Back to Journals » Local and Regional Anesthesia » Volume 16
Regional Anesthetic Use in Trans-Hiatal Esophagectomy. Are They Worth Consideration? A Case Series
Authors Mitchell W , Roser T, Heard J, Logarajah S, Ok J, Jay J, Osman H, Jeyarajah DR
Received 19 November 2022
Accepted for publication 12 June 2023
Published 11 July 2023 Volume 2023:16 Pages 99—111
DOI https://doi.org/10.2147/LRA.S398331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Stefan Wirz
William Mitchell,1,* Thomas Roser,1,* Jessica Heard,2 Shankar Logarajah,2 John Ok,1,2 John Jay,2 Houssam Osman,1,2 D Rohan Jeyarajah1,2
1Burnett School of Medicine at Texas Christian University, Fort Worth, TX, USA; 2Methodist Richardson Medical Center, Richardson, TX, USA
*These authors contributed equally to this work
Correspondence: D Rohan Jeyarajah, Tel +1 972 619 3500, Email [email protected]
Background: Esophagectomy traditionally has high levels of perioperative morbidity and mortality due to surgical techniques and case complexity. While thoracic epidural analgesia (TEA) is considered first-line for postoperative analgesia after esophagectomy, complications can arise related to its sympathectomy and mobility impairment. Additionally, it has been shown that postoperative outcomes are improved with early extubation following esophagectomy. Our aim is to describe the impact of transversus abdominis plane (TAP) blocks on extubation rates following esophagectomy when uncoupled from TEA.
Methods: This is a case series of 42 patients who underwent trans-hiatal esophagectomy between 2019 and 2022 who received a TAP block without TEA. The primary outcomes of interest were the rates of extubation within the operating room (OR) and reintubation. Secondary outcomes included: intensive care unit (ICU) and hospital length of stay (LOS), opioid pain medication use, post-operative hypotension, fluid administration, postoperative pain scores, development of anastomotic leak, and 30-day readmission.
Results: The mean age at operation was 63 years and 97.6% of patients were represented by American Society of Anesthesia (ASA) physical status class III or IV. Thirty-four (81%) patients immediately extubated postoperatively. Nine patients (21.4%) underwent reintubation during their hospital course. Only seven patients (16.7%) required vasopressors postoperatively. The median LOS was five days in the ICU and 10 days in the hospital. TAP block alone was found to be equivalent to TAP with additional regional blocks (TAP+) on the basis of immediate extubation, reintubation, ICU and hospital LOS, and reported postoperative pain.
Conclusion: The results of this study demonstrated immediate extubation is possible using TAP blocks while limiting post-operative hypotension and fluid administration. This was shown despite the elevated comorbidity burden of this study’s population. Overall, this study supports the use of TAP blocks as a possible alternative for primary analgesia in patients undergoing trans-hiatal esophagectomy.
Trial Registration: This study includes participants who were retrospectively registered. IRB# 037.HPB.2018.R.
Keywords: trans-hiatal, esophagectomy, transversus abdominis plane, TAP, quadratus lumborum, QL, block, epidural, ERAS, analgesia
Background
Esophagectomy for both malignant and benign diseases are known to carry significant perioperative risk. In fact, several studies have shown the perioperative morbidity to be as high as 59% with a 90-day mortality risk of 4.5%.1 The elevated level of morbidity and mortality associated with esophagectomy is polyfactorial, but thought to be related to patient comorbidities, operative technique, and the devastating nature of the associated postoperative complications. Opioid-based analgesia in tandem with thoracic epidural analgesia (TEA) is generally considered the first-line approach to postoperative analgesia for esophagectomy. In addition to providing adequate analgesia, some studies suggest a theoretical benefit by reducing splanchnic blood flow thus reducing the chances of developing an anastomotic leak.2,3 However, current literature remains inconclusive on this potential benefit.2,3 Due to the strong analgesia TEA provides, it has been incorporated into the enhanced recovery after surgery (ERAS) pathway in most hospitals.4
TEA is not without complications, including urinary retention, respiratory depression, hypotension, and impaired mobility which may slow postoperative recovery.5,6 Importantly, resulting hypotension is often treated with increased fluid administration in the perioperative setting and may lead to bowel edema, ileus, and ischemia-related anastomotic breakdown. Hypotension occasionally necessitates early removal of TEA, resulting in insufficient postoperative analagesia.4,7 Moreover, many centers require intensive care unit (ICU) monitoring for the duration of TEA utilization and a dedicated acute pain management service for its proper titration.7–9 During a time with strained hospital resources and bed availability, these can be significant constraints, particularly in non-academic hospital settings. Finally, concerns exist regarding epidural hematoma development with TEA, often delaying prophylactic anticoagulation initiation.4,7
