Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Refractory Bullous Systemic Lupus Erythematosus Successfully Treated with Rituximab: A Case Report and Literature Review
Authors Ratanapokasatit Y , Seree-Aphinan C , Chanprapaph K
Received 6 January 2023
Accepted for publication 25 March 2023
Published 4 April 2023 Volume 2023:16 Pages 883—890
DOI https://doi.org/10.2147/CCID.S403866
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Yanisa Ratanapokasatit, Chutima Seree-Aphinan, Kumutnart Chanprapaph
Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Kumutnart Chanprapaph, Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok, 10400, Thailand, Tel +662-201-1141, Fax +662-201-1211, Email [email protected]
Abstract: Bullous systemic lupus erythematosus (BSLE) is a rare blistering skin manifestation of systemic lupus erythematosus (SLE). Dapsone is reported to be helpful in mild-to-moderate BSLE cases; however, its use may be limited or prohibited due to particular complications such as drug hypersensitivity, dose-dependent hemolytic anemia, and other significant hematologic abnormalities. Rituximab, an anti-CD20 monoclonal antibody, has been reported with off-label use in BSLE patients, but data are still limited. Hence, our objective is to explore the efficacy of rituximab among these patients. Herein, we report a 21-year-old Thai woman presented with blistering eruption on the oral cavity, scalp, trunk, and extremities for 1 month. The investigations revealed a positive direct Coomb’s test, an elevated erythrocyte sedimentation rate (ESR), and a positive antinuclear antibody (ANA). Skin biopsy showed focal interface dermatitis. Direct immunofluorescence (DIF) illustrated mixed linear and granular deposition of immunoglobulin (Ig)G, IgM, IgA, and C3 along the dermo-epidermal junction (DEJ). Enzyme-linked immunosorbent assay (ELISA) showed circulating antibodies to type VII collagen. She was diagnosed with severe BSLE and autoimmune hemolytic anemia (AIHA) refractory to several oral immunosuppressants but was successfully treated with rituximab. The authors also performed a review of the literature on prior BSLE cases managed with rituximab.
Keywords: anti-CD20 monoclonal antibody, autoimmune bullous diseases, systemic lupus erythematosus, bullous systemic lupus erythematosus, subepidermal blistering eruption
Introduction
BSLE is an uncommon autoimmune subepidermal blistering eruption that can occur in patients with SLE. It typically affects young women in the second to fourth decades of life.1–4 Although 59% to 85% of SLE patients develop cutaneous manifestations, BSLE affects less than 5% of lupus patients1,5–8 and approximately 2–3% of all subepidermal autoimmune bullous skin diseases.9 A large cohort of studies showed that 0.19–0.41% of SLE and cutaneous lupus erythematosus patients develop BSLE.8,10 The highest incidence is in those of African descent. However, it can present in all ages, races, and genders.11
BSLE typically presents with a subepidermal tense vesiculobullous eruption that heals without scarring. It affects mainly the trunk, upper extremities, neck, face, and vermillion borders but can also occur anywhere.1 Some cases of BSLE are associated with increased SLE activity, particularly lupus nephritis.1,12–14 BSLE is characterized histologically by a subepidermal blister, with a predominantly neutrophilic dermal infiltrate. Immunofluorescence examination shows deposition of IgG, lgA, IgM, and complement (C) along the basement membrane zone (BMZ).8,15 The presence of circulating antibody on the dermal side of the BMZ can be presented. A major target antigen in patients with BSLE is the anchoring fibril component type VII collagen in the dermis.8,16 Treatment with dapsone, glucocorticoids, antimalarial agents, and other immunosuppressives has shown variable efficacy. Off-label use of rituximab has led to successful outcomes in refractory BSLE cases.2,4,17,18 Rituximab depletes mature B-cells that are CD20 positive and therefore likely to reduce the quantity of circulating anti-type VII collagen antibodies, which are responsible for bullae formation in BSLE.1 However, there are still limited studies regarding the use of rituximab in severe BSLE. In addition, the duration of remission, rate of relapse, and whether repeated infusion is necessary remains unclear. We, herein, report a 21-year-old Thai woman with severe BSLE successfully treated with rituximab and include a review of the literature on prior BSLE cases managed with rituximab. The search for English-language publications was conducted using the PubMed database. The specific search term was “bullous systemic lupus erythematosus [text word]” (no exclusions were utilized); thereafter, a manual search of case reports/series and review articles reporting rituximab treatment as well as each article’s references was undertaken to ensure a more complete sample of existing literature.
