Back to Journals » Infection and Drug Resistance » Volume 15
Refractory Osteomyelitis Caused by Mycobacterium aubagnense and Its L-Form: Case Report and Review of the Literature
Authors Cheng J , Zhang L, Huang Q, Li C, Yu J, Zhu M
Received 9 September 2022
Accepted for publication 29 November 2022
Published 13 December 2022 Volume 2022:15 Pages 7317—7325
DOI https://doi.org/10.2147/IDR.S388629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jianghao Cheng, Long Zhang, Qian Huang, Chaodan Li, Jianhua Yu, Mingli Zhu
Hangzhou Xixi Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
Correspondence: Mingli Zhu, Email [email protected]
Purpose: To report a rare case of tibial osteomyelitis caused by Mycobacterium aubagnense and its L-form, to systematically review non-tuberculous mycobacteria (NTM) infections of the bones, and to summarize the medication guidelines for infections with NTM and its L-forms.
Methods: Case report and literature review.
Results: We report a 31-year-old HIV-positive man who developed osteomyelitis caused by M. aubagnense and its L-form. Culture, electron microscopy, polymerase chain reaction assay, and a reversion test confirmed the existence of M. aubagnense. The patient was treated with surgical debridement and a combination of systemic antibiotics, and continued to take antiretroviral treatment. Some clinical improvement was noted shortly after the initiation of this treatment. Resolution of osteomyelitis was achieved after 10 months. We also systematically reviewed cases of NTM osteomyelitis in the PubMed database and compared antibiotic sensitivity between L-forms and their prototype bacteria. We have summarized the treatment regimens for infections of the bone and bone marrow caused by NTM and their L-forms.
Conclusion: We have reported the first case of refractory osteomyelitis caused by M. aubagnense and its L-form in a patient with immune deficiency, reviewed the literature on NTM osteomyelitis, and compared the antibiotic sensitivity of L-forms and their prototype bacteria.
Keywords: non-tuberculous mycobacteria, HIV, bone and bone marrow infection
Introduction
Osteomyelitis refers to the infectious inflammation of the bones and bone marrow. Its etiology mainly includes bloodstream infection, traumatic infection, blood circulation disorder, and low host immune function. Almost all microorganisms, including viruses, bacteria, and fungi, can cause osteomyelitis.1 Many pathogens such as Staphylococcus aureus,2 Salmonella,3 Candida,4 non-tuberculous mycobacteria (NTM),5 Cryptococcus,6 Bartonella,7 and Mycobacterium tuberculosis8 have been reported to cause osteomyelitis. NTM have recently garnered increased research attention because they can cause infections in immunocompromised patients, in whom, the infection presents as a disseminated extrapulmonary form. Compared to tuberculosis (TB), NTM infections must be treated for a much longer duration.
L-form bacteria are cell wall-deficient bacteria that are produced by bacterial mutation; these forms can be induced experimentally in many bacterial species by treatment with antibiotics, lytic enzymes, and/or certain amino acids that interfere with the bacterial cell wall or its synthesis.9 In one study, Mycobacterium bovis was reported to produce L-form variants under stress conditions,10 suggesting that NTM can easily transform into L-forms under the influence of antibiotics and other factors. L-forms can also revert to their prior state, albeit at a low frequency.11 L-form bacteria need to grow and reproduce in a hypertonic environment, so their successful culture requires special culture conditions, and considerable skill and experience, or these variants are quite easy to miss. Thus far, no cases of osteomyelitis caused by Mycobacterium aubagnense and its L-form have been reported. In this study, we report a case of tibial osteomyelitis caused by M. aubagnense and its L-form that was successfully managed using surgical debridement and antibiotic therapy for 10 months. The present case highlights the importance of considering NTM and their L-forms, when investigating chronic, relapsing osteomyelitis. We also conducted a literature review of cases of NTM osteomyelitis, and compared the antibiotic sensitivities of L-forms and their prototype bacteria. We concluded that appropriate pharmacotherapy along with surgical debridement is the optimal method to treat NTM osteomyelitis and avoid relapses. We advise that clinicians remain vigilant when evaluating uncommon forms of osteomyelitis.
