Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Refractory Folliculitis Decalvans Treatment Success with a Novel Surgical Excision Approach Using Guarded High-Tension Sutures
Authors Umar S , Waterman A, Ton D, Shitabata P
Received 19 May 2023
Accepted for publication 20 August 2023
Published 1 September 2023 Volume 2023:16 Pages 2381—2390
DOI https://doi.org/10.2147/CCID.S422077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Sanusi Umar,1– 3 Ade Waterman,3 Donna Ton,3 Paul Shitabata1,2,4
1Department of Medicine, University of California at Los Angeles, Los Angeles, CA, USA; 2Division of Dermatology, Department of Medicine, Harbor-UCLA Medical Center, Torrance, CA, USA; 3Dr. U Hair and Skin Clinic, Manhattan Beach, CA, USA; 4Dermatopathology Institute, Torrance, CA, USA
Correspondence: Sanusi Umar, Dr. U Hair and Skin Clinic, 2121 N. Sepulveda Avenue, Suite 200, Manhattan Beach, CA, 90266, USA, Tel +1 310 318-1500, Fax +1 310 318-1590, Email [email protected]
Purpose: Folliculitis decalvans (FD) is a difficult-to-treat, localized scarring alopecia characterized by an expanding area of chronically inflamed purulent plaques or masses. Current treatment modalities vary and often result in only temporary remission. There are no reports of surgical therapies for FD. Here, we describe FD treatment using surgical excision and second-intention healing aided by guarded high-tension sutures.
Methods: Five patients (one woman and four men) with histologically confirmed FD were treated by surgical lesion excision. All wounds were allowed to heal via second-intention. Guarded high-tension sutures were employed to minimize tissue tears while aiding and guiding wound contraction.
Results: All wounds healed with a 47– 83% spatial contraction of the maximum wound diameters. Three patients healed entirely by second-intention, while two required a minor skin graft to close the wound completely. No disease recurrence was noted at 10– 24 months.
Conclusion: Surgical excision with second-intention healing aided by guarded high-tension sutures effectively treated small and extensive FD lesions with no recurrence at long-term follow-up. To our knowledge, this is the first report of successful surgical treatment of FD.
Plain Language Summary: Current treatment modalities of folliculitis decalvans, including steroids, antibiotics, and isotretinoin, are suboptimal, typically resulting in recurrence following the withdrawal of treatments. In this case series, we report a first account of treatment success of refractory folliculitis decalvans using surgical excision with second intention healing aided by guarded high-tension suturing. The outpatient procedure resulted in long-term remission at 10– 24 months follow-up.
Keywords: folliculitis decalvans, treatment, surgical treatment, alopecia, primary cicatricial alopecia, acne keloidalis nuchae, lichen planopilaris, surgery, therapy, second intention healing, tension sutures, scaring alopecia, hair loss
Introduction
Folliculitis decalvans (FD) is a debilitating condition that causes chronic inflammation and primary scarring alopecia. FD mainly affects middle-aged men and results in cicatricial alopecia and pustular plaques or masses. FD accounts for 10.5% of cicatricial alopecia cases and 2.8% of all alopecia cases.1 While the etiology of FD has not been fully elucidated, Staphylococcus aureus has been reported to play a role in the disease.2 Currently, there is no standard treatment for FD. Moreover, treatment options are suboptimal, often resulting in only temporary remission.2–7 These therapeutic options include systemic antibiotics,1–6 topical and intralesional corticosteroids,1,3–6 isotretinoin,6,7 tumor necrosis factor-alpha inhibitors,8 and photodynamic therapy,1 with the most promising of these treatments (isotretinoin) showing a high relapse rate (>50%) within 2.5–3 months of stopping treatment.7 While the continuation of low-dose isotretinoin may control the disease in up to 33% of cases,7 it is not a viable path for many patients given isotretinoin’s poor toxicity profile.9
Longer-term remission has been reported, mainly in small, uncontrolled studies, after permanent hair elimination via laser hair removal10,11 or radiation,12 emphasizing the disease’s folliculocentric nature. However, the dynamics of laser functions suggest that their application may be limited to thin or atrophic lesions with follicular presence.10,11,13 Furthermore, radiation is inapplicable in most instances because of its higher potential for adverse effects (including skin cancer). Although both lesions and hair can be removed using surgical methods, there have been no reports on surgical therapies for FD. Here, we describe surgical excision and closure by second intention healing (SIH) aided by guarded high-tension sutures, which were used to resolve FD in five patients in whom years of various conventional treatments had failed.
