Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Reflectance Confocal Microscopy and Dermoscopy For the Diagnosis and Treatment of Cutaneous Larva Migrans
Authors Tang N , Huang QA, Cai LH, Deng DM, Niu M
Received 21 December 2022
Accepted for publication 4 April 2023
Published 17 April 2023 Volume 2023:16 Pages 1019—1023
DOI https://doi.org/10.2147/CCID.S401982
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Nan Tang,1,* Qing-An Huang,2,* Lan-Hua Cai,1 Dong-Mei Deng,3 Mu Niu4
1Departments of Traditional Chinese Medicine, Guangzhou Red Cross Hospital of Jinan University, Guangzhou, Guangdong, People’s Republic of China; 2Department of General Ward, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 3Health Management Center, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China; 4Department of Cosmetic Dermatology, The Fifth People’s Hospital of Hainan Province, Haikou, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mu Niu, Department of Cosmetic Dermatology, The Fifth People’s Hospital of Hainan Province, Haikou, People’s Republic of China, Email [email protected] Dong-Mei Deng, Health Management Center, The Fifth People’s Hospital of Hainan Province, Haikou, People’s Republic of China, Email [email protected]
Abstract: We describe a 39-year-old woman with a 1-month-old linear erythema diagnosed with cutaneous larva migrans by reflectance confocal microscopy (RCM). This case reveals that the great significance of diagnosing and treating cutaneous larva migrans (CLM) by RCM and dermoscopy, which might provide novel insights into dermatological clinical practice.
Keywords: reflectance confocal microscopy, cutaneous larva migrans, linear erythema
Introduction
Cutaneous larva migrans (CLM) is a creeping eruption caused by larval skin migration of nematodes, flukes (trematodes), and tapeworms (cestodes). It is endemic in tropical and subtropical regions, with high incidence during spring and summer.1 The prevalence of CLM as an occupational disease is high in workers exposed to animals (ie, dogs and cats), which potentially harbor ancylostomatids, and workers in contact with potential contaminated soil or sand, such as breeders, farmers, agriculturists, and gardeners.2 Thus, even in the absence of animal contact, certain professions may be considered at high risk of infection. CLM is diagnosed based on its clinical characteristics. Noninvasive diagnostic techniques have been increasingly used in dermatological clinical practice.3 Therefore, we report an autochthonous case of CLM diagnosed by reflectance confocal microscopy (RCM).
Case Presentation
A 39-year-old woman, who is a sanitation worker, unmarried and nulliparous, was admitted to our hospital because of linear-erythema with accompanying pruritus and formication in her left upper arm for 1 month. The lesion appeared after she had cleared the grass in the highway greening zone. The linear erythema was misdiagnosed as eczema in her local hospitals. She also received a topical therapy with mometasone furoate cream to alleviate pruritus. One week before the admission, the skin lesion gradually spread to the proximal part of the upper limbs, and the intense pruritus severely affected her sleep, work, and quality of life. Hence, she came to our hospital. She denied hepatitis, tuberculosis, or other infectious diseases, and had no history of smoking, drinking, allergies, or surgery. On admission, dark red, linear, spiral patches were seen on the lateral side of the upper arm. The lesion was well-circumscribed with a slightly raised border, and no ulcerations or erosions (Figure 1A and B).
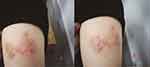 |
Figure 1 Clinical presentation of the patient on admission (A and B) dark red, linear, spiral patches on the lateral side of the upper arm). |
Laboratory testing showed completely normal humoral and cellular immune function. A blood test revealed a white blood cell count of 7.10 × 109/L (reference range: 4.0–10.0 × 109/L), eosinophil count of 1.3 × 109/L (reference range: 0.02–0.35 × 109/L), and eosinophil percentage of 18.5% (reference range: 0.005–0.050%). Her routine urine test was normal. At the same time, we performed a dermoscopy examination of the patient’s lesion, and found that linear, light-yellow, and dark red block area of unequal width was a tunnel burrow, formed in the stratum corneum by larva migration (Figure 2A). We also saw translucent round structures from the apex of the linear lesion, which had several high refractive dots internally, which might be the larval body or paws (Figure 2B).