Case Report
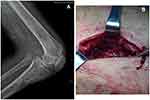
A 31-year-old man presented to our outpatient clinic with redness, swelling, and severe pain in both knees as well as an inability to walk since one year ago. The patient who has been HIV positive for 7 years had a history of severe prurigo nodularis for four years and had tubercle bacillus bloodstream infection two years ago. His antiretroviral treatment was successful. He did not have any relevant family history or history of genetic diseases. After admission, the patient underwent bilateral curettage for tibial osteomyelitis under epidural anesthesia. An anterior incision was made at the proximal end of the tibia. Bone destruction was observed at up to a depth of approximately 2 cm inside the tibial tubercle, and a large amount of purulent secretion was discharged from the anterior tibial incision. The inflammatory granulation tissue and scar tissue were removed. A small curette was used to eliminate the inflammatory granulation tissues and pus in the remaining dead space, and the removed material was placed in a sterile bag. Bone cement was used to fill in the tibial defect, and the wound surface was covered with VSD dressing and fixed on the peripheral skin. Continuous vacuum aspiration was performed to drain the wound (Figure 1). Culture of the wound drainage fluid showed no growth of mycobacteria or other bacteria after 1 week, but a small number of red acid-fast positive spheroids were detected on smear microscopy (Figure 2). Considering that the patient had a history of tuberculous bloodstream infection and had symptoms of low fever and fatigue, we initially suspected a diagnosis of tuberculous osteomyelitis. The patient was immediately administered anti- multidrug-resistant tuberculosis (MDR-TB) treatment, but the response was not good. After 21 days of culture, no M. tuberculosis growth was observed, and the Xpert MTB/RIF and T-SPOT.TB tests were negative, indicating that there was no TB infection and explaining the patient’s lack of response to the anti-MDR-TB treatment. As some red acid-fast spheroids had been observed on microscopy, we now considered the possibility of infection with the L-forms of M. tuberculosis or NTM. Culture, microscopic examination, electron microscopy (Figure 3), polymerase chain reaction (PCR) assays and a reversion test (Figure 4) confirmed the presence of NTM and its L-forms. The patient was therefore treated with moxifloxacin, protionamide, linezolid, isoniazid, rifampicin, and ethambutol for 10 months, according to the results of the drug-sensitivity test. After this treatment, the wounds appeared clean, with no evidence of redness, swelling, heat, or pain. Thus, amputation of the affected legs was avoided.
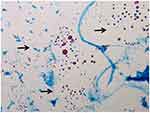 |
Figure 2 Microscopic morphology (400 x) of L-form NTM. (red spheroids, arrows). |
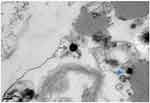 |
Figure 3 Morphology of L-form NTM (blue arrows) on electron microscopy. |
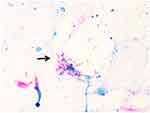 |
Figure 4 Acid-fast bacilli, slightly curved, with a tendency to branch (arrows) are observed on microscopy after the reversion test. |
Discussion
Disseminated NTM infection has been a major cause of mortality and comorbidity in patients with human immunodeficiency virus (HIV) infection. Osteomyelitis is commonly caused by S. aureus and M. tuberculosis;12 NTM osteomyelitis is quite rare, and is usually observed following trauma. Thus far, there have been no reports about osteomyelitis caused by M. aubagnense and its L-form in patients with acquired immunodeficiency syndrome (AIDS).
NTM include more than 160 ubiquitous, environmental, acid-fast-staining bacterial species, some of which may cause disease in humans. Mycobacteroides abscessus, Mycobacterium avium complex (MAC), Mycobacterium kansasii, Mycobacterium malmoense, and Mycobacterium xenopi are clinically the most important species.13 According to a survey released by the CDC in the United States, the incidence rate of NTM infections (per 100,000 persons) was 1.8 during 1981–1983, which increased to 9.1 in 1997 and 14.1 in 2003, and has reached 17.7 according to recent data.14 This indicates that the incidence and prevalence of NTM infections are increasing. NTM infections most commonly present as pulmonary infections (5.6 per 100,000 persons), followed by skin and soft-tissue infections (0.9 per 100,000), and rarely cause osteomyelitis (0.3 per 100,000 persons).14
One of the most important predisposing factors for NTM infection is an immunocompromised state, such as that seen in patients with HIV infection, those using immunosuppressive agents, those with mutations related to the interleukin-12 (IL-12)–interferon gamma (IFN-γ) axis, and those with hematological malignancies. In the US, approximately 43% of AIDS patients had disseminated NTM infections in the pre-anti-retroviral therapy era.15 MAC are the most common species isolated from patients with systemic immunosuppression and NTM infection.16 A recent study reported that the prevalence of mycobacterial disease in patients with interferon gamma receptor 1 (IFN-γR1) deficiency was 95%, and that MAC was the most commonly isolated mycobacterial pathogen from these patients.17 The mean prevalence of NTM infections in patients with hematological malignancies is 1.2%,18 which is far higher than the prevalence in the normal population.16 This suggests that NTM may be an opportunistic infection in patients with hematological malignancies.
