Elevated fasting insulin is associated with cardiovascular and metabolic risk in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13(3):2098–2105.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