 |
Figure 2 Dermoscopy images: (A) a tunnel with red arrows formed by larva migration. (B) high refractive dots with red arrows, which may be larval body or paws. |
The lesion was assessed by RCM which showed edema in the epidermis and superficial dermis, and a well-circumscribed, oval-shaped tunnel in the stratum corneum or granular layer, which could extend to the superficial dermis (Figure 3A). At one end of the tunnel, there was a well-delineated ovoid larval body and claw-like structures (Figure 3B).
Based on clinical presentation, dermoscopy, and RCM, the patient was finally diagnosed with CLM. The patient was treated with cryotherapy. Attention should be paid to the scope, time, depth, and frequency of cryotherapy, to avoid obvious scars, which could be formed by excessive treatment. Liquid nitrogen was applied to the patient’s both ends of the skin rash several times using a cotton swab. After 3 days, the linear rash continued to migrate, requiring extending cryotherapy to all lesions. Seven days later, the rash still spread to the proximal part of the left upper arm and left shoulder (Figure 4A and B). Thus, dermatoscopy and RCM were performed to accurately locate the position of the larvae in the skin lesions, which were then frozen. After 14 days, the patient’s pruritus significantly reduced, and the rash did not spread. Three weeks later, the lesion was completely cured, and the skin rash was completely resolved. At this time, a routine blood test showed, a white blood cell count of 8.18 × 109/L (reference range: 4.0–10.0 × 109/L), eosinophil count of 0.3 × 109/L (reference range: 0.02–0.35 × 109/L), and eosinophil percentage of 3.7% (reference range: 0.5–5.0%), and routine urine test was normal. No recurrence was noted after 2 months (Figure 5A and B).
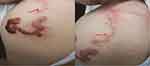 |
Figure 4 Clinical presentation of the patient after one week of the treatment (A and B) the rash still spread to the proximal part of the left upper arm and left shoulder with red arrows). |
 |
Figure 5 Clinical presentation of the patient after the treatment (A) after one month of the treatment. (B) after two months of the treatment). |
Discussion
CLM, or creeping eruption, is caused by various animal hookworm (helminths) species, such as Ancilostoma braziliense, A scaris suum, and Bunostomum phlebotum, which is acquired by direct contact with soil contaminated with dog or cat feces.4 Humans are infected through contact with animals, plants, water, or soil that has been contaminated by larvae, and most reports indicate that it is caused by hookworms.5,6 The larvae enter the local skin by releasing proteases. The typical skin lesions are non-specific dermatitis such as papules, pimples or erythema first appearing in the side of larva penetration. After a few days of latency, the larvae begin to dig forward, and gradually form linear erythema that is first bright red and then dark red, slightly elevating on the skin surface. It can move forward in a straight line or meander, spreading from several millimeters to several centimeters forward every day, which can cause local itching and burning pain during larval migration. Therefore, the diagnosis of this disease mainly depends on the characteristic clinical manifestations.7 However, due to the atypical skin lesions seen in some patients, they are easily misdiagnosed with papular urticaria, urticarial vasculitis, chronic migratory erythema, and tinea corporis.8 The application of emerging skin imaging technologies such as dermatoscopy and RCM has great value in diagnosing and differential diagnosis. Dermoscopy is a fast and convenient non-invasive means of examining the surface of the skin, overcoming the disadvantage of the naked eye that can not detect various morphological features, thereby improving the clinical diagnostic accuracy of various skin diseases.9 Thus, we examined the skin lesions by dermoscopy and found a linear pale yellow-dark red mass with varying widths, presumably a tunnel formed by larval migration. A round translucent structure was seen at the top of the lesion, which was considered a larval worm. In RCM, different structures in the skin are used to obtain different reflection coefficients for the laser. It can perform real-time and dynamic imaging of the epidermis and superficial dermis, and display different microstructures in the skin with different light and shade.10 Its scanning depth, from the surface of the skin to the dermal papilla and shallow layer of about 300 μm, has been used to assist in the diagnosis and evaluation of various skin diseases. However, there are few reports on the use of RCM in the diagnosis of creeping rash. We detected the patient’s skin lesions by RCM and found an oval tunnel with clear boundaries in the stratum corneum or granular layer. At one end of the tunnel, a well-defined oval, well-defined worm body and its claw-like structures were visible, and the larval position was accurately marked. Combined with the patient’s occupation, nature of work, and typical skin lesions, the diagnosis of creeping rash was established.