Invasive infections caused by NTM have included lymphatic, skin, bone, and soft-tissue infections in addition to pulmonary involvement.19 NTM infections are common among individuals with immunodeficiency, diabetes, or alcohol addiction. To better assess the clinical presentation of NTM osteomyelitis and its relevance, we studied the main causative agents, diagnostic tools, treatment options, and treatment duration after diagnosis among patients with NTM osteomyelitis. We searched the PubMed database for reports of NTM osteomyelitis published between 1995 and August 2022 and assessed the full texts of the retrieved papers for relevance and eligibility.
The pooled data provided that 21 cases reports (Table 1). The mean age of the 21 patients was 52 ± 22.8 years and 52.4% of the reported cases occurred in male patients. The NTM infection sites among the 21 patients were as follows: vertebrae, 10 patients; clavicle, 3 patients; tibia, ankle, femur, and fibula, 2 patients each; and frontal bone, wrist, elbow, metacarpal, skull, and sternum, 1 patient each. Five patients had multiple infection sites. The following pathogenic microorganisms were identified: MAC, 4 patients; Mycobacteroides abscessus, 3 patients; Mycobacteroides chelonae infections and Mycobacterium riyadhense, 2 patients each; and Mycobacterium monacense, Mycobacterium farcinogenes, Mycobacterium phlei, Mycobacterium haemophilum, Mycobacterium fortuitum, Mycobacterium kansasii, Mycobacterium goodii, Mycobacterium flavescens, Mycobacterium avium, and Mycobacterium senegalense,1 patient each. Vertebral osteomyelitis caused by NTM is more common among immunosuppressed patients than among immunocompetent patients,20 and was found in 5 of 7 (71.4%) patients with various degrees of immunosuppression in our review. Trauma, including fracture, burns, dermatosis, and impact injury, is one of the most important causes of NTM infection. In our study, many patients (36.4%) developed NTM infection of the tibia or other skeletal sites after surgery or trauma. In all patients, the diagnostic work-up involved imaging methods, and culture, biopsy, and molecular biology methods were essential for identifying the pathogen. Once NTM osteomyelitis is diagnosed, surgical debridement is the most effective treatment.21 Adjunctive surgical therapy with debridement was reported in most of the cases in our review (81.0%), with obvious clinical improvement. In addition, individualized antimycobacterial chemotherapy is also essential for treatment. The 4 most commonly used drugs in our review were clarithromycin (42.9%), amikacin (28.6%), ciprofloxacin (23.8%), and rifampicin (23.8%). In general, individualized antimycobacterial chemotherapy was successful; most patients recovered well, and only 1 patient died before receiving medicine. However, the treatment duration was long, at a minimum of 5 months and a maximum of 2 years in our review (Table 1).
 |
Table 1 Characteristics of Cases of NTM Osteomyelitis Identified in the Literature Review |
The transition of bacteria to cell wall-deficient L-forms in response to stress factors has been assumed to be a potential mechanism for the survival of microbes.22 Under unfavorable conditions, many bacterial species can develop a state called an L-form, which has lost the peptidoglycan cell wall. Therefore, L-forms are completely resistant to most antibiotics that work specifically on cell wall synthesis. L-form bacteria can persist in humans even under the action of antibiotics.23–25 NTM often change into L-forms after treatment, which leads to increased drug resistance, with some patients failing to recover despite years of treatment.
L-form bacteria can be induced by cell wall-targeting antibiotics and require a medium with high osmolality for survival and growth. Except for the cell wall, the pathogenic factors of L-forms are similar to those of the prototype bacteria. There have been sporadic reports of L-forms being involved in a wide range of often chronic or recurrent infections.26 L-forms of S. aureus,27 Pseudomonas aeruginosa,28 Neisseria gonorrhoeae,29 Salmonella,30 and M. tuberculosis31 have been reported. We compared the antibiotic sensitivity of bacterial prototypes and their L-forms.