Alternatively, transversus abdominis plane (TAP) and quadratus lumborum (QL) blocks with local anesthetics may provide effective opioid-sparing relief of postoperative surgical incision pain without exposing patients to TEA specific related side effects.10–12 One study compared the efficacy of TAP blocks for esophagectomy against TEA in patients undergoing esophagectomy and found equivocal pain scores while effectively avoiding the complications associated with TEA.12
In order to adequately prepare patients to undergo esophagectomy, a multidisciplinary approach is paramount. The physician most appropriate to lead this multidisciplinary, perioperative team in the care of thoracic patients is the anesthesiologist due to the type of training they receive, and their expertise in the assessment, evaluation, and preparation of patients with complex comorbidities for surgery.13,14 With the direction of the anesthesiologist, adequate post-operative analgesia strongly favors early mobilization and rapid recovery.7 The anesthesiologist also plays an important role intraoperatively in minimizing the risk of pulmonary complications and anastomotic leak through implementation of “protective anesthesia” measures which rely on protective ventilation strategies and judicious fluid management.7
Several studies have confirmed that early postoperative clinical outcomes are improved with immediate extubation in the operating room (OR) following esophagectomy.15,16 However, most available literature utilize TEA as the primary form of analgesia.15,16 One retrospective review by Levy et al demonstrated that TAP blocks may decrease perioperative fluid requirements while still achieving adequate pain control but did not address ventilator liberation and patient status leaving the OR.12 While it is known that early extubation in the OR is possible after esophagectomy utilizing TEA and paravertebral blocks, there is no current literature on early extubation of patients using TAP blocks.4,8,12,17
The aim of this study is to describe this high-volume, single site experience performing trans-hiatal esophagectomy utilizing regional anesthesia as the primary mode of analgesia including TAP blocks, QL blocks, and erector spinae blocks, in the absence of TEA in order to investigate the safety and efficacy of immediate extubation in these case types.
Methods
This is a retrospective, IRB-approved, study of patients who underwent trans-hiatal esophagectomy at a single, high-volume, private institution from January 2019 to January 2022. Informed consent was waived by the IRB due to the retrospective nature of the study, the negligible risk posed to patients, and this research did not require FDA regulation. Patient confidentiality was maintained at all times. Data was collected only by authorized personnel via a password and hospital-firewall protected computer and maintained in its de-identified form. All consecutive patients who underwent esophagectomy during the study period were identified via electronic medical record (EMR). Patients were excluded if they did not receive a preoperative TAP block. The use of additional regional block types beyond TAP was noted but did not result in study exclusion. Both surgeon and anesthesiologist were familiar with current ERAS guidelines specific to esophagectomy, and these standards were adhered to with appropriate variations based on clinical discretion and urgency of the procedure.4
Data Collection
For all patients who met inclusion criteria, the following information was obtained from the electronic medical record:
- Demographics: This includes sex, age at the time of surgery, tobacco abuse, and the presence of the following pulmonary comorbidities: asthma, chronic obstructive pulmonary disease (COPD), pulmonary hypertension, restrictive lung disease, and obstructive sleep apnea (OSA). Other medical comorbidities collected include hypertension, congestive heart failure, coronary artery disease, chronic kidney disease, and diabetes mellitus.
- Preoperative measures: This includes American Society of Anesthesiologists physical status (ASA) classification, body mass index (BMI), and timing of neoadjuvant radiation in relation to surgery.
- Surgical intraoperative measures: This includes type of conduit utilized, surgical access method, operative time, estimated blood loss (EBL), volume of transfused blood products, urine output, IV fluids, and overall fluid balances.
- Anesthesia intraoperative measures and medications: This includes duration of mean arterial pressure (MAP) less than 60 mmHg, duration of tidal volume (Vt) >8 cc/kg ideal body weight, duration of time the fraction of inspired oxygen (FiO2) was >80%, duration of positive end expiratory pressure (PEEP) greater than 5 cm H2O, mechanical ventilation time, types of regional blocks performed, and morphine milligram equivalents (MME) required. All data is reported in a minute-by-minute manner collected from the anesthetic record case start to end in the EMR. Medication information included vasopressor requirements at the conclusion of the case and the amount of norepinephrine, ketamine, magnesium, acetaminophen, and methocarbamol utilized.
- Postoperative measures: This includes ability to extubate in OR, event of reintubation during entire hospital stay, reported postoperative pain scores as recorded by the anesthesiologists and nursing teams through postoperative day (POD) 3, presence of postoperative hypotension, fluid bolus volumes, anastomotic leak, vasopressor requirement, ICU length of stay (LOS) and total hospital LOS.