Methods
All patients provided written informed consent for surgical excision. All procedures were conducted per the Declaration of Helsinki (revised in 2013) Institutional Review Board approval was not required or sought as this study was not a prospective or systematic investigation of FD treatment but described the general principles for surgical excision of scalp lesions.
All patients in this retrospective study who underwent surgical excision for FD lesions had failed prior therapies, rejected the continuation of drug treatments, failed, declined, or were poor candidates for laser or radiation therapy.
Patients with thick mass lesions devoid of follicles were also considered poor candidates for laser hair removal treatment.
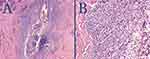
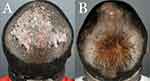
The surgeries were performed in a single Los Angeles clinic between March 2021 and June 2022. The diagnosis of FD was based on clinical examination and histologic findings (Figure 1).
Local anesthesia was achieved by injecting 1% lidocaine and a 1:200,000 epinephrine solution into the lesion margins. This was then further diluted with Ringer’s lactate at a 10:1 ratio and injected into the supragaleal plane of the entire lesion to achieve tumescent anesthesia and hydrodissection.
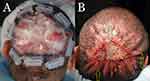
The lesions were excised using an electrosurgical tip with a 0.5–1 cm margin carried to the galeal plane (Figure 2A). Hemostasis was achieved using electrocoagulation and suturing. No undermining was performed. Hair follicles in the wound margins were deliberately destroyed by cautery to minimize the potential for isomorphic reactivation of FD from inflammation of entrapped hairs in the scar of the healing wound margins.14
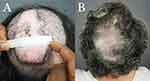
The wound edges were pulled as close to each other as possible (but no less than 1 cm) under high tension. Finally, they were fixed in place using size 0 polydioxanone sutures placed in a horizontal mattress fashion, bolstered by a medical-grade plastic guard, which the lead author designed to minimize tissue tear (Athena Suture Guards®; Dr. U Devices, Manhattan Beach, CA).15 (Figure 2B) Long suture tails were instituted to enable retightening as needed (Figures 2C and 3A).
The patients were discharged on a 7-day course of oral cephalosporin with instructions to return the following day for wound inspection and hemostasis surveillance.
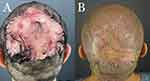
The wounds were left open to allow complete closure by SIH. Daily wound care was performed using a solution of diluted acetic acid in distilled water for cleaning and Aquaphor® or bacitracin ointment (Figures 2C and 3A). Residual open wounds, often minor, if found at 8–12 weeks, were closed by applying a small split-thickness skin graft (STSG) (Figure 3B). High-tension sutures were removed once they became lax owing to wound contraction, typically between 4–6 weeks, with the option of retightening at 4 weeks. The remaining scalp was assessed for commonly associated conditions such as acne keloidalis nuchae (AKN)16–18 and scalp-wide histologic evidence of perifollicular infundibulo-isthmic lymphocytic infiltrates and fibrosis, an lichen planopilaris (LPP)-like condition19–21 These conditions were treated separately after completing FD treatment.
Results
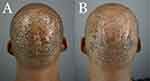
Demographic and treatment detail are summarized in Table 1. Five patients (4 men and 1 woman) had their FD lesions excised using the described surgical method. The average age was 37 years (range 32–47). All achieved complete remission at 10–24 months of follow-up (Tables 1, 2 and Figures 4–8).
 |
Table 1 Demographic and Pre-Surgical Data on Five Patients That Underwent Surgical Excision of FD Lesions with SIH Aided by Guarded High-Tension Suturing |
 |
Table 2 Surgical and Post-Surgical Data on Five Patients That Underwent Surgical Excision of FD Lesions with SIH Aided by Guarded High-Tension Suturing |
Two of the five patients with coronal wound diameters exceeding 10 cm required minor STSG for complete wound closure (Table 2 and Figure 3). The other three patients, with 10 cm or less coronal wound diameters, did not require skin grafting (Table 2, Figures 4, 7, and 8). In both cases requiring STSG, the skin grafts measured between 20–33% of the excision wound size (Table 1). A maximal wound diameter contraction of 47–83% was noted. All patients tolerated the procedure well without side effects, and remained in complete remission over a mean (SD) follow-up period of 17 months (6.1).
Discussion
Our case series demonstrates the successful treatment of refractory FD of various sizes by surgical excision and second-intention healing aided by high-tension suture guards with long-term remission. We are not aware of any reports of FD treatment by surgical excision.
