Back to Journals » International Journal of General Medicine » Volume 13
Relation Between Aortic Stiffness Index and Distensibility with Age in Hypertensive Patients
Authors Nabati M , Namazi SS, Yazdani J, Sharif Nia H
Received 10 March 2020
Accepted for publication 15 May 2020
Published 11 June 2020 Volume 2020:13 Pages 297—303
DOI https://doi.org/10.2147/IJGM.S253357
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Maryam Nabati,1 Seyed Shojaeddin Namazi,2 Jamshid Yazdani,3 Hamid Sharif Nia4
1Department of Cardiology, Faculty of Medicine, Cardiovascular Research Center, Mazandaran University of Medical Sciences, Sari, Iran; 2Student Research Committee, Faculty of Medicine, Cardiovascular Research Center, Mazandaran University of Medical Sciences, Sari, Iran; 3Department of Biostatics, Faculty of Health, Mazandaran University of Medical Sciences, Sari, Iran; 4Department of Nursing, Mazandaran University of Medical Sciences, Sari, Iran
Correspondence: Seyed Shojaeddin Namazi
Student Research Committee, Faculty of Medicine, Cardiovascular Research Center, Mazandaran University of Medical Sciences, Sari, Iran
Tel/Fax +98-11-33324002
Email [email protected]
Background: Systolic and diastolic blood pressure is associated with physiologic changes of aortic wall and left ventricular structure. We aimed to evaluate aortic stiffness index and distensibility, global longitudinal strain (GLS), post systolic index (PSI) in hypertensive patients and compare these parameters with normotensive subjects.
Patients and Methods: Eighty-two patients (42 hypertensive compared with 40 normotensive subjects) with preserved left ventricular ejection fraction and without significant coronary artery disease were enrolled in the study. Systolic and diastolic blood pressure was measured by automated BP measurement system. Aortic stiffness index and distensibility, GLS and PSI were measured by transthoracic echocardiography and compared in both study groups.
Results: Aortic stiffness index (0.097 vs 0.069) and E/e´ (8.16 vs 6.56) were significantly higher in hypertensive patients, respectively (p< 0.05). Aortic distensibility (cm2/dyn) (0.28 vs 0.42) and e´ (cm/s) (8.25 vs 9.52) were significantly lower in hypertensive patients than normotensive subjects (p< 0.05). PSI and GLS were not significantly different between both study groups. Aortic stiffness index and distensibility had significant correlation with age in normotensive subjects while this correlation was not statistically significant in hypertensive patients.
Conclusion: Hypertension is associated with diastolic dysfunction and abnormal aortic wall compliance. Age-related aortic wall changes can present early in hypertensive patients.
Keywords: hypertension, myocardial strain, postsystolic shortening, echocardiography
Introduction
Systolic and diastolic hypertension (HTN) is a well-known risk factor and the leading cause of morbidity and mortality in patients with cardiovascular disease (CVD). HTN is associated with several potentially fatal geometrical and structural heart abnormalities such as Left Ventricle Hypertrophy (LVH), systolic and diastolic dysfunction and also aortic stiffness, enlargement, and dissection.1,2 Aortic and ventricular remodeling themselves play an essential pathophysiological role in the development of heart failure (HF).3 HF similarly changes the great vessels architecture (in especially aortic stiffness and dilation which are one of the most important predictors for CVD mortality). Moreover, Aortic stiffness has been considered as a subclinical target organ damage with prognostic value in hypertensive patients.3,4 A number of studies proposed that increased aortic stiffness is associated with persistent hypertension in some subjects.5,6 This vicious circle between HTN, aortic stiffness/distensibility and heart failure would be continued without any intervention whereas, early detection and appropriate interventions can reduce these complications.3,7
Over recent years, speckle tracking global longitudinal strain (GLS) was introduced as a quantitative method for measurement of LV function.8,9
GLS can be employed to assess systolic and diastolic dysfunction at initial stages of myofibril deformation before this process could be defined by a 2-D echocardiogram. Furthermore, GLS is more available and reproducible and has comparable accuracy for myocardial viability assessment to myocardial perfusion imaging (MPI) and cardiovascular magnetic resonance (CMR) imaging.9,10
In this study, we aimed to compare aortic stiffness, distensibility, and longitudinal strain of LV in hypertensive and normotensive subjects with preserved LVEF. Some studies demonstrated that myocardial fibrosis has an association with post-systolic index (PSI) and this correlation was attributed to impaired LV relaxation and increased LV stiffness.11,12 Accordingly, in this study, we also aimed to determine whether there is any association between PSI with aortic-related variables and other echocardiographic findings.