Anesthetic Management
Arterial line access and central line access with subsequent blood pressure monitoring were performed in every case after induction. All patients were intubated using single lumen endotracheal tubes, as one lung ventilation was not anticipated due to surgical approach. Pressure controlled volume guaranteed ventilation was utilized for every patient. We used modified goal-directed fluid therapy (GDFT) utilizing respiratory variation and responsiveness to fluid challenges to direct fluid administration. Prior to 2019, strict GDFT had been found to cause a higher-than-expected rate of acute renal injury and postoperative hypotension requiring additional fluids in PACU and POD1 at this institution. To avoid this, the anesthesiologist began administering a more liberalized GDFT which was called modified GDFT, typically adding 1 liter of crystalloid beyond GDFT. The neuromuscular blockade was reversed at the end of every case prior to extubation.
Anesthetic Blocks
All regional anesthetic blocks were performed in a bilateral fashion under ultrasound guidance using either 0.25% bupivacaine or 0.5% bupivacaine with 1.3% liposomal bupivacaine, appropriately dosed by patient weight. Performance of TAP blocks included both a lateral approach along with rectus sheath blocks, often referred to as a four-quadrant TAP block, placed according to the New York Society of Regional Anesthesia for TAP blocks.13 This four-quadrant TAP block, allowed for supraumbilical cutaneous and infraumbilical pain coverage.8,18,19 The QL blocks, in this case, a QL1 variation, were performed using a lateral approach, approximately 2 cm superior to the iliac crest bilaterally.10 The QL block produces a broad distribution of local anesthetic, resulting in a large area of blocked sensory input (T7 through L1 in most cases).10 A single erector spinae plane (ESP) block was also performed on one patient that was positioned upright and seated in the OR prior to induction.
TAP and QL blocks were placed while the patient was positioned supine in the OR after induction and prior to first incision. Beginning with the TAP block, the patient’s entire abdomen was prepped and draped. Ultrasound imaging was performed with the transducer oriented longitudinally parallel to the subcostal margin and 2 cm lateral from the xiphoid process. After the external oblique, internal oblique, and transversus abdominis muscles were identified, the needle was inserted in plane to the ultrasound probe. The needle was advanced past the internal oblique muscle and aspiration was performed to ensure the needle was not in a blood vessel. With correct needle position, the local anesthetic was injected under ultrasound guidance to demonstrate continued spread within the potential space and then repeated on the contralateral side. The attention was then turned to the QL blocks with the ultrasound probe applied to the flank just cephalad to the iliac crest and just posterior to the mid axillary line. The same three abdominal muscle layers were identified and traced posteriorly to identify the thoracolumbar fascia. The needle was then inserted in plane and advanced through the abdominal muscles until it reached the anterolateral edge of the QL muscle. The local was injected under ultrasound guidance to demonstrate continued spread within the potential space and then repeated on the contralateral side. ESP blocks were performed using a deep needle approach with injection of the local anesthetic deep to the erector spinae muscles.20 For analysis purposes, patients that received a TAP block with the addition of any other regional block combination are referred to as TAP+.
Transhiatal Esophagectomy
Robotic-assisted laparoscopic trans-hiatal esophagectomy was performed using the Da Vinci Xi platform (Intuitive Surgical; Sunnyvale, CA) and two surgical teams. Five trocars are placed in the upper abdomen: supraumbilical midline, left midclavicular region, left midaxillary region, and far right subcostal region. With the patient in steep reverse Trendelenburg, mobilization of the greater curvature of the stomach is performed and the esophagus is encircled. Dissection is attempted trans-abdominally to the level of the carina. After the cervical esophagus is bluntly dissected via a left neck incision, the esophagus is cervically transected and extracted trans-abdominally through a limited midline incision.
In an open approach, the abdominal mobilization was performed through an upper abdominal midline incision. This incision is approximately two to three times as large as the incision required for specimen extraction. All esophagectomies received a generous Kocher maneuver and Heineke-Mikulicz style pyloroplasty. In all cases, a stapled anastomosis is created via the posterior proximal stomach and proximal cervical esophagus. A nasogastric tube and cervical drain were placed prior to the completion of each case.