Second-intention healing is an optimal surgical approach for addressing excised lesions located in relatively concave body surfaces.22–24 However, FD, which typically occurs on the convex or flat surface of the vertex and mid-scalp, does not benefit from a concave surface. Despite the disadvantageous convex location, all five FD excision wounds showed significant wound contraction, suggesting a role for the guarded high-tension suturing technique.
In second-intention healing, wound closure occurs through the combined processes of wound contraction (results in wound size reduction) and epithelization (results in no reduction in wound size). The lead author has shown in a previous publication that the application of tension tilts the process in favor of wound contraction, which has the effect of the wound edges closing in towards each other, reducing the final scar formed.22 The mechanism by which tension causes wound contraction is supported by studies that have shown that under tension, fibroblasts transform to proto-myofibroblasts and eventually myofibroblasts25,26 which are crucial to the ability of the wound to contract.27 Another benefit of using tension sutures, is that, it enables the surgeon to control the final shape of the wound by manipulating tension at various locations along tension sutures.22 This process is primarily mediated by contractile forces induced by the tension sutures, which prompt the deposition of collagen and fibronectin-rich extracellular matrix (ECM) by the resident fibroblasts. The newly synthesized collagen and ECM, together with the fibroblasts, exhibit distinctive alignment patterns parallel to the wound bed and in accordance with the anticipated lines of mechanical stress.24,27
In all patients, the objective of permanently eliminating FD-diseased tissue was achieved. Wounds with coronal diameters ≤10 cm closed completely without skin grafting. In two patients where the coronal wound diameter exceeded 10 cm, a skin graft of a significantly smaller area, 20–33% of the original wound, was applied. Our 17-month follow-up without ancillary FD treatment suggests that the observed complete remission was long-lasting.
Other surgical approaches we considered and dismissed included tissue expansion to harness flaps for closing the excision wound. This is limited by high invasiveness, the requirement for general anesthesia, and a complication rate of over 27%.28 In addition, the theoretical potential of the surgical trauma of such a procedure to cause an isomorphic activation of LPP disease29 in the rest of the scalp already showing LPP-like histology was a deterring consideration. Another option was excision with skin grafting, which is a two-stage surgery that creates an uncontracted Alopecic plaque of the same or larger size than the original lesions, bearing a striking contrast with the surrounding skin in color, texture and lack of hair.30,31 Both tissue expansion and extensive skin grafting procedures require a specialized skill set and a significant investment in equipment. The outpatient methodology described in this paper for excision followed by SIH aided by guarded high-tension sutures confers some benefits, including maintaining the wound margins under continuous high tension in the direction of closure. This latter feature encourages wound contraction and guides closure toward the desired scar esthetics.26,32 Furthermore, suture guards minimize suture cut-through by redistributing and diffusing the high-tension vectors away from their natural tendencies of dragging the suture skin entry points toward the wound edges and cutting through. Traditional horizontal mattress sutures, with or without gauze-reinforced bolsters, if performed under considerably high tension for a sustainable amount of time, would drag suture entry points toward the wound edge, tearing through the tissue.
Apart from the unwelcome effect of the wound, suture tear-through neutralizes the high tension needed to keep the edges close to the desired proportions set by the surgeon. The result would be a diminution or even failure of the process, poor esthetics caused by new scars from the suture tears, and a final scar now off-kilter and wider due to failed or poorly controlled contraction. Another concern when using traditional horizontal vertical mattress suturing under very high tension with or without gauze bolsters is tissue strangulation due to strangulated vasculature; the plastic suture guard features foot pads (Figure 2) to minimize the pressure exerted on underlying tissue, thus canceling the risk of strangulation. Furthermore, the porous nature of gauze or cotton bolsters presents an added risk of infection if left in a wet wound environment for the 4-week period they are required to be in place. Lastly, current suture guards are often designed for short-term use in wounds that are closed primarily by stitching.33 Also, the sutures on the bridging apparatus in current suture bridges would cross the wounds at their surfaces, thereby obstructing access to the wound bed and complicating wound care and hygiene necessary during the long SIH period.34 A suture guard designed for use in a horizontal mattress fashion ensured unobstructed access to the open wound, allowing the daily wound care needed to maintain wound hygiene over the weeks required for its closure.
Wound undermining was avoided to provide the high-tension suture guards with a firm base to sit. Additionally, the risk of undermined wounds compromises the blood supply to the wound edges resulting in hematoma, hemorrhage, and possible necrosis.35 All of these could be accentuated in a wound whose edges are subjected to considerable tension forces.
Our experience with Patient #1 suggests that the healing course can be prolonged in those with poor healing tendencies, evidenced by delayed or suboptimal granulation tissue formation. Using wound healing accelerators such as platelet-rich plasma36 may be beneficial in such instances.