Patients and Methods
This case–control study was conducted among patients presented with angina or equivalent angina during May 2018–Feb 2019 to Fatemeh Zahra Hospital, the academic heart training center in the sari, Mazandaran province, in northern Iran. According to the Eren13 study (µ1 = 18, µ2= 11, σ= 8) the study sample size was estimated based on α= 0.05, β= 0.1and d= 0.8, 34 for each case and control group by Gpower 3.1.7.
Study Population
The initial evaluation consisted of history taking and physical examination was done, and 12 lead standard Electrocardiography (ECG) was obtained. Exercise ECG test, stress echocardiography or single-photon emission computed tomography (SPECT) was done according to patients’ ability to exercise and ECG interpretability. Patients with positive-mentioned noninvasive ischemic test underwent coronary angiography (CAG).
In order to minimize the confounding effect of ischemia on study results, we excluded all patients in whom coronary angiography showed moderate or significant stenotic lesions and those with up to mild CAD (less than 50% stenosis) enrolled in the study. The study was according to the Helsinki Declaration guidelines. From all participants, written informed consent was obtained and the study was approved by the ethics committee of Mazandaran University of Medical Sciences (ethic number: IR.MAZUMS.REC.1397.1285).
After 8–10 hrs fasting blood samples were analyzed for measurement of fasting blood sugar, triglyceride (TG), Cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), blood urea nitrogen (BUN) and creatinine (Cr)
Patients with a history of HTN and under treatment with anti-hypertensive agents during admission categorized as case group. And those without HTN and with constant systolic and diastolic blood pressure (BP) less than 140/90 mmHg during hospitalization period, considered as control subjects.
Patients’ demographic variables including age, gender, previous medical and drug history were recorded. Height (cm) and weight (kg) were measured and body mass index (BMI) (kg/m2) was calculated for each patient.
After CAG and before patient’s discharge, systolic and diastolic BP and pulse pressure (PP) was measured again (in order to reduce the probable effect of stressors on blood pressure before CAG at arrival time, we measured BP again before echocardiography) after 5 min resting in sitting position, with the left arm at the heart level by an automated BP measurement system. Patients were advised to avoid consuming tea, coffee, cigarettes smoking or have vigorous physical activity at least 30 min before BP measurement.
Patients with known history of CAD (CABG or PCI), severe systolic or diastolic heart failure (HF), constant atrial or ventricular arrhythmia, conductive heart abnormalities such as bundle branch blocks, autoimmune disease, connective tissue disorders, significant valvular heart regurgitation, moderate or severe valvular stenosis, congenital heart disease, previous open-heart surgery or transcatheter heart interventions, dilated and restrictive cardiomyopathies, constrictive pericarditis, peripheral artery disease, pulmonary HTN, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), history of cancer and anti-cancer or chemotherapeutic agents consumption, and those with poor echocardiographic visualization were excluded from the study.
Echocardiographic Measurement
TTE was performed at rest in left lateral decubitus for all subjects by a fellowship of echocardiography that was not informed about the patients’ data by using an ACUSON SC2000 with a 4V1c transducer (Siemens Medical Solutions USA Inc., Mountain View, CA) position. Grayscale images were obtained in standard views (Apical 2, 3 and 4 chamber parasternal long axis and short axis views). The peak longitudinal strain was measured by an average of epicardial and endocardial tracing of all 18 myocardial segment borders in various standard views by using semi-automated analysis software.
Left ventricular ejection fraction (LVEF) was determined by modified Simpson’s technique from apical 2 and 4 chamber views using the following equation:

Pulse wave (PW) Doppler echocardiography and tissue doppler imaging (TDI) was used to determining myocardial diastolic function according to 2016 European Cardiology Society (ESC) guideline for heart failure.14 The diameter of ascending aorta was measured by two-dimensional M-mode echocardiogram in parasternal long-axis view, 3 cm above the aortic valve level between the trailing edge and leading edge of the anterior and posterior aorta, respectively. The aortic strain was defined by aortic systolic (AoS) diameter (max aortic valve opening) and Aortic diastolic (AoD) diameter (peak of QRS complex) from the formula:15,16 AoS _ AoD/AoD
Aortic distensibility was measured according to below formula:15,16
Aortic Distensibility (cm2/dyn) = (2×aortic strain)/(a. systolic pressure − diastolic pressure). (Aortic strain was measured by echo and systolic and diastolic pressure was measured by automated BP system).