Data Analysis
Continuous parametric data were compared using Student’s t-test while a Mann–Whitney U-test was used for non-parametric continuous data. Categorical variables were analyzed using Chi-square tests where appropriate. Multiple linear regression analysis was performed to identify the correlation and impact of key variables in ICU LOS and hospital LOS. Proportions were reported as percentage, parametric continuous variables reported as the mean with interquartile range (IQR), and non-parametric continuous variables reported as the median with range. A p-value less than 0.05 was considered statistically significant. All multiple linear regression analysis and Mann–Whitney U-tests were performed using IBM SPSS Version 28 (Predictive Analytics Software, Armonk, NY) while Student’s t-test and Chi-square tests were performed using Microsoft Excel Workbook Version 16.64 (Microsoft Corporation, Redmond, WA).
Results
Forty-three patients were identified to meet inclusion criteria. One patient was excluded due to failure to receive a preoperative TAP block. Figure 1 shows a study flow chart of the study population and includes which regional block group was most affected by reintubation. Of the 42 patients that met inclusion criteria, the primary operative indication was esophageal cancer, although 3 patients with benign disease were included in this study (1 with recurrent stricture, 2 with achalasia).
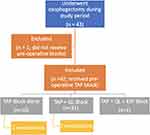 |
Figure 1 Study flow chart. Abbreviations: TAP, transversus abdominis plane; QL, quadratus lumborum block; ESP, erector spinae plane block. |
Table 1 shows the demographic data of the entire study population. The mean age at operation was 63 years, and there was a male predominance (85.7%). 73.2% of the study population were ASA III and 23.9% were ASA IV. Pre-existing lung conditions were present in 42.9% of the patients with OSA (23.8%) and COPD (14.3%) being the most common. The most common pre-existing medical conditions were hypertension (78.6%) and tobacco use (64.3%). The predominant indication for surgery was due to esophageal cancer (92.8%).
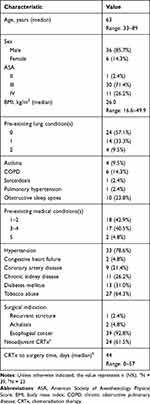 |
Table 1 Patient Characteristics |
Table 2 displays the intraoperative surgical, anesthetic, and medical management of the study population. All patients underwent a trans-hiatal esophagectomy. 76.2% of cases were robotic assisted. Median operative time was 146 minutes overall, with open esophagectomy being slightly quicker than robotic assisted cases (131 vs 147 minutes, respectively). Median duration of tidal volume >8mL/kg occurred for 101 minutes of the case. FiO2 duration >80% occurred for a median duration of 43 minutes of the case. Of note, this includes pre-intubation oxygenation and following extubation while in the OR. Median duration of PEEP >5cm H2O was 0 minutes. Regarding blood pressure parameters, patient experienced minimal duration of MAP <60mmHg (median 4 minutes) and only 14 patients (33%) required intraoperative norepinephrine use. Only 2 patients required any vasopressor use at the end of the case. With regard to volume status, median total fluid delivered was 4150 mL, with median fluid balance at the end of the case being 2938 mL. The median blood loss of 300 mL with receipt of 2 units of blood.
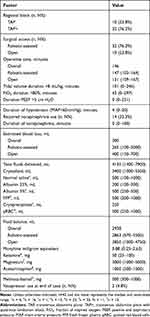 |
Table 2 Intraoperative Factors |
Regarding multimodal intraoperative pain control medications, the median patient received 0.88mg/kg MME, 50mg of ketamine, 3000mg of magnesium, 1000mg of acetaminophen, and 500mg of methocarbamol. Of note, not every patient received all of the medication types. Ketamine and magnesium were the most commonly administered (n = 23 and n = 33, respectively).
Table 3 includes the postoperative measures of interest. Thirty-four patients (81%) were able to be immediately extubated in the OR. During the entire course of the patient’s hospital stays, there were nine instances (21.4%) of reintubation. Five (11.9%) of the nine patients underwent reintubation within 48 hours of surgery: one due to hypoxemia following immediate extubation in the OR, 2 due to pneumonia, 1 due to pulmonary embolism, and 1 due to hypotension during atrial fibrillation with rapid ventricular response. The other 4 instances of reintubation occurred outside of the perioperative period from POD 3–30: 1 patient due to hypoxemia following delayed extubation, 2 patients due to pneumonia, and 1 patient due to pulmonary embolism. Post-operative pulmonary complications regardless of need for reintubation included 9 total instances of pneumonia, 3 of pleural effusion requiring thoracentesis, 2 pulmonary embolisms, and 1 instance of significant upper airway edema.
 |
Table 3 Postoperative Outcomes |
Only 7 patients (16.7%) required the use of vasopressors postoperatively due to hypotension. Incidence of hypotension only occurred 11 times from POD 1–3, limiting fluid boluses administered. Seven patients (16.7%) were found to have an anastomotic leak. The median hospital LOS was 10 days (range: 7–48 days) and the median stay in the ICU was 5 days (range: 0–48 days). There was a 30-day mortality rate of 2.4%.