The treatment of creeping rash mainly consists of physical therapy, such as liquid nitrogen freezing, surgical treatment, and laser treatment, and drug deworming, such as albendazole and thibendazole. Since larval tunneling is limited to the epidermis and mostly stays near the end of the lesion, liquid nitrogen treatment mainly freezes both ends of the lesion. In this case, we first treated the two ends of the frozen lesion, but the lesion continued to expand, without excluding the possibility of the larvae moving back and forth in the lesion. Thus, we expanded the frozen range to all lesions, but the lesion continued to spread to the proximal part of the arm and shoulder. For more accurate treatment, we used dermoscopy and RCM detection to accurately locate and mark the larvae, and then cryotherapy was performed. After 14 days, the itching was relieved, the sense of something crawling on the skin disappeared, and the rash did not continue to spread. There was no recurrence of the rash after 2 months of follow-up.
Conclusion
This was a rarely reported case of using dermoscopy and RCM to diagnose and treat CLM, which have high diagnostic accuracy, allowing us to achieve an obvious therapeutic effect. Thus, dermatoscopy and RCM have great significance for the diagnosis and treatment of prurigo and provide a new approach to clinical diagnosis and treatment.
Ethics Statement
The patient in this manuscript provided written informed consent to the publication of case details and accompanying images.
Acknowledgments
We would like to thank the patient and physicians for participating in our study.
Funding
This project was supported by Hainan Province Clinical Medical Center, and the Science and Technology Program of Guangzhou, China (item number_202102020766).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yap FB. Creeping eruption. Int J Infect Dis. 2010;14(6):e545. doi:10.1016/j.ijid.2009.07.006
2. Stufano A, Foti C, Lovreglio P, et al. Occupational risk of cutaneous larva migrans: a case report and a systematic literature review. PLoS Negl Trop Dis. 2022;16(5):e0010330. doi:10.1371/journal.pntd.0010330
3. Campoli M, Cortonesi G, Tognetti L, Rubegni P, Cinotti E. Noninvasive imaging techniques for the diagnosis of cutaneous larva migrans. Skin Res Technol. 2022;28(2):374–376. doi:10.1111/srt.13126
4. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216(1):14–23. doi:10.1159/000109353
5. Hla Aye MT, Kyaw AY, Rubel AR, Han MB, Mani BI, Chong VH. Cutaneous larva migrans. QJM. 2022;115:1–2. doi:10.1093/qjmed/hcac193
6. Purdy KS, Langley RG, Webb AN, Walsh N, Haldane D. Cutaneous larva migrans. Lancet. 2011;377(9781):1948. doi:10.1016/S0140-6736(10)61149-X
7. Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129(5):588–591. doi:10.1001/archderm.129.5.588
8. Dhanaraj M, Ramalingam M. Cutaneous larva migrans masquerading as tinea corporis: a case report. J Clin Diagn Res. 2013;7(10):2313. doi:10.7860/JCDR/2013/5359.3510
9. Micali G, Lacarrubba F, Massimino D, Schwartz RA. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64(6):1135–1146. doi:10.1016/j.jaad.2010.03.010
10. Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49(1):7–19. doi:10.1002/lsm.22600
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