Aortic stiffness index was also measured using parasternal long-axis view 3–4 cm above the aortic valve level by TTE and defined according to the subsequent equation:16
Arterial stiffness index β=Ln (SBP/DBP)/strain (Ln: natural logarithm)
PSI was calculated according to the below equation:12

Interventricular septal thickness and left atrial (LA) diameter were measured at end-diastole and peak systole, respectively, from parasternal long-axis view. Valvular regurgitation severity (defined as no, mild, moderate and severe) and pulmonary arterial pressure was determined based on the guidelines of the American Society of Echocardiography (ASE).17,18
Statistical Analysis
Data were analyzed by SPSS25 and MedCalc19.1. Continues variables with normal distribution were expressed as mean ± standard deviation (SD) and analyzed by independent Student’s t-test. Categorical variables were compared between groups using chi-square tests and Fisher’s exact tests. Association between continuous normally distributed variables was analyzed using the Pearson correlation test. A p value less than .05 was considered statistically significant.
Result
The study population included 82 patients, 29 males (35.4%) and 53 females (64.6%) who were considered for coronary angiography because of the evidence of ischemia on non-invasive studies with no significant stenosis on coronary angiography between 2018 and 2019. Patients were divided into two groups: hypertensive (n= 42) and normotensive (n=40). The mean age of patients was 55.82 ± 10.28 years (range 35–76 years) and 12 patients (14.6%) had DM. The mean BMI was 28.80± 4.81 kg/m2 and the mean systolic blood pressure (SBP), diastolic blood pressure (DBP) and pulse pressure (PP) were 123.68± 18.28, 76.33± 10.44 and 47.35± 13.99 mmHg, respectively. Demographics and laboratory characteristics of the study population are presented in Table 1.
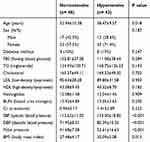 |
Table 1 Demographic and Measures of Common Cardiovascular Risk Factors of the Study Population Categorized as Having or Not Having Hypertension |
As shown in Table 1, the hypertensive group were older (P =.018) and they had higher SBP, DBP and PP (P <.001) than the patients in the normotensive group. Furthermore, body mass index (BMI) in the hypertensive group was higher than normotensive patients (P =.013). The echocardiographic characteristics of the study population are presented in Table 2.
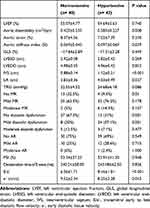 |
Table 2 Echocardiographic Variables of the Study Population Categorized as Having or Not Having Hypertension |
Among echocardiographic variables, IVS was thicker (P <0.001) and LA diameter was larger (P = 0.037) in hypertensive patients and they had lower e’ velocity (P = 0.015) and higher E/e’ ratio (P <0.001) than normotensive group. Also, hypertensive patients had a higher prevalence of MR (P = 0.01) and diastolic dysfunction (P <0.001) compared to normotensive patients. There was no statistically significant difference for the aortic strain, GLS, and PSI between two study groups (P = 0.33, 0.44 and 0.94, respectively). However, aortic distensibility was lower (P = 0.008) and aortic stiffness index was higher (P = 0.039) among hypertensive patients compared with the normotensive group.
We used the Pearson correlation coefficient to assess the association between the different echocardiographic parameters and age for each group, separately. Tables 3 and 4 show correlations between echocardiographic variables and age among two study groups with details.
 |
Table 3 Correlation Between Different Echocardiographic Variables and Age in Patients with Hypertension |
 |
Table 4 Correlation Between Different Echocardiographic Variables and Age in Normotensive Patients |
The study results showed, there was a direct correlation between age and aortic stiffness index and an inverse correlation between age and aortic distensibility in the normotensive group (P <0.001 and r = 0.611 and P < 0.001 and r = −0.567), respectively. Nevertheless, the hypertensive group did not show any significant correlation between age neither with aortic stiffness index nor to aortic distensibility.