Case Report
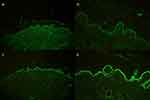
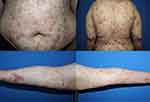
A 21-year-old Thai female diagnosed with severe BSLE and autoimmune hemolytic anemia (AIHA) presented with blistering eruption on the lips, hard palate, scalp, trunk, upper and lower extremities with desquamation of the palms and soles for 1 month. She also had non-scarring alopecia and arthralgia. Physical examination revealed multiple tense vesicles on lips, hard palate, and scalp; multiple tense bullae on erythematous base at trunk and extremities, accompanied with erythematous desquamated plaques on the palms and soles (Figure 1). Laboratory findings demonstrated hemolytic anemia with a positive direct Coomb’s test, an elevated ESR, and positive ANA with fine speckled (titer 1:320) and nucleolar (titer 1:80) pattern. A skin biopsy from left arm showed focal interface dermatitis. Direct immunofluorescence (DIF) illustrated mixed linear and granular deposition of IgG, IgA, and C3 as well as granular deposition of IgM along the dermo-epidermal junction, IgM deposition at superficial blood vessels, few cytoid bodies of IgM and IgA, and perifollicular deposition of C3 (Figure 2). ELISA showed circulating antibodies to type VII collagen (>200 RU/mL). She was initially treated with dapsone (25–50 mg/day), prednisolone (30–40 mg/day), colchicine (0.6 mg/day), and clobetasol cream twice daily for 1 months. Then, hydroxychloroquine (200 mg/day) and azathioprine (50mg/day) were added. The treatment response was monitored for another 2 months. However, the cutaneous lesions persisted with multiple new tense bullae appearing on back, trunk, scalp, and extremities. Thereafter, the patient was administered with 1 g of pulse methylprednisolone for 3 days and 500 mg of intravenous cyclophosphamide (IVCY) every 2 weeks for 4 sessions. The combination treatment with IVCY and oral immunosuppressants was continued for 2 months. The dosage of oral prednisolone was increased to 60 mg/day, while dapsone was limited to 50 mg/day due to hematological concerns. Additionally, mycophenolate mofetil (1000 mg/day), cyclophosphamide (100 mg/day), and methotrexate (10 mg/week) were added for 1 month. Nevertheless, the cutaneous disease remained refractory to the treatment. She finally received a rheumatoid arthritis protocol for rituximab consisting of two 1000 mg infusions 2 weeks apart. The patient responded well to rituximab, the oral lesions improved within 7 days after the first dose, and the skin lesions improved within 9 days after the second dose. Most of the lesions healed with post-inflammatory hyperpigmentation within 1 month, as shown in Figure 3. Prednisone was successfully tapered, and other immunosuppressants were decreased to minimal dose. Nevertheless, she developed a flare of BSLE at 6 months following the initial course of rituximab. She underwent another course of two infusions of rituximab 1000 mg administered 2 weeks apart and is currently in remission under minimal immunosuppressive therapy for 6 months.
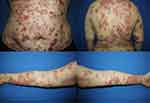 |
Figure 1 A 21-year-old female diagnosed with severe BSLE and autoimmune hemolytic anemia. Multiple tense bullae on erythematous base at trunk and extremities. |
Discussion
BSLE is a rare cutaneous manifestation of SLE, which is a LE-nonspecific vesiculobullous disease with an antibody targeting type VII collagen and histological evidence of neutrophilic dermatitis.1,19 The diagnostic criteria for BSLE were first described by Camisa and Sharma in 198312 and revised in 1988.14 These criteria included (1) a diagnosis of SLE based on the American College of Rheumatology (ACR) criteria; (2) the presence of vesicles and/or bullae; (3) the presence of histopathological features similar to those of dermatitis herpetiformis (DH); (4) DIF findings showing IgG, IgM, or IgA at the BMZ; and (5) IIF findings negative or positive for circulating autoantibodies against the BMZ. In 1995, Yell et al defined BSLE as “an acquired subepidermal blistering disease in a patient with SLE, with immune reactants at the BMZ on either DIF or IIF”. They subdivided BSLE into three classes based on the presence of antibodies to type VII collagen and location of the antibodies against the BMZ.20,21 In our case, the differential diagnoses include bullous pemphigoid, linear IgA bullous dermatosis, DH, and epidermolysis bullosa acquisita. She was diagnosed with SLE by fulfilling the EULAR/ACR criteria22 and had compatible clinical and immunopathological findings for BSLE. The patient was further classified as having type I BSLE due to the presence of circulating autoantibodies to type VII collagen, which is the most common subtype.