The association of FD with AKN is well established with studies, including two published by the lead author reporting a 7–24% association and suggesting a possible common pathoetiology.16–18Three out of five patients in our cohort had separate AKN lesions in their nape zones that were treated by either an electrosurgical knife or punch excision (Table 1). Furthermore, the presence of an LPP-like disease in the remaining non-FD scalp is a recognized phenomenon19–21 and may influence prognosis; this should be investigated in all FD patients using trichoscopy and histology and treated as a separate entity when found.37 In our patients, LPP-like zones were treated with topical tacrolimus (1 week alternating with 2 weeks off) or a topical botanical (Gashee®, FineTouch Laboratories. Manhattan Beach, CA) daily.38
Limitations
The study was limited by the retrospective nature of this case series and the limited number of patients.
Conclusion
Surgical excision with second intention healing aided by the described guarded high-tension suture apparatus effectively treated small and extensive refractory FD lesions with long-term remission in our patient cohort. Further validation in larger controlled studies is warranted. To our knowledge, this is the first report of successful surgical treatment success of FD.
Abbreviations
FD, Folliculitis decalvans; SIH, second intention healing; STSG, split-thickness skin graft; AKN, acne keloidalis nuchae; LPP, lichen planopilaris.
Data Sharing Statement
In addition to photographic evidence showing interval changes in the manuscript, complete medical records of the presented cases are stored in the treating clinic, in line with the standard of care.
Ethics and Consent
All patients who underwent FD excision provided written informed consent for participation and publication. All procedures were conducted in accordance with the Declaration of Helsinki (revised in 2013). Institutional Review Board approval was not required or sought as this study was not a prospective or systematic investigation of FD treatment but described the general principles for surgical excision of scalp lesions.
Acknowledgments
We are particularly grateful to our patients.
Funding
There is no funding to report.
Disclosure
Dr. Umar owns shares, patents, and patent applications in Dr. U Devices and FineTouch Laboratories Inc (Manhattan Beach, CA) and reports a patent issued to Dr U Devices. The other authors declare that they have no conflicts of interest in this work.
References
1. Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5(5):309–315. doi:10.1159/000496708
2. Otberg N, Kang H, Alzolibani AA, Shapiro J. Folliculitis decalvans. Dermatol Ther. 2008;21(4):238–244. doi:10.1111/j.1529-8019.2008.00204.x
3. Bunagan MJ, Banka N, Shapiro J. Retrospective review of folliculitis decalvans in 23 patients with the course and treatment analysis of long-standing cases. J Cutan Med Surg. 2015;19(1):45–49. doi:10.2310/7750.2014.13218
4. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140(2):328–333. doi:10.1046/j.1365-2133.1999.02675.x
5. Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79(5):878–883. doi:10.1016/j.jaad.2018.05.1240
6. Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29(9):1816–1821. doi:10.1111/jdv.13052
7. Aksoy B, Hapa A, Mutlu E. Isotretinoin treatment for folliculitis decalvans: a retrospective case-series study. Int J Dermatol. 2018;57(2):250–253. doi:10.1111/ijd.13874
8. Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with Adalimumab. Int J Trichol. 2019;11(6):241–243. doi:10.4103/ijt.ijt_92_19
9. Pile HD, Sadiq NM. Isotretinoin. In: StatPearls (Internet). Treasure Island, FL: StatPearls Publishing; 2023.
10. Meesters AA, Van der Veen JP, Wolkerstorfer A. Long-term remission of folliculitis decalvans after treatment with the long-pulsed Nd: YAG laser. J Dermatolog Treat. 2014;25(2):167–168. doi:10.3109/09546634.2013.826340
11. Parlette EC, Kroeger N, Ross EV. Nd: YAG laser treatment of recalcitrant folliculitis decalvans. Dermatol Surg. 2004;30(8):1152–1154. doi:10.1111/j.1524-4725.2004.30344.x
12. Elsayad K, Kriz J, Haverkamp U, et al. Treatment of folliculitis decalvans using intensity-modulated radiation via tomotherapy. Strahlenther Onkol. 2015;191(11):883–888. doi:10.1007/s00066-015-0891-6
13. Umar S. Selection criteria and techniques for improved cosmesis and predictable outcomes in laser hair removal treatment of acne keloidalis nuchae. JAAD Case Rep. 2019;5(6):529–534. doi:10.1016/j.jdcr.2019.02.034
14. Annessi G. Tufted folliculitis of the scalp: a distinctive clinicohistological variant of folliculitis decalvans. Br J Dermatol. 1998;138(5):799–805. doi:10.1046/j.1365-2133.1998.02216.x
15. Umar S. Retention suture assembly. United States patent application number US11607212B2;. 2019 Available from: https://patents.google.com/patent/US11607212B2/en.