Table 4 compares the pain scores between the patients based on the regional block they received pre-operatively. The median pain score was noted to be 1 in the TAP group and 0 in the TAP+ group (p = 0.139) on initial postoperative assessment in the PACU vs scores of 3 and 4, respectively (p = 0.648), on POD 1. Scores were available for less than half of the cohort on POD 2 and 3, and as such statistical analysis on the basis of pain scores beyond POD1 was not conducted.
 |
Table 4 Postoperative Pain Score Compared by TAP Vs TAP+ |
Table 5 compares the differences between TAP block alone vs TAP+ and immediate vs delayed extubation groups. No significance was identified when comparing TAP block alone vs TAP+ on the basis of early extubation rate (p = 0.503), ICU (p = 0.998) or total hospital LOS (p = 0.941), and intraoperative opiate consumption (p = 0.593). Differences were noted in FiO2 requirements, with the TAP+ group requiring significantly less supplemental oxygen intraoperatively (p = 0.007). When comparing immediate vs delayed extubation, the immediate extubation group required statistically fewer intraoperative transfusions (p = 0.029). Additionally, they received statistically more methocarbamol and acetaminophen intraoperatively (p < 0.001); however, this was not the case with ketamine or magnesium. The extubation rate was not significantly affected by the presence of pulmonary comorbidities (p = 0.403). It was also not affected by vasopressor requirements, including the norepinephrine administration (p = 0.143) or duration of norepinephrine administration (p = 0.612) intraoperatively. Immediate extubation did not impact ICU (p = 0.385) or total hospital length of stay (p = 0.334). Anesthesiologist variation in FiO2, tidal volume, PEEP, and total fluids delivered were also insignificant.
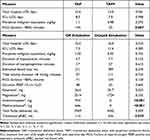 |
Table 5 Statistically Significant Data from Analysis Using t-Test and Chi-Square Analysis |
Multiple linear regression to predict ICU LOS was calculated based on duration of intraoperative norepinephrine use, presence of ASA IV (p = 0.121), presence of reintubation, presence of POD 1 hypotension, and volume of perioperative IVFs (p = 0.023) (F(5,36)= 14.23, p < 0.001) with an R2 of 0.664. For each 10 minutes of intraoperative norepinephrine use, the average ICU length of stay increased by one day (β= 0.43, t(42)= 4.19, p < 0. 001). Reintubation was associated with an average increase in ICU length of stay of 12.12 days (β= 0.50, t(42)= 4.59, p < 0. 001) while the presence of POD 1 hypotension corresponded to an increase in 12.94 days (β= 0.38, t(42)= 3.89, p < 0. 001). Prediction of total hospital length of stay was calculated based on ICU LOS, ASA IV status, and intraoperative EBL (p = 0.137) (F(3,38)= 59.04, p < 0.001). The R2 was found to be 0.823. For every day spent in the ICU, the associated total hospital LOS increased by an average of 0.81 days (β= 0.94, t(42)= 13.2, p < 0. 001). The presence of ASA IV predicted an increased total hospital LOS of 4.27 days (β= 0.22, t(42)= 3.00, p = 0. 005).
Discussion
This study is the first of its kind to specifically demonstrate that successful immediate extubation is possible after trans-hiatal esophagectomy using TAP blocks as the primary source of postoperative analgesia rather than TEA. OR extubation has been well documented in literature when involving the use of TEA and this study was able to achieve an OR extubation rate of 81%, demonstrating that trans-hiatal esophagectomy patients can be successfully extubated in the OR by means of TAP blocks without additional neuraxial anesthesia.4,16 This rate of extubation is notable in a population with an ASA median of 3 as it compares to a study by Lanuti et al who described a 90% immediate extubation rate using TEA in a population with a median ASA of 2.16 Additionally, the reintubation rate of this study was 21% which is nearly identical to the findings of 20% reintubation reported in a systematic review of 490 esophagectomy patients utilizing TEA by Serafim et al.17
The expected disadvantage of TEA is that an induced sympathectomy often leads to difficulty managing post-operative hypotension. In a study by Imai et al, 31% of patients required the use of vasopressors during the course of their ICU stay.15 In this study, however, there were limited postoperative hypotensive complications requiring vasopressor use among TAP or TAP+ block patients (16.7%). There were only 11 instances of hypotension across POD1-3 and as such fluid boluses were able to be limited, potentially preventing higher incidence of anastomotic leak beyond the seven instances recorded.21
There was only one statistically significant difference identified in any measured outcome between patients who underwent TAP block alone vs TAP+ and this was the minutes of FiO2 >80% (p = 0.007), in which the TAP+ group required much less oxygenation. While one could argue that higher intraoperative oxygen demand could correlate with less effective intra-operative analgesia from a TAP block in comparison to TAP+, PACU and POD1 pain scores were equivalent between groups and as such, this finding may be incidental. Overall, the TAP and TAP+ groups demonstrated themselves equivocal. Due to the relatively limited number of patients who underwent TAP alone (n = 10), it is possible that with a larger sample size clinical differences between blocks would become clearer.
