Back to Archived Journals » Core Evidence » Volume 9
Regorafenib: an evidence-based review of its potential in patients with advanced liver cancer
Received 28 February 2014
Accepted for publication 26 March 2014
Published 17 July 2014 Volume 2014:9 Pages 81—87
DOI https://doi.org/10.2147/CE.S48626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Sujan Ravi,1 Ashwani K Singal2
1Department of Internal Medicine, 2Division of Gastroenterology and Hepatology, University of Alabama at Birmingham, Birmingham, AL, USA
Abstract: Hepatocellular carcinoma (HCC) is the second-most common cause of cancer-related death in the world. In spite of HCC surveillance with repeated imaging, about 50% of patients are diagnosed at an advanced stage and are not amenable to curative treatment options. Sorafenib, a multikinase inhibitor, remains the standard of care for advanced HCC. Over the last 5 years, several other medications have been tested in Phase III trials. However, they have not shown any added benefit over sorafenib. Regorafenib, another multikinase inhibitor, has demonstrated inhibition of a broader range of kinases, along with higher inhibition potential in preclinical models. After its safety and pharmacological properties was studied in Phase I trials, a Phase II study evaluating the role of Regorafenib in patients with advanced HCC who progressed on sorafenib therapy demonstrated efficacy and a manageable safety profile. A Phase III trial is ongoing, and its result will help us better evaluate the role of Regorafenib in patients with advanced HCC.
Keywords: Regorafenib, hepatocellular carcinoma, HCC, advanced HCC, multikinase inhibitors
Introduction
Hepatocellular carcinoma (HCC) is the second-commonest cause of cancer-related death, and accounted for 746,000 world deaths in 2012. HCC is the fifth-commonest cancer in men and the ninth-commonest cancer in women, accounting for 782,000 new cases in 2012.1 Male sex and advancing age are common predisposing demographic factors, with the highest incidence rates reported from developing countries. Approximately 90% of HCC cases are associated with underlying chronic liver disease and liver cirrhosis, due to such risk factors as chronic hepatitis from hepatitis B and C virus infection, alcohol abuse, and aflatoxin exposure.2 Symptomatic tumors usually present at an advanced stage. In spite of regular screening and surveillance of patients at high risk for development of HCC, only about 30%–60% of cases can be diagnosed at a stage amenable to curative treatment options (Figure 1).3 In this review, we focus on the management of advanced HCC, especially the current status of Regorafenib, a multikinase inhibitor.
  | Figure 1 Barcelona Clinic Liver Cancer staging system and treatment strategy. |
What is advanced hepatocellular carcinoma?
Staging of HCC is essential to determine the treatment modality as well as the prognosis. This is a rather complex process in the case of HCC, as the staging system should take into account the 1) tumor stage (number and size of nodules, vascular invasion, extrahepatic spread), 2) liver function (Child–Pugh class, bilirubin, albumin, portal hypertension), 3) functional status, as determined by the Eastern Cooperative Oncology Group (ECOG),4 and 4) the patient’s symptoms. The Barcelona Clinic Liver Cancer (BCLC) classification system is used worldwide, and takes into account all these variables. Apart from being extensively validated, this system connects between the staging and treatment options, providing well-laid out algorithms for managing HCC (Figure 1).5 The American Association for the Study of Liver Diseases and European Association for the Study of the Liver2 practice guidelines endorse the use of BCLC classification.6 The Model for End-Stage Liver Disease score provides good prognostic information among patients with cirrhosis, and is used worldwide to list patients for liver transplantation. However, it does not serve the same purpose in staging and treatment of HCC.7 Tumor-node metastasis classification includes evidence of vascular invasion.8 In the case of HCC, this is not easy to determine in the absence of availability of resected surgical specimens. The Okuda classification does not encompass patients with early or indeterminate HCC.9 Further, these systems do not take liver function or ECOG status into consideration, important variables for decision making in the management of HCC. The BCLC staging system classifies HCC into five stages: 0, A, B, C, and D (Figure 1). Patients with advanced HCC are categorized as BCLC stage C. These patients have portal vein invasion and/or extrahepatic spread ± cancer-related symptoms, but with good performance status (ECOG 1–2).2 They have an expected median survival of 6 months.10 Currently, the only approved treatment for advanced-stage HCC is sorafenib (Figure 1).