Unlike the prototype bacteria, L-forms are resistant to a series of antibiotics acting on the cell wall, such as penicillins and cephalosporins. Therefore, for infections involving L-forms, we can select antibiotics that act on both the bacterial cell wall and plasma membrane, such as vancomycin and neomycin; antibiotics that act on the plasma membrane and interfere with protein synthesis, such as amikacin and gentamicin; and antibiotics that inhibit bacterial nucleic acid synthesis, such as ofloxacin and levofloxacin. L-forms can infect various parts of the human body and can revert to the prototype bacteria with cell walls during treatment. Therefore, we should adopt combined therapy, choosing antibiotics that target both the prototype bacteria and the L-form bacteria.
In our report, the patient underwent received vacuum sealing drainage treatment, under epidural anesthesia. The short-term pus culture did not grow any pathogen, but acid-fast bacilli were initially observed on the smear. Reports indicate that under the action of anti-TB drugs such as ethambutol, the synthesis disorder of mycobacterial cell wall leads to the production of L-forms, which can survive longer than the classical forms under adverse conditions.31 Therefore, for osteomyelitis patients with a poor response to treatment, NTM and its L-forms should also be considered in addition to TB and MDR-TB infection.
The identification of the specific NTM species in the clinical specimen is crucial since the treatment regimens differ strikingly among different NTM strains. In the present case, we have found that liquid culture media grow NTM bacterial more rapidly than solid media, and pus samples need to be digested to better cultivate NTM and its L-form. Nevertheless, we still took 4 weeks to obtain the culture results. PCR-based sequencing has become the gold standard for the identification of mycobacterial species.32 In the absence of a microbial pathogen, we obtained an adequate amount of specimens and performed PCR assays, and finally identified the pathogen as M. aubagnense. On microscopy and electron microscope, we found spherical bacteria, and conducted a reversion test to confirm the existence of the L-form.
Currently, there are no consistent guidelines for the treatment of vertebral osteomyelitis caused by NTM because few clinical trials have an adequate number of patients to compare different therapeutic regimens.20 The appearance of L-form NTM lacking cell walls increased the difficulty of diagnosis and treatment in the present case. We chose rifampin (which inhibits bacterial DNA- dependent RNA polymerase),33 linezolid (which inhibits bacterial protein synthesis through binding to rRNA),34 ethambutol, protionamide, isoniazid, and moxifloxacin (a fluoroquinolone) for combination therapy. We also performed surgical resection, drainage of the abscess, and provided VSD coverage for the wound, and continued highly active antiretroviral therapy. The patient recovered after 10 months of treatment. Our report describes the treatment recommendations for mainly M. aubagnense infection, but the optimal duration of antibiotic treatment remains unclear.
Conclusion
In conclusion, osteomyelitis caused by L-form NTM is unusual in clinical practice. Clinicians should be aware of the potential for this uncommon infection, so that they can avoid misdiagnosis or delayed diagnosis. This article is the first to report a case of refractory osteomyelitis caused by M. aubagnense and its L-form. We reiterate that L-form mycobacterial infection should be considered when acid-fast positive spheroids are found on microscopy, even if the mycobacterial culture is negative, because mycobacteria can transition into L-forms under the influence of long-term anti-TB drugs treatment. We can confirm the existence of L-forms through electron microscopy, reversion tests, and PCR assays. Extensive surgical debridement is key to eliminating the offending pathogen, and antimycobacterial therapy is also important. Among anti-NTM antibiotic drugs, rifampicin, protionamide, linezolid, and other drugs have obvious effects on L-form NTM.
Abbreviations
NTM, non-tuberculosis mycobacteria; HPLC, high-performance liquid chromatography; NGS, next-generation sequencing; DHT, DNA hybridization technology; WGS, whole genome sequencing; NAC, nucleic acid chromatography; MAC, Mycobacterium avium complex; FOX, cefoxitin; CIP, ciprofloxacin; CLARY, clarithromycin; IPM, imipenem; LZD, linezolid; MXF, moxifloxacin; AZT, azithromycin; RFP, rifampicin; INH, isoniazid; PZD, pyrazinamide; VAN, vancomycin; AZM, azithromycin; MIN, minocycline; EMB, ethambutol; AMK, amikacin; MEM, meropenem; DOX, doxycycline; LVFX, levofloxacin; TMP/SMX, trimethoprim/sulfamethoxazole; CLO, clofazolamide; DAP, daptomycin; NAP, naproxen; AMX, amoxicillin.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This case report was approved by the ethics committee of Hangzhou Xixi Hospital Affiliated to Zhejiang Chinese Medical University. The patient provided written informed consent. The study was carried out in accordance with the principles of the Declaration of Helsinki. The first author vouches for the completeness and accuracy of the data and for the fidelity of the study to the protocol.