This study resulted in a median pain score overall of 0 on a 10-point scale for pain reported while in the PACU. While pain scores were recorded consistently in the PACU following surgery in the PACU and on POD1, there was no statistical significance between TAP and TAP+ groups. POD 2–3 lacked sufficient reporting to provide meaningful statistical analysis and reliable interpretation. Of note, in a study comparing TAP to TEA by Levy et al, POD1 scores were 4.34 in those receiving TEA and 5.79 in those receiving TAP blocks (P = 0.06).12 While this comparison data suggests an increase in analgesic capabilities of TEA over TAP, our results of a median POD 1 pain score of three with TAP and four with TAP+ blocks suggest block density within the first 24 hours after surgery remains adequate compared to TEA, however inclusion of a direct comparison group with reliable pain score reporting through POD 3 would be required to substantiate accurate clinical impact and comparison to TEA in future studies.
While this study did not reflect a difference in intraoperative MME consumption between TAP and TAP+ groups or in immediate extubation rates, it remains unclear whether there would be clinically meaningful differences based on other measures such as early postoperative MME requirements or insufficient pain control-associated complications. This potential difference warrants further investigation. Regardless, the median 0.88 mg/kg MME used intraoperatively demonstrated a lack of excessive opiate requirements in order to address a patient’s response to surgical stimulation and pain when using these regional blocks. Conclusions cannot be drawn in the post-operative period concerning opiate consumption as patient-controlled analgesia (PCA) without EMR recorded totals was implemented on the wards and the data were unavailable for analysis.
When looking at medication-related factors that might play a role in extubation, both acetaminophen and methocarbamol were used in statistically greater amounts among patients who underwent immediate extubation in the OR (p < 0.001). In fact, no patient who underwent delayed extubation received a dose of either medication. Due to the retrospective nature of the study, the reason for this discrepancy is unclear. These findings serve to highlight the importance of further investigating how multimodal pain control within the increasingly common ERAS pathways contribute to overall patient recovery.
The total volume of IVF administration was fairly consistent among all patients with median fluid balance of 2938 mL at the end of the case. Conservative measures with fluid balance may have partially contributed to the limited number of anastomotic leaks identified in the study group (16.7%) especially when compared to literature such as Hoelzen et al in which robotic, hybrid, and open approaches yielded noticeably higher rates of leak (23.5%, 22% and 37% respectively).22 Additionally, Deana et al highlight clearly how intraoperative fluid balance correlates positively with post-operative complications in esophagectomy.23 Related to fluid balance, there were significant differences in the volume of blood product administration between immediate and delayed extubation groups. Patients who were extubated in the OR immediately received on average 114 mL of pRBC whereas patients who were unable to be extubated received on average 536 mL of pRBC (p = 0.029). This finding is likely reflective of a more “complicated” surgical course rather than reflective of anesthetic management as patients that received more blood were, unsurprisingly, found to have higher reported intraoperative EBL volumes.
Concerning ventilatory parameters, while not statistically significant there difference in FiO2 requirements between immediate and delayed extubation groups may be clinically significant. This may represent issues in the oxygen diffusion capacity due to an underlying lung pathology, individual variation in practice among anesthesia providers, or may be a spurious finding. Most of the high fraction of FiO2 delivery was surrounding time of intubation (to adequately pre-oxygenate the patient prior to induction) as well as the timing of extubation (to liberate the patient off of the ventilator). No case required continuous FiO2>80%. At the anesthesiologist’s discretion, additional O2 use intraoperatively was implemented in the event that pleura was surgically breached, or ventilation was challenged based on patient comorbidity to maintain adequate oxygen saturation. Other parameters of elevated PEEP, excess tidal volume, and duration of mechanical ventilation had no statistically significant correlations with ability for immediate extubation.