Molecular mechanisms of HCC and targeted therapies
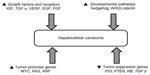
Hepatocarcinogenesis is a complex multistep process involving several pathways with activation of growth factors and their receptors, such as vascular endothelial growth factor (VEGF),11,12 fibroblast growth factor (FGF), epidermal growth factor (EGF),13 and insulin like growth factor;14,15 such oncogenes as RAS with activation of Ras-mitogen-activated protein kinase and RAF oncogenes;16,17 such developmental pathways as Wnt/β-catenin and hedgehog pathways;16,18,19 and inactivation or dysregulation of various tumor-suppressor genes (Figure 2).
Identification of these pathways has provided new treatment targets, with avenues for development of pharmaceutical agents for treatment of advanced-stage HCC that are not amenable to curative treatment options of resection, liver transplantation, or tumor ablation. Demonstration of efficacy and safety of sorafenib, a multikinase inhibitor of angiogenesis (VEGF and platelet-derived growth factor [PDGF] receptors) and tumor proliferation (Raf kinase) in a randomized placebo-controlled double-blind large multicenter study for advanced HCC changed the paradigm of management of HCC patients.20 In a dose of 400 mg twice daily, sorafenib compared to placebo was useful in improving the median overall survival (10.7 versus 7.9 months, P<0.001), with a shorter time to radiologic progression (5.5 versus 2.8 months, P<0.001). Side effects, including hand–foot skin rash, diarrhea, weight loss, and hypophosphatemia, were frequent with sorafenib, but were manageable in most cases.
Median improvement was limited to about 3 months only, indicating the need for newer drugs for the treatment of advanced HCC patients. Since then, many Phase II or III studies have been performed with newer drugs. All Phase III studies with sunitinib (angiogenesis inhibitor),21 linifanib (angiogenesis kinase inhibitor),22 and brivanib (inhibitor of VEGF and FGF receptors)23 failed in demonstrating superiority of these agents over sorafenib. Further, all these agents had a poorer side-effect profile compared to sorafenib. With the rationale of multiple pathways being involved in hepatocarcinogenesis, a combination of agents has been tried for the treatment of advanced HCC. A Phase III study with sorafenib (VEGF- and PDFG-receptor inhibitor) and erlotinib (EGF-receptor inhibitor) combination failed to be superior to a sorafenib and placebo combination.24 Given the unavailability of more effective treatment options, sorafenib has remained the standard of care for the treatment of advanced HCC over the last 5 years.
Regorafenib, a multikinase inhibitor like sorafenib, is being currently studied in the treatment of patients with advanced HCC who fail to respond to sorafenib. Based on lessons from the sorafenib study and Phase III trials with other drugs, Regorafenib in the treatment of advanced HCC is currently being studied, avoiding the limitations of previous trials. First of all, all the newer drugs have been entered into Phase III studies without prior assessment in preclinical, Phase I, or Phase II studies. It is now suggested that newer drugs to be tested for advanced HCC should go through all phases in a stepwise fashion before beginning a Phase III trial. Further, it is suggested that Phase I studies on newer drugs be performed in cirrhotic patients with establishment of the right dose and pharmacokinetics of the drug in this population.6 Secondly, overall survival was the primary endpoint in the sorafenib study. Underlying cirrhosis present in 70%–90% of HCC patients may confound assessment of cause of patient mortality in HCC patients.25 Therefore, it is recommended that time to progression be assessed as the primary outcome. Although this translates well with overall survival, results of post hoc analysis from sorafenib studies would provide robust evidence of time to progression as a valid surrogate marker for overall survival. Finally, mechanisms of a ceiling effect of sorafenib with disease stabilization remain unknown. Therefore, newer drugs should be tested among patients who progress on sorafenib therapy. In this regard, brivanib use among patients who have not responded to sorafenib failed to show efficacy compared to placebo treated patients (median overall survival of 9.4 versus 8.2 months, P=0.33).26 Adverse events were also more frequent in the experimental arm compared to patients in the placebo arm. This review of the use of Regorafenib in advanced HCC is timely and relevant, as its use has overcome many of the limitations with previously tested newer drugs, including demonstration of preclinical efficacy, Phase I dose-finding studies in HCC patients, and Phase II studies in HCC patients before moving into Phase III study.