Consent for Publication
Signed consent was obtained for the publication of the case details from the patient.
Acknowledgments
We thank the patient who participated in this study for his cooperation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Beck-Broichsitter BE, Smeets R, Heiland M. Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis. 2015;28(3):240–245. doi:10.1097/qco.0000000000000155
2. Cobb LH, Park J, Swanson EA, et al. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS One. 2019;14(11):e0220421. doi:10.1371/journal.pone.0220421
3. Zhan C, Du J, Chen L. Salmonella osteomyelitis in a previously healthy neonate: a case report and review of the literature. Ital J Pediatr. 2018;44(1):28. doi:10.1186/s13052-018-0464-2
4. Krishnamoorthy M, Othman NAN, Hassan NEB, Hitam SB. Candida skull base osteomyelitis: a case report and literature review. Acta Medica. 2020;63(2):82–85. doi:10.14712/18059694.2020.22
5. Jaime-Villalonga A, Saul Z, Miljkovic G. Mycobacterium arupense finger osteomyelitis: case report. Int J Infect Dis. 2020;92:226–227. doi:10.1016/j.ijid.2020.01.027
6. Matsuki T, Miyamoto S, Yamashita T. Cryptococcal osteomyelitis of the Zygomatic bone: a case report. BMC Infect Dis. 2020;20(1):399. doi:10.1186/s12879-020-05123-2
7. Puri K, Kreppel AJ, Schlaudecker EP. Bartonella osteomyelitis of the acetabulum: case report and review of the literature. Vector Borne Zoonotic Dis. 2015;15(8):463–467. doi:10.1089/vbz.2014.1758
8. Mannepalli S, Mitchell-Samon L, Guzman N, Relan M, McCarter YS. Mycobacterium tuberculosis osteomyelitis in a patient with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): a case report. Cases J. 2010;3:67. doi:10.1186/1757-1626-3-67
9. Errington J, Mickiewicz K, Kawai Y, Wu LJ. L-form bacteria, chronic diseases and the origins of life. Philos Trans R Soc Lond B Biol Sci. 2016;371(1707):20150494. doi:10.1098/rstb.2015.0494
10. Slavchev G, Michailova L, Markova N. Stress-induced L-forms of Mycobacterium bovis: a challenge to survivability. New Microbiol. 2013;36(2):157–166.
11. Billings G, Ouzounov N, Ursell T, et al. De novo morphogenesis in L-forms via geometric control of cell growth. Mol Microbiol. 2014;93(5):883–896. doi:10.1111/mmi.12703
12. Corrah TW, Enoch DA, Aliyu SH, Lever AM. Bacteraemia and subsequent vertebral osteomyelitis: a retrospective review of 125 patients. Qjm. 2011;104(3):201–207. doi:10.1093/qjmed/hcq178
13. Wassilew N, Hoffmann H, Andrejak C, Lange C. Pulmonary disease caused by non-tuberculous mycobacteria. Respiration. 2016;91(5):386–402. doi:10.1159/000445906
14. Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12):e124–e129. doi:10.1086/648443
15. Ristola MA, von Reyn CF, Arbeit RD, et al. High rates of disseminated infection due to non-tuberculous mycobacteria among AIDS patients in Finland. J Infect. 1999;39(1):61–67. doi:10.1016/s0163-4453(99)90104-4
16. Mirsaeidi M, Vu A, Leitman P, et al. A patient-based analysis of the geographic distribution of mycobacterium avium complex, mycobacterium abscessus, and mycobacterium kansasii infections in the United States. Chest. 2017;151(4):947–950. doi:10.1016/j.chest.2017.02.013
17. Marazzi MG, Chapgier A, Defilippi AC, et al. Disseminated Mycobacterium scrofulaceum infection in a child with interferon-gamma receptor 1 deficiency. Int J Infect Dis. 2010;14(2):e167–e170. doi:10.1016/j.ijid.2009.03.025
18. Chen CY, Sheng WH, Lai CC, et al. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31(6):1059–1066. doi:10.1007/s10096-011-1407-7
19. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
20. Petitjean G, Fluckiger U, Schären S, Laifer G. Vertebral osteomyelitis caused by non-tuberculous mycobacteria. Clin Microbiol Infect. 2004;10(11):951–953. doi:10.1111/j.1469-0691.2004.00949.x
21. Shimizu H, Mizuno Y, Nakamura I, Fukushima S, Endo K, Matsumoto T. Vertebral osteomyelitis caused by non-tuberculous mycobacteria: case reports and review. J Infect Chemother. 2013;19(5):972–977. doi:10.1007/s10156-013-0550-8
22. Markova N, Slavchev G, Michailova L, Jourdanova M. Survival of Escherichia coli under lethal heat stress by L-form conversion. Int J Biol Sci. 2010;6(4):303–315. doi:10.7150/ijbs.6.303
23. Wittler RG, Malizia WF, Kramer PE, Tuckett JD, Pritchard HN, Baker HJ. Isolation of a Corynebacterium and its transitional forms from a case of subacute bacterial endocarditis treated with antibiotics. J Gen Microbiol. 1960;23:315–333. doi:10.1099/00221287-23-2-315
24. Domingue GJ, Woody HB. Bacterial persistence and expression of disease. Clin Microbiol Rev. 1997;10(2):320–344. doi:10.1128/cmr.10.2.320
25. Clasener H. Pathogenicity of the L-phase of bacteria. Annu Rev Microbiol. 1972;26:55–84. doi:10.1146/annurev.mi.26.100172.000415
26. Errington J. Cell wall-deficient, L-form bacteria in the 21st century: a personal perspective. Biochem Soc Trans. 2017;45(2):287–295. doi:10.1042/bst20160435
27. Han J, He L, Shi W, et al. Glycerol uptake is important for L-form formation and persistence in Staphylococcus aureus. PLoS One. 2014;9(9):e108325. doi:10.1371/journal.pone.0108325
28. Hubert EG, Potter CS, Hensley TJ, Cohen M, Kalmanson GM, Guze LB. L-forms of Pseudomonas aeruginosa. Infect Immun. 1971;4(1):60–72. doi:10.1128/iai.4.1.60-72.1971
29. Roberts RB. L form of Neisseria gonorrhoeae. J Bacteriol. 1966;92(6):1609–1614. doi:10.1128/jb.92.6.1609-1614.1966
30. Yang C, Li H, Zhang T, Chu Y, Zuo J, Chen D. Study on antibiotic susceptibility of Salmonella typhimurium L forms to the third and forth generation cephalosporins. Sci Rep. 2020;10(1):3042. doi:10.1038/s41598-020-59456-8
31. Slavchev G, Michailova L, Markova N. L-form transformation phenomenon in Mycobacterium tuberculosis associated with drug tolerance to ethambutol. Int J Mycobacteriol. 2016;5(4):454–459. doi:10.1016/j.ijmyco.2016.06.011
32. Somoskovi A, Salfinger M. Nontuberculous mycobacteria in respiratory infections: advances in diagnosis and identification. Clin Lab Med. 2014;34(2):271–295. doi:10.1016/j.cll.2014.03.001
33. Campbell EA, Korzheva N, Mustaev A, et al. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell. 2001;104(6):901–912. doi:10.1016/s0092-8674(01)00286-0
34. Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;12:1759–1767. doi:10.2147/dddt.S164515
35. Ashraf S, Mendoza C, Salman SH, Kelly P, Adrish M, Rare A. Case of Mycobacterium monacense osteomyelitis: a case report. Cureus. 2021;13(3):e14199. doi:10.7759/cureus.14199
36. Al Farsi F, Al Adawi B, Ba Tahir H, et al. Mycobacterium farcinogenes osteomyelitis of the proximal tibia: a case report. IDCases. 2021;25:e01194. doi:10.1016/j.idcr.2021.e01194
37. Saad MM, Alshukairi AN, Qutub MO, Elkhizzi NA, Hilluru HM, Omrani AS. Mycobacterium riyadhense infections. Saudi Med J. 2015;36(5):620–625. doi:10.15537/smj.2015.5.11226
38. McGee AW, Dean CS, Ignatiuk A, Savelli C, Kleck CJ. Mycobacterium phlei Vertebral Osteomyelitis. J Am Acad Orthop Surg Glob Res Rev. 2019;3(12):5. doi:10.5435/JAAOSGlobal-D-18-00069