While systemic steroids and antimalarials are the standard treatments for the cutaneous manifestations of SLE, dapsone is the mainstay of treatment in BSLE, with a rapid clinical response.5 However, it is not uncommon for patients to be unresponsive to dapsone. Our patient was treated with dapsone in combination with several immunosuppressants for 3 months without clinical improvement. In addition, like this case the use of dapsone may be limited or prohibited in BSLE with significant hematologic abnormalities such as AIHA. Recently, rituximab, an anti-CD20 monoclonal antibody, has emerged as an effective therapy for treating a wide spectrum of diseases that include various rheumatic, gastrointestinal, and cutaneous diseases, and has shown promising results in BSLE cases refractory to dapsone, corticosteroids, or immunosuppressants.1,5 Rituximab depletes mature B-cells that are CD20 positive and therefore likely reduces the quantity of circulating anti-type VII collagen antibodies in the case of BSLE.
Rituximab was approved by the US Food and Drug Administration (FDA) for the treatment of malignancies, rheumatoid arthritis and later for pemphigus vulgaris.2,23 It has been used off label to treat BSLE cases that are resistant to dapsone, corticosteroids, and other immunosuppressants. However, there are limited case reports demonstrating rituximab treatment outcome and the time of treatment response in severe BSLE cases. In addition, long-term efficacy of rituximab is not known. Table 1 shows demographic data, clinical, laboratory, treatment, and outcome of existing severe BSLE cases treated with rituximab.
 |
Table 1 Demographic Data, Clinical, Laboratory Characteristics, Treatment, and Outcome of Severe BSLE Treated with Rituximab |
In 2011, Alsanafi et al reported a case of 61-year-old African American woman with overlapping SLE/Sjogren syndrome presented with refractory BSLE who was successfully treated with rituximab. The skin lesions improved within 10 days after the first dose and cleared by day 15 after the second dose. She remained free of recurrence of blistering lesions for 6 months.2 In 2013, Anyanwu et al reported successful treatment of refractory BSLE in a 42-year-old woman who had bullous lupus lesions cleared for 2 years after receiving rituximab treatment.17 In 2019, Lowe et al reported an 18-year-old African woman with BSLE whose bullous eruption resolved after administering rituximab and was in remission under low-dose prednisolone (5mg) for a duration of 2 years.4 The most recent case report demonstrated a 39-year-old woman with severe BSLE who was successfully treated with low-dose rituximab at a dose of 500 mg 2 weeks apart (due to cost considerations). Skin lesions resolved completely after 3 months and remained stable at 1-year post-treatment.18 Similar to these previous reports, our case showed promising resolution of cutaneous lesions after rituximab treatment in severe BSLE that has limited response to dapsone, corticosteroid, antimalarial agent, and other immunosuppressants. However, the response to rituximab was relatively short and relapsed occurred after 6 months. Therefore, the existing evidence suggests a potential role for treatment of refractory BSLE with rituximab, but we also emphasize that its effectiveness may be short and patients may require repeated infusions.
Conclusion
We report a case of severe BSLE refractory to low-dose of dapsone, antimalarial agent, corticosteroid, and several immunosuppressants that finally responded well to rituximab and emphasize the potential role of rituximab in the treatment of refractory BSLE through a review of the literature.
Ethics Approval and Consent to Participate
This article was performed in accordance with the principles of Declaration of Helsinki. Ethical review and approval were not required to publish the case details in accordance with local legislation and institutional requirements. Written informed consent was obtained from the patient for publication of this case report and any accompanying images as per our standard institutional rules.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15(6):517–524. doi:10.1007/s40257-014-0098-0
2. Alsanafi S, Kovarik C, Mermelstein AL, Werth VP. Rituximab in the treatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17(3):142–144. doi:10.1097/RHU.0b013e318214f30c
3. Tincopa M, Puttgen KB, Sule S, Cohen BA, Gerstenblith MR. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27(4):373–376. doi:10.1111/j.1525-1470.2010.01179.x
4. Lowe CD, Brahe CA, Green B, Lam TK, Meyerle JH. Bullous systemic lupus erythematosus successfully treated with rituximab. Cutis. 2019;103(6):E5–E7.
5. Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:167064. doi:10.1155/2015/167064
6. Rutnin S, Chanprapaph K. Vesiculobullous diseases in relation to lupus erythematosus. Clin Cosmet Investig Dermatol. 2019;12:653–667. doi:10.2147/CCID.S220906
7. Tankunakorn J, Sawatwarakul S, Vachiramon V, Chanprapaph K. Stevens-Johnson syndrome and toxic epidermal necrolysis-like lupus erythematosus. J Clin Rheumatol. 2019;25(5):224–231. doi:10.1097/RHU.0000000000000830
8. Chanprapaph K, Sawatwarakul S, Vachiramon V. A 12-year retrospective review of bullous systemic lupus erythematosus in cutaneous and systemic lupus erythematosus patients. Lupus. 2017;26(12):1278–1284. doi:10.1177/0961203317699714
9. Bernard P, Vaillant L, Labeille B, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol. 1995;131(1):48–52. doi:10.1001/archderm.1995.01690130050009
10. Pons-Estel GJ, Quintana R, Alarcón GS, et al. A 12-year retrospective review of bullous systemic lupus erythematosus in cutaneous and systemic lupus erythematosus patients. Lupus. 2018;27(10):1753–1754. doi:10.1177/0961203318776104
11. Sprow G, Afarideh M, Dan J, Hedberg ML, Werth VP. Bullous systemic lupus erythematosus in females. Int J Womens Dermatol. 2022;8(3):e034. doi:10.1097/JW9.0000000000000034
12. Camisa C, Sharma HM. Vesiculobullous systemic lupus erythematosus. Report of two cases and a review of the literature. J Am Acad Dermatol. 1983;9(6):924–933. doi:10.1016/S0190-9622(83)70210-0
13. Malcangi G, Brandozzi G, Giangiacomi M, Zampetti M, Danieli MG. Bullous SLE: response to methotrexate and relationship with disease activity. Lupus. 2003;12(1):63–66. doi:10.1191/0961203303lu241cr
14. Camisa C. Vesiculobullous systemic lupus erythematosus. A report of four cases. J Am Acad Dermatol. 1988;18(1 Pt 1):93–100. doi:10.1016/S0190-9622(88)70014-6
15. de Risi-Pugliese T, Cohen Aubart F, Haroche J, et al. Clinical, histological, immunological presentations and outcomes of bullous systemic lupus erythematosus: 10 New cases and a literature review of 118 cases. Semin Arthritis Rheum. 2018;48(1):83–89. doi:10.1016/j.semarthrit.2017.11.003
16. Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29(4):649–653. doi:10.1016/j.det.2011.06.002
17. Anyanwu CO, Ang CC, Werth VP. Oral mucosal involvement in bullous lupus. Arthritis Rheum. 2013;65(10):2622. doi:10.1002/art.38051
18. Akpabio AA, Otike-Odibi BI. Severe bullous systemic lupus erythematosus successfully treated with low dose rituximab: a case report from sub-Saharan Africa. Reumatismo. 2020;72(2):115–119. doi:10.4081/reumatismo.2020.1274
19. Chanprapaph K. Management of cutaneous lupus erythematosus. In: Chanprapaph K, editor. Cutaneous Lupus Erythematosus. Division of Dermatology, Department of Internal Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University; 2022:414–466.
20. Yell JA, Allen J, Wojnarowska F, Kirtschig G, Burge SM. Bullous systemic lupus erythematosus: revised criteria for diagnosis. Br J Dermatol. 1995;132(6):921–928. doi:10.1111/j.1365-2133.1995.tb16950.x
21. Qiao L, Zhang B, Zheng W, et al. Clusters of clinical and immunologic features in patients with bullous systemic lupus erythematosus: experience from a single-center cohort study in China. Orphanet J Rare Dis. 2022;17(1):290. doi:10.1186/s13023-022-02445-z
22. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–1159. doi:10.1136/annrheumdis-2018-214819
23. Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150(7):703–708. doi:10.1001/jamadermatol.2013.6739
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.