Structure pharmacokinetics and pharmacodynamics of Regorafenib
Regorafenib is a novel multikinase inhibitor belonging to the group of biaryl urea chemicals. Its chemical name is 4-(4-[{(4-chloro-3-[trifluoromethyl]phenyl)carbamoyl}amino]-3-fluorophenoxy)-N-methylpyridine-2-carboxamide hydrate. The structure of Regorafenib (Figure 3) is very similar to sorafenib, except for a fluorine atom in the center phenyl ring.27,28 This structural change results in a broader spectrum of kinase inhibition and a higher inhibition potential (Table 1).29,30 Studies using Regorafenib have shown potent inhibition of angiogenic and stromal receptor tyrosine kinases, including VEGFR-1, VEGFR-2, VEGFR-3, PDGFRβ, FGFR-1, and tyrosine kinase with immunoglobulin and epidermal growth-factor homology domain 2. It has also shown activity against oncogenic receptor tyrosine kinases and intracellular signaling kinases.31
`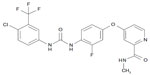  | Figure 3 Structure of Regorafenib. 4-(4-[{(4-Chloro-3-[trifluoromethyl]phenyl)carbamoyl}amino]-3-fluorophenoxy)-N-methylpyridine-2-carboxamide. |
  | Table 1 Biochemical activity of regorafenib and sorafenib: target inhibition |
The bioavailability of Regorafenib is 69% after oral administration in tablet form, and 83% when given as an oral solution. The drug is metabolized in the liver by cytochrome P450 3A4 and uridine 5′-diphospho-glucuronosyltransferase 1A9 into two active metabolites – N-oxide-Regorafenib and N-desmethyl-Regorafenib – and excreted primarily in the feces.32 The plasma concentration of Regorafenib and its metabolites showed multiple peaks in relation to time. It had an initial time to maximum concentration of 1–6 hours, a secondary maximum of 6–8 hours, and a tertiary maximum of 24 hours. The terminal half-life of Regorafenib was 20–40 hours, resulting in accumulation of the drug after multiple doses. Pharmacodynamic assessment showed dose-dependent reduction of plasma VEGFR-2 during cycles 1–3.33
Efficacy of Regorafenib in advanced HCC
Preclinical studies have demonstrated the potential for Regorafenib as oral therapy for human cancers with tolerable side effects.31 Its activity against angiogenic, stromal, and oncogenic kinases was studied in in vitro and in vivo models. The benefit from preclinical models led to the evaluation of Regorafenib in clinical trials. In a Phase I study, Regorafenib was used as monotherapy in patients with advanced solid tumors, including HCC.33 Based on safety profile and pharmacological data, the recommended dose from was found to be 160 mg daily for 3 weeks every 4 weeks, with a 1-week gap between the two cycles. The most common adverse effects were reported to be dermatologic reaction, hypertension, and diarrhea, as experienced with sorafenib and other multikinase inhibitors.