16. Umar S, Ton D, Carter MJ, Shitabata P. Unveiling a Shared Precursor Condition for Acne Keloidalis Nuchae and Primary Cicatricial Alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315–2327.
17. Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14(4):E61–E67.
18. Doche I, Coelho EQ, Quaresma MV, et al. Acne keloidalis nuchae and folliculitis decalvans: same process affecting the follicle or coexisting diseases? A retrospective study. Int J Dermatol. 2019;58(10):e200–e203. doi:10.1111/ijd.14565
19. Egger A, Stojadinovic O, Miteva M. Folliculitis decalvans and lichen planopilaris phenotypic spectrum-A series of 7 new cases with focus on histopathology. Am J Dermatopathol. 2020;42(3):173–177. doi:10.1097/DAD.0000000000001595
20. Yip L, Barrett TH, Harries MJ. Folliculitis decalvans and lichen planopilaris phenotypic spectrum: a case series of biphasic clinical presentation and theories on pathogenesis. Clin Exp Dermatol. 2020;45(1):63–72. doi:10.1111/ced.13989
21. Doche I, Hordinsky MK, Valente NS, et al. Evidence for lymphocytic inflammation in non-lesional scalp of folliculitis decalvans: an observational study of 25 patients. J Eur Acad Dermatol Venereol. 2022;36(2):e109–e111. doi:10.1111/jdv.17649
22. Umar S, David CV, Castillo JR, Queller J, Sophia Sandhu S. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7(5):e2215. doi:10.1097/GOX.0000000000002215
23. Maranda EL, Simmons BJ, Nguyen AH, Lim VM, Keri JE. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6(3):363–378. doi:10.1007/s13555-016-0134-5
24. Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2(3):92–106. doi:10.1016/0738-081X(84)90031-2
25. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi:10.1038/nrm809
26. Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–1020. doi:10.1016/S0002-9440(10)61776-2
27. Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–550. doi:10.1007/BF02147594
28. Azzi JL, Thabet C, Azzi AJ, Gilardino MS. Complications of tissue expansion in the head and neck. Head Neck. 2020;42(4):747–762. doi:10.1002/hed.26017
29. Montpellier RA, Donovan JC. Scalp trauma: a risk factor for lichen planopilaris? J Cutan Med Surg. 2014;18(3):214–216. doi:10.2310/7750.2013.13020
30. Cosman B, Wolff M. Acne keloidalis. Plast Reconstr Surg. 1972;50(1):25–30. doi:10.1097/00006534-197207000-00004
31. Galarza LI, Azar CA, Al Hmada Y, Medina A. Surgical management of giant acne keloidalis nuchae lesions. Case Reports Plast Surg Hand Surg. 2021;8(1):145–152. doi:10.1080/23320885.2021.1982392
32. Hinrichsen N, Birk-Sørensen L, Gottrup F, Hjortdal V. Wound contraction in an experimental porcine model. Scand J Plast Reconstr Surg Hand Surg. 1998;32(3):243–248. doi:10.1080/02844319850158561
33. Ferrell K, Lear W. Intraoperative tissue expansion to allow primary linear closure of 2 large adjacent surgical defects. Cutis. 2020;106(5):250–252. doi:10.12788/cutis.0105
34. Kazmer DO, Eaves FF. Force modulating tissue bridges for reduction of tension and scar: finite element and image analysis of preclinical incisional and nonincisional models. Aesthet Surg J. 2018;38(11):1250–1263. doi:10.1093/asj/sjy079
35. Melis P, Noorlander ML, Bos KE. Tension decrease during skin stretching in undermined versus not undermined skin: an experimental study in piglets. Plast Reconstr Surg. 2001;107(5):1201–1205. doi:10.1097/00006534-200104150-00016
36. Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, et al. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018;9(1):10. doi:10.3390/jfb9010010
37. Bakhshoudeh B, Salehi M, Sadeghi R. Therapeutic updates on Lichen planopilaris and frontal fibrosing alopecia: a systematic review. Rev Clin Med. 2018;5:76–94.
38. Umar S, Kan P, Carter MJ, et al. Lichen planopilaris responsive to a novel phytoactive botanical treatment: a case series. Dermatol Ther. 2022;12(7):1697–1710. doi:10.1007/s13555-022-00749-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.