In the study by Imai et al, examining the rate of immediate extubation in the OR (n = 42) following esophagectomy using TEA, they reported a median ICU stay of 3 days (IQR: 2.75–3) and median hospital stay of 22.5 days (IQR: 16.5–27.75) with an average ASA score of 1.5 amongst the population.15 ICU LOS is similar between our study (median of 5 days) and this comparison study due to the similar rates of reintubation; this is especially notable in light of our studies’ average ASA score of 3.3. Regression analysis of our study demonstrated that the primary driver for increased ICU LOS was reintubation. While our regression model did not identify ASA classification as a statistically significant factor in ICU LOS, ASA IV was correlated with an increased total hospital LOS. Of note, the results of our study demonstrated a shorter total hospital LOS (median of 10 days) despite this substantially heavier comorbidity burden of ASA 3.3, although this is likely multifactorial and not a result directly explained by the use of TAP block as the primary source of analgesia instead of TEA.
This study has several limitations. First, this is a retrospective study from a single center and thus has an intrinsic risk of bias.
Second and most significantly, this study lacks a comparison group of patients receiving TEA. Thus, we required reliance on literature comparison, and inference of the clinical relevance of TAP, setting out to demonstrate viability and efficacy for immediate extubation using only regional anesthesia for analgesia as opposed to directly comparing the efficacy of regional anesthesia against neuraxial anesthesia.
Third, while the TAP block plans to provide effective surgical incision site pain relief, there are limitations of regional anesthetic ability to address soft tissues/organ related pain in the patient, where TEA may partially address this.24
Fourth, the study group includes both open and robot assisted trans hiatal esophagectomy. Variability in midline incision may have impact on local complications, EBL, and post-operative pain scores, however the difference in minimally invasive and robotic-assisted techniques is less significant with trans-hiatal esophagectomy than it is with a McKeown or Ivor Lewis technique where thoracotomy is involved.
Fifth, while this study was performed over a relatively short period of time, theoretically limiting the bias incurred due to unaccounted for changes over time, there were known significant changes in care due to the impact of COVID-19 during the course of the study. Namely, these included the intermittent use of non-dedicated anesthesiologists, hospital bed availability, and delays in patient care due to covid infections.
Finally, when compared to other published studies, this study is small to moderate in size and overall statistical inferences are limited by the number of available patients for analysis. Further publications of outcomes utilizing TAP block without TEA using control groups will be required to further solidify the use of regional anesthetic techniques in esophagectomy.
Conclusions
This study was able to specifically demonstrate that regional TAP blocks can maintain the anesthesiologist’s ability to immediately extubate patients following esophagectomy while providing adequate analgesia and limiting post-operative hypotension and fluid administration, presenting a possible alternative to TEA. This finding was born out despite the patient population of this study exhibiting severe comorbid health conditions and an elevated age at the time of surgery. This data should be used as a basis for further studies and exposes avenues for potentially viable alternatives of the existing ERAS pathways for esophagectomy patients.
Abbreviations
TEA, thoracic epidural analgesia; ERAS, enhanced recovery after surgery; ICU, intensive care unit; TAP, transversus abdominis plane; ESP, erector spinae plane; QL, quadratus lumborum; OR, operating room; COPD, chronic obstructive lung disease; OSA, obstructive sleep apnea; ASA, American Society of Anesthesiologists physical status; EBL, estimated blood loss; pRBC, packed red blood cell products; IVF, intravenous fluids; MAP, mean arterial pressure; Vt, tidal volume; FiO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; MME, morphine milligram equivalents; LOS, length of stay; ANOVA, analysis of variance; IQR, interquartile; PACU, post anesthesia care unit; POD, postoperative day; TAP+, combined TAP and all other regional blocks; MME, Morphine milligram equivalents; SBT, Spontaneous breathing trial; ARDS, Acute respiratory distress syndrome.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article.
Ethics Approval and Consent to Participate
This study has approval from the IRB protocol #037.HPB.2018.R at Methodist Richardson Medical Center. This study complies with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Texas Christian University Open Access Fund.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015;262:286–329. doi:10.1097/SLA.0000000000001098
2. Low DE, Allum W, De Manzoni G, et al. Guidelines for perioperative care in Esophagectomy: enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg. 2019;43(2):299–330. doi:10.1007/s00268-018-4786-4
3. Caldwell MT, Murphy PG, Page R, et al. Timing of extubation after oesophagectomy. Br J Surg. 1993;80:1537–1539. doi:10.1002/bjs.1800801214
4. Solis-Velasco MA, Ore Carranza AS, Stackhouse KA, et al. Transversus abdominis plane block reduces pain and narcotic consumption after robot-assisted distal pancreatectomy. HPB (Oxford). 2019;21(8):1039–1045. doi:10.1016/j.hpb.2018.12.005
5. Robertson SA, Skipworth RJ, Clarke DL, et al. Ventilatory and intensive care requirements following oesophageal resection. Ann R Coll Surg Engl. 2006;88(4):354–357. doi:10.1308/003588406X98694