Regorafenib has been tested in Phase II trials as monotherapy for renal cell cancer, HCC, gastrointestinal stromal tumors, and metastatic colorectal carcinoma.34–37 In a prospective open-label Phase II study in patients with advanced HCC (BCLC stage B or C) who progressed on sorafenib therapy, 36 patients were enrolled from 13 centers across Europe and Asia.34 Regorafenib was used in a dose of 160 mg for 3 weeks, and repeated again after a break of 1 week. The primary endpoint of the study was the safety of Regorafenib. Secondary endpoints were efficacy (defined as time to progression), objective tumor-response rate (complete response + partial response), disease-control rate (complete response + partial response + stable disease), and overall survival. Response in this study was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST).38 The median time to progression was 4.3 (2.9–13.1) months, with median overall survival of 13.8 (9.3–18.3) months. About 65% and 44% of patients were alive without HCC progression at 3 and 6 months, respectively, after starting treatment. Similar overall survival rates were 88% and 79%, respectively. The best response based on mRECIST was partial response in one patient (3%), stable disease in 25 patients (69%), and progressive disease in five patients (14%), giving an overall response of 3% and a disease-control rate of 72%.
Safety of Regorafenib in advanced HCC
Safety data on Regorafenib use for advanced HCC are derived from the Phase II study. Although limited by lack of placebo arm in this study, the safety data are similar to the drug’s use in other cancers and to sorafenib safety data in HCC. Thirty-five (97%) of the 36 patients in the Phase II study had at least one adverse event. The most common adverse events included diarrhea, fatigue, and hand–foot skin reaction (Table 2). Most of these adverse events were manageable with supportive measures, dose reductions, and treatment interruption. Fourteen patients (39%) had biochemical abnormalities, such as proteinuria, acidosis, bilirubin, creatinine, hypoalbuminemia, and hypophosphatemia. Twenty-one patients had a grade 3 or higher adverse event. Five of these were reported as adverse reactions and related to the use of Regorafenib.
  | Table 2 Adverse-effect profile of Regorafenib |
Over a median duration of 19.5 (2–103) weeks of Regorafenib administration, 33 of 36 patients discontinued treatment before the data-cutoff date. Twenty of these discontinued treatment for adverse events (Table 2), ten due to disease progression, two due to consent withdrawal, and one patient died on therapy. Among the patients who discontinued treatment due to adverse events, seven of them were due to adverse reactions and related to Regorafenib. A total of seven deaths occurred during the study period. However, these were considered not to be related to Regorafenib. Drug-related liver injury is a known serious adverse event from tyrosine-kinase inhibitors.39,40 Two patients in the study died from liver dysfunction; however, neither of these deaths was thought to be related Regorafenib.
Summary and future perspectives
The Regorafenib Phase II study lacks the strengths of the sorafenib study of large sample size and randomized double-blind placebo-controlled design. However, time to progression as the primary endpoint, response evaluation by mRECIST criteria with emphasis on tumor enhancement, and a step-by-step approach of going through all the phases will help us better understand the role of this drug in the treatment of advanced HCC.
Regorafenib is currently being evaluated in a Phase III study in patients with advanced HCC who have progressed on sorafenib treatment (ClinicalTrials.gov identifier NCT01774344, RESORCE [REgorafenib after SORafenib in patients with hepatoCEllular carcinoma] trial). With a target enrollment of about 530 patients (2:1 Regorafenib:placebo), this randomized study has been recruiting participants all over the world since May 2013, and is estimated to finish enrollment by October 2016. The primary endpoint of the study is overall survival and secondary outcomes, being time to progression, progression-free survival, objective tumor response, and disease control. Successful completion and results of the ongoing Phase III Regorafenib study among sorafenib progressors may potentially influence the treatment algorithms among patients with advanced HCC.