PSI had direct correlation with age (P value = 0.012, r = 0.409) and GLS (P = 0.020, r = 0.367) in normotensive individuals while, PSI in hypertensive patients was not significant correlated with age and GLS. These correlations are demonstrated well by the scatter plot in Figures 1 and 2.
 |
Figure 1 Scatter plots of the correlation between aortic stiffness index and age in hypertensive and normotensive groups. |
 |
Figure 2 Scatter plots of the correlation between aortic distensibility and age in hypertensive and normotensive groups. |
The study results also showed, there was an inverse correlation between aortic stiffness index and e’ velocity and a direct correlation between aortic distensibility and e’ velocity in the normotensive patients (P =.001 and r= -.531 and P <.001 and r=.563). While the hypertensive group did not show any significant correlation between e’ velocity neither with aortic stiffness index and nor with aortic distensibility (Figures 3 and 4).
 |
Figure 3 Scatter plots of the correlation between aortic stiffness index and e’ velocity in hypertensive and normotensive groups. |
 |
Figure 4 Scatter plots of the correlation between aortic distensibility and e’ velocity in hypertensive and normotensive groups. |
Discussion
Our study showed GLS had a significant inverse and direct correlation, respectively, with early diastolic tissue velocity (e’) and deceleration time (DT) in the hypertensive group. Our findings were consistent with the study reported by others.19–21
Our study results showed that mild diastolic dysfunction and E/e’ ratio was significantly higher and e’ was meaningfully lower in hypertensive patients compared to the control group. Our finding was concordant with the reported findings by Mottram et al.22 They evaluated arterial stiffness in hypertensive patients and their relation to diastolic heart failure. Their result showed mild diastolic dysfunction and arterial compliance were significantly higher and lower, respectively, in hypertensive patients and these variables progressed with increasing age. They also found that decreased aortic compliance in hypertensive patients associated with increased aortic SBP and decreased aortic DBP.22
Our study showed hypertensive patients had lower aortic distensibility and higher aortic stiffness index than those without HTN. This finding is consistent with the previous studies.22,23 Functional and structural changes of the aortic wall can decrease aortic compliance and present as an initial detectable manifestation of target organ damage in hypertensive patients.24,25
In a study by Asmar et al, they evaluated the aortic distensibility in treated hypertensive patients and compared them with the normotensive population. Their study showed although decreasing blood pressure can reduce arterial alterations, but cannot reverse this change completely.26 Their results were similar to our finding. In our study, all hypertensive patients were already under treatment with antihypertensive drugs whoever, BP was not completely controlled for all patients. Our findings showed, despite antihypertensive medication, aortic stiffness and distensibility remained impaired among hypertensive patients.
Our study also showed aortic stiffness index had a direct correlation and aortic distensibility had an inverse correlation with age in normotensive individuals. However, patients with HTN did not show any correlation between these variables and age. This also can be due to the direct remodeling effect of HTN on aortic wall irrespective of age. Indeed, increased aortic stiffness and reduced aortic distensibility can be due to extracellular matrix changes of the aortic wall which happen early in hypertensive patients.
Our study showed several echocardiographic variables in normotensive patients had a significant correlation with age including diastolic dysfunction, and aortic compliance. These variables impaired in normotensive patients due to physiological aging. Whereas age was significantly correlated only with diastolic dysfunction but not with PSI and aortic compliance in the hypertensive group. This also can be due to the direct effect of HTN on LV structure which presents early in hypertensive disease irrespective of the aging process.
As a consequence, normotensive individuals may have normal aortic compliance and diastolic function until senility while hypertensive patients are susceptible to decreased aortic compliance, impaired PSI and diastolic dysfunction at an earlier age. It seems that blood pressure effects on the arterial and ventricular structure may be higher than age-related changes.
Our results showed that both case and control groups had similar LVEF and mildly impaired GLS. These findings are particularly important in this regard that we enrolled individuals with positive noninvasive ischemia detection test and without significant CAD. But in this study, we did not exclude coronary microvasculature disease for participants and this may be the probable cause of mildly impaired GLS in both study groups. Choosing patients with negative non-invasive tests as a control group, instead of a positive noninvasive test with normal CAG, may give more attractive results.
Limitations
This single-center study was limited by its small sample size and another limitation may be the fact that we just enrolled individuals with preserved LVEF and those with concomitant structural heart diseases such as severe LV dysfunction and severe valvular regurgitation and atrial or ventricular arrhythmia were excluded from the study whereas these abnormalities may be induced by HTN and can affect the study results. Thereby, these findings may not be completely extended to all hypertensive patients. Thus, future studies with considering larger sample size and concomitant diseases, which cannot be attributed to other causes except HTN are recommended.