39. Gray ME, Liu PW, Wispelwey B. Mycobacterium Avium complex vertebral osteomyelitis in the absence of HIV infection: a case report and review. BMC Infect Dis. 2018;18(1):235. doi:10.1186/s12879-018-3143-4
40. Moral MZ, Desai K, Arain AR, O’Leary RE, Haddad SF, Lawrence JP. Mycobacterium abscessus-associated vertebral osteomyelitis in an immunocompetent patient: a rare case report and literature review. Spinal Cord Ser Cases. 2019;5:53. doi:10.1038/s41394-019-0197-5
41. Elsayed S, Read R. Mycobacterium haemophilum osteomyelitis: case report and review of the literature. BMC Infect Dis. 2006;6:70. doi:10.1186/1471-2334-6-70
42. Wong KP, Tang ZH, Tan GM. Mycobacterium fortuitum and Mycobacterium abscessus infections in the foot and ankle in two immunocompetent patients. Biomedicine. 2020;10(4):52–56. doi:10.37796/2211-8039.1021
43. Bhatt K, Banavathi K. Mycobacterium kansasii osteomyelitis - a masquerading disease. JMM Case Rep. 2018;5(1):e005114. doi:10.1099/jmmcr.0.005114
44. Breda L, de Michele G, Nozzi M, De Sanctis S, Di Marzio D, Chiarelli F. Non-tuberculous mycobacterial osteomyelitis: an unusual cause of Hip pain in immunocompetent children. Rheumatol Int. 2009;29(12):1487–1489. doi:10.1007/s00296-009-0844-4
45. Kadota N, Shinohara T, Hino H, et al. Mycobacterium abscessus ssp. abscessus infection progressing to empyema from vertebral osteomyelitis in an immunocompetent patient without pulmonary disease: a case report. BMC Pulm Med. 2019;19(1):100. doi:10.1186/s12890-019-0860-4
46. Diaz A, Ardura MI, Wang H, Antonara S, Ouellette CP. Osteomyelitis Due to Mycobacterium goodii in an Adolescent, United States. Emerg Infect Dis. 2020;26(11):2781–2783. doi:10.3201/eid2611.200206
47. Dong S, Lin Z, Wu S, Cai L. Spondylitis following bloodstream dissemination of Mycobacterium chelonae disseminated in an immunocompetent patient: a case report and literature review. J Int Med Res. 2021;49(9):3000605211047766. doi:10.1177/03000605211047766
48. Mastroianni A. Mycobacterium flavescens vertebral osteomyelitis in an immunocompetent host. Infez Med. 2003;11(2):97–101.
49. Harp GM, Achcar RD, Drummond WK. Necrotizing osteomyelitis in a man with disseminated Mycobacterium chelonae infection. ID Cases. 2018;12:71–73. doi:10.1016/j.idcr.2018.03.013
50. Omori K, Kitagawa H, Tadera K, et al. Vertebral osteomyelitis caused by Mycobacteroides abscessus subsp. abscessus resulting in spinal cord injury due to vertebral body fractures. J Infect Chemother. 2022;28(2):290–294. doi:10.1016/j.jiac.2021.09.013
51. Garcia DC, Sandoval-Sus J, Razzaq K, Young L. Vertebral osteomyelitis caused by Mycobacterium abscessus. BMJ Case Rep. 2013;2013:bcr2013009597–bcr2013009597. doi:10.1136/bcr-2013-009597
52. Johnson JE, Gupton MS, Finn J, Deivaraju C. Chronic multifocal Osteomyelitis - is Mycobacterium avium complex really the culprit? A case report in an adult female. J Orthop Case Rep. 2021;11(9):86–89. doi:10.13107/jocr.2021.v11.i09.2428
53. Xu X, Lao X, Zhang C, et al. Chronic Mycobacterium avium skin and soft tissue infection complicated with scalp osteomyelitis possibly secondary to anti-interferon-γ autoantibody formation. BMC Infect Dis. 2019;19(1):203. doi:10.1186/s12879-019-3771-3
54. Maupin J, Cantrell A, Kupiec K, Melendez DP, Haleem AM. Mycobacterium senegalense osteomyelitis of the distal Tibia: a case report. J Bone Jt Infect. 2019;4(3):140–145. doi:10.7150/jbji.33321
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.