Further studies and data are needed to clearly define 1) criteria for progression on sorafenib, 2) accurate surrogate endpoints that best translate into survival, and 3) biomarkers to help personalize treatment of advanced HCC patients. For example, the c-Met tyrosine-kinase inhibitor tivantinib showed better efficacy in patients with high Met expression. Similarly, anti-glypican 3 monoclonal antibody was most effective among patients with high glypican 3 expression in tumor tissue. However, the lack of accessible tumor tissue, given the frequent noninvasive accurate diagnosis of HCC, and the heterogeneity of intratumoral tissue for these markers makes these approaches difficult. In this regard, studies using liquid biopsy or serum assays using proteomic, metabolomic, or genetic approaches are critical for identifying accurate biomarkers for response to these drugs and personalizing use to patients who are likely to best respond to them. Given its more broader and potent kinase inhibition, Regorafenib, like sorafenib, may also be tested in combination with transcatheter arterial chemoembolization for treating BCLC stage B or C HCC and for prevention of HCC recurrence after curative treatment of HCC.41–44
In conclusion, HCC remains a serious public health problem. Current treatment options for advanced HCC patients are limited, and provide a maximum survival of about a year. Sorafenib chemotherapy remains the standard of care as of today. With the guidelines laid for study-design strategies, use of better primary end points, and identifying biomarkers for personalizing use of chemotherapy to best responders will hopefully change the paradigm of treatment of advanced HCC and improve its outcome.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012: Estimated Cancer Incidence and Mortality Worldwide. Geneva: WHO; 2012. | |
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. | |
Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20–S37. | |
Eastern Cooperative Oncology Group. ECOG performance status. 2006. Available from: http://ecog.dfci.harvard.edu/general/perf_stat.html. Accessed April 8, 2014. | |
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. | |
Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. | |
Huo TI, Lin HC, Hsia CY, et al. The model for end-stage liver disease based cancer staging systems are better prognostic models for hepatocellular carcinoma: a prospective sequential survey. Am J Gastroenterol. 2007;102(9):1920–1930. | |
Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20(6):1527–1536. | |
Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56(4):918–928. | |
Cabibbo G, Enea M, Attanasio M, Bruix J, Craxi A, Camma C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274–1283. | |
Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41(5):864–880. | |
Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68(16):6779–6788. | |
Ito Y, Takeda T, Sakon M, et al. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer. 2001;84(10):1377–1383. | |
Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25(27):3787–3800. | |
Tovar V, Alsinet C, Villanueva A, et al. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol. 2010;52(4):550–559. | |
Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27(1):55–76. | |
Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19(49):5548–5557. | |
Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674–687. | |
Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25(2):212–225. | |
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. | |
Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. | |
Cainap C, Qin S, Huang WT. Phase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2013;30 Suppl 34:249. | |
Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. | |
Zhu AX, Rosmorduc O, Evans J, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma (HCC). Eur J Cancer. 2012;48 Suppl:2. | |
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. | |
Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516. | |
Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. | |
Fabian MA, Biggs WH 3rd, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23(3):329–336. | |
Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–844. | |
Strumberg D, Schultheis B. Regorafenib for cancer. Expert Opin Investig Drugs. 2012;21(6):879–889. | |
Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. | |
Solimando DA Jr, Waddell JA. Drug monographs: bosutinib and Regorafenib. Hosp Pharm. 2013;48(3):190–194. | |
Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of Regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18(9):2658–2667. | |
Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412–3419. | |
George S, Wang Q, Heinrich MC, et al. Efficacy and safety of Regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol. 2012;30(19):2401–2407. | |
Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106(11):1722–1727. | |
Eisen T, Joensuu H, Nathan PD, et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: a single-group phase 2 trial. Lancet Oncol. 2012;13(10):1055–1062. | |
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. | |
Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10(8):794–800. | |
de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75–S87. | |
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29(30):3960–3967. | |
Park JW, Koh YH, Kim HB, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatoly. 2012;56(6):1336–1342. | |
Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117–2127. | |
Abdel-Rahman O, Elsayed ZA. Combination trans arterial chemoembolization (TACE) plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review of the literature. Dig Dis Sci. 2013;58(12):3389–3396. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.