Conclusion
Aortic stiffness index and distensibility and also LV filling pressure and relaxation in hypertensive patients were significantly impaired compared to normotensive individuals. Also, aortic wall changes, PSI and diastolic dysfunction which are seen physiologically with increasing age in the normal population may be presented early in hypertensive patients.
Acknowledgments
This project was supported by Mazandaran University of Medical Sciences. We want to hereby extend our sincere gratitude to all of the authorities of selected heart center who helped to make this research possible.
Disclosure
The authors declared no conflicts of interest with respect to the research, authorship and/or publication of this article.
References
1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi:10.1016/S0140-6736(05)17741-1
2. Devereux RB, Bella J, Boman K, et al. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Blood Press. 2001;10(2):74–82. doi:10.1080/08037050152112050
3. Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta-analysis. Heart Fail Rev. 2015;20(3):291–303. doi:10.1007/s10741-015-9471-1
4. Kuipers AL, Miljkovic I, Barinas-Mitchell E, et al. Arterial stiffness and hypertension status in afro-caribbean men. J Hypertens. 2019;37(3):546–554. doi:10.1097/HJH.0000000000001909
5. Sehgel NL, Zhu Y, Sun Z, et al. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol. 2013;305(9):H1281–H7. doi:10.1152/ajpheart.00232.2013
6. Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014;64(1):13–18. doi:10.1161/HYPERTENSIONAHA.114.00921
7. Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5(8):543–551. doi:10.1016/j.jchf.2017.04.012
8. Krishnasamy R, Isbel NM, Hawley CM, et al. Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS One. 2015;10(5):e0127044. doi:10.1371/journal.pone.0127044
9. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–1680. doi:10.1136/heartjnl-2014-305538
10. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11(2):260–274.
11. Brainin P, Haahr-Pedersen S, Sengeløv M, et al. Presence of post-systolic shortening is an independent predictor of heart failure in patients following ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging. 2018;1–10.
12. Nabati M, Mahmoudi P, Yazdani J, Parsaee H. Postsystolic index for distinguishing coronary artery disease in left bundle branch block. Echocardiography. 2019;36(4):687–695. doi:10.1111/echo.14313
13. Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90(1):37–43. doi:10.1136/heart.90.1.37
14. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi:10.1002/ejhf.592
15. Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45(3):426–431. doi:10.1161/01.HYP.0000157818.58878.93
16. Cho JY, Kim KH. Evaluation of arterial stiffness by echocardiography: methodological aspects. Chonnam Med J. 2016;52(2):101–106. doi:10.4068/cmj.2016.52.2.101
17. Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi:10.1016/S0894-7317(03)00335-3
18. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi:10.1016/j.echo.2010.05.010
19. Galderisi M, Lomoriello VS, Santoro A, et al. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. J Am Soc Echocardiogr. 2010;23(11):1190–1198. doi:10.1016/j.echo.2010.07.010
20. Ishizu T, Seo Y, Kameda Y, et al. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension. 2014;63(3):500–506. doi:10.1161/HYPERTENSIONAHA.113.02149
21. Galderisi M, Trimarco B. Global longitudinal strain: a novel hallmark of cardiac risk in arterial hypertension. J Hypertens. 2016;34(6):1050–1051. doi:10.1097/HJH.0000000000000920
22. Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91(12):1551–1556. doi:10.1136/hrt.2004.046805
23. Contaldi C, Imbriaco M, Alcidi G, et al. Assessment of the relationships between left ventricular filling pressures and longitudinal dysfunction with myocardial fibrosis in uncomplicated hypertensive patients. Int J Cardiol. 2016;202:84–86. doi:10.1016/j.ijcard.2015.08.153
24. Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. 2012;1(4):1–10. doi:10.1258/cvd.2012.012016
25. Rose J-L, Lalande A, Bouchot O, et al. Influence of age and sex on aortic distensibility assessed by MRI in healthy subjects. Magn Reson Imaging. 2010;28(2):255–263. doi:10.1016/j.mri.2009.07.001
26. Asmar R, Benetos A, London G, et al. Aortic distensibility in normotensive, untreated and treated hypertensive patients. Blood Press. 1995;4(1):48–54. doi:10.3109/08037059509077567
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