6. O’Grady M, Firth R, Roberts R. Intensive care unit utilisation post-oesophagectomy. N Z Med J. 2020;133(1510):56–61.
7. Lassen K, Coolsen MME, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2013;37:240–258. doi:10.1016/j.clnu.2012.08.011
8. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery. JAMA Surg. 2017;152:292. doi:10.1001/jamasurg.2016.4952
9. Imai T, Abe T, Uemura N, et al. Immediate extubation after esophagectomy with three-field lymphadenectomy enables early ambulation in patients with thoracic esophageal cancer. Esophagus. 2018;15(3):165–172. doi:10.1007/s10388-018-0608-x
10. Levy G, Cordes MA, Farivar AS, Aye RW, Louie BE. Transversus abdominis plane block improves perioperative outcome after esophagectomy versus epidural. Ann Thorac Surg. 2018;105(2):406–412. doi:10.1016/j.athoracsur.2017.08.046
11. Bottiger BA, Esper SA, Stafford-Smith M. Pain management strategies for thoracotomy and thoracic pain syndromes. Semin Cardiothorac Vasc Anesth. 2014;18:45–56. doi:10.1177/1089253213514484
12. Jain K, Jaiswal V, Puri A. Erector spinae plane block: relatively new block on horizon with a wide spectrum of application - a case series. Indian J Anaesth. 2018;62(10):809–813. doi:10.4103/ija.IJA_263_18
13. New York School of Regional Anesthesia. Truncal and cutaneous blocks. Available from: www.nysora.com/techniques/ultrasound-guided-techniques/3253-truncal-and-cutaneous-blocks.html.
14. Niraj G, Kelkar A, Hart E, Kaushik V, Fleet D, Jameson J. Four quadrant transversus abdominis plane block and continuous transversus abdominis plane analgesia: a 3-year prospective audit in 124 patients. J Clin Anesth. 2015;27:579–584. doi:10.1016/j.jclinane.2015.07.005
15. Dhanjal S, Tonder S. Quadratus lumborum block. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available from https://www.ncbi.nlm.nih.gov/books/NBK537212/.
16. Lanuti M, de Delva PE, Maher A, et al. Feasibility and outcomes of an early extubation policy after esophagectomy. Ann Thorac Surg. 2006;82(6):2037–2041. doi:10.1016/j.athoracsur.2006.07.024
17. Serafim MCA, Orlandini MF, Datrino LN, et al. Is early extubation after esophagectomy safe? A systematic review and meta-analysis. J Surg Oncol. 2022;126(1):68–75. doi:10.1002/jso.26821
18. Deana C, Vetrugno L, Bignami E, Bassi F. Peri-operative approach to esophagectomy: a narrative review from the anesthesiological standpoint. J Thorac Dis. 2021;13(10):6037–6051. doi:10.21037/jtd-21-940
19. Della Rocca G, Vetrugno L, Coccia C, et al. Preoperative evaluation of patients undergoing lung resection surgery: defining the role of the anesthesiologist on a multidisciplinary team. J Cardiothorac Vasc Anesth. 2016;30(2):530–538. doi:10.1053/j.jvca.2015.11.018
20. Fabbi M, Hagens ERC, van Berge Henegouwen MI, Gisbertz SS. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus. 2021;34(1):doaa039. doi:10.1093/dote/doaa039
21. New York School of Regional Anesthesia. Epidural anesthesia and analgesia. New York School of Regional Anesthesia; 2022. Available from: https://www.nysora.com/topics/regional-anesthesia-for-specific-surgical-procedures/abdomen/epidural-anesthesia-analgesia/.
22. Hoelzen JP, Sander KJ, Sesia M, et al. Robotic-assisted esophagectomy leads to significant reduction in postoperative acute pain: a retrospective clinical trial. Ann Surg Oncol. 2022;29(12):7498–7509. doi:10.1245/s10434-022-12200-0
23. Deana C, Vetrugno L, Stefani F, et al. Postoperative complications after minimally invasive esophagectomy in the prone position: any anesthesia-related factor? Tumori. 2021;107(6):525–535. doi:10.1177/0300891620979358
24. Richards ER, Kabir SI, McNaught CE, MacFie J. Effect of thoracic epidural anaesthesia on splanchnic blood flow. Br J Surg. 2013;100(3):316–321. doi:10.1002/bjs.8993
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
