Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Reevaluation of the Efficacy of First Line Regimen for Helicobacter pylori
Authors Tariq H, Patel H , Kamal MU , Abbas N , Ameen M , Azam S, Kumar K, Ravi M, Vootla V, Shaikh D , Amanchi V, Hussain AN, Makker J
Received 20 November 2019
Accepted for publication 7 January 2020
Published 22 January 2020 Volume 2020:13 Pages 25—33
DOI https://doi.org/10.2147/CEG.S239343
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Wing-Kin Syn
Hassan Tariq, 1, 2 Harish Patel, 1, 2 Muhammad Umar Kamal, 1 Naeem Abbas, 1, 2 Muhammad Ameen, 1 Sara Azam, 1 Kishore Kumar, 1, 2 Madhavi Ravi, 1, 2 Vamshidhar Vootla, 1, 2 Danial Shaikh, 1, 2 Vamsi Amanchi, 1 Ali N Hussain, 3 Jasbir Makker 1, 2
1Department of Medicine, BronxCare Health System, Bronx, NY 10457, USA; 2Division of Gastroenterology, BronxCare Health System, Bronx, NY 10457, USA; 3Baruch College, City University of New York (CUNY), New York, NY 10010, USA
Correspondence: Muhammad Umar Kamal
BronxCare Health System, 1650 Selwyn Ave, Suite #10C, Bronx, NY 10457, USA
Tel +1 718-960-1234
Fax +1 718-960-2055
Email [email protected]
Background: Helicobacter pylori is a common cause of gastritis, peptic ulcer disease, and non-ulcer dyspepsia, and is also associated with gastric adenocarcinoma and mucosa associated lymphoid tissue lymphoma. Despite being known about for more than 30 years, finding an effective therapeutic strategy against it remains a challenge.
Aim: There are no US studies evaluating the efficacy of a Levofloxacin based therapy for H. pylori infection. We here intend to study the efficacy of Levofloxacin based triple antibiotic regimen as compared to Clarithromycin based triple therapy and Bismuth based quadruple therapy in our patient population.
Methods: This is a retrospective single center observational study. Patients with Helicobacter pylori infection who underwent treatment for H. pylori with one of the three therapies, i.e. Clarithromycin triple, Bismuth Quadruple or Levofloxacin triple, were included in the study and the eradication rates were compared. The confirmation of the H. pylori was done 4 weeks after the completion of anti-microbial therapy.
Results: A total of 177 individuals underwent the H. pylori treatment in our retrospective review. Of these, 54% (n=97) of patients were treated with Clarithromycin based triple therapy (Group 1), 35% (n=63) were treated with Levofloxacin based regimen (Group 2), and the remaining 11% (n=17) were treated with Bismuth based quadruple therapy (Group 3). The eradication rates were significantly higher in patients treated with Clarithromycin based triple therapy as compared to Levofloxacin based triple therapy and Bismuth quadruple therapy (78.3% vs 49.2% vs 41.1% P=0.001).
Conclusion: In conclusion, our study shows significantly lower eradication rates with Levofloxacin triple therapy among a selected US population. Thus, it may not be a good first-line therapy among this US population and the Clarithromycin based regimen may still be used successfully.
Keywords: Helicobacter pylori, clarithromycin, levofloxacin, quadruple therapy, peptic ulcer
Introduction
Helicobacter pylori (H. pylori) is a common cause of gastritis, peptic ulcer disease, and non-ulcer dyspepsia, and is also associated with gastric adenocarcinoma and mucosa associated lymphoid tissue lymphoma.1,3 Despite being known for more than 30 years, finding an effective therapeutic strategy against it remains a challenge. Given the high morbidity associated with this disease, it is of paramount importance to utilize the most effective and potent eradication regimen.2,4
The seroprevalence of H. pylori is about 80% in developing countries and 20–50% in developed countries.5 It commonly infects older people, and Mexican Americans have the highest prevalence among all ethnic groups.2
Treatment of H. pylori and its eradication is strongly recommended due to the serious complications associated with untreated H. pylori infection.6 Several treatment regimens have been utilized thus far for its eradication. A Clarithromycin (CLA) based regimen to treat H. pylori has been utilized for several decades and is one of the commonly utilized first line therapies. The efficacy of the CLA based therapy is affected by several factors, including patient’s adherence, areas of bacterial colonization, host CYP2C19 polymorphisms, gastric acid secretion, and, most importantly, H. pylori resistance to CLA.7,8 Pre-treatment antimicrobial susceptibility for the antibiotic therapy has shown better H. pylori eradication rates, but it is not yet the standard of care.9
CLA based therapies are currently recommended in both the US and Europe, due to adequate eradication rates of 75–80%,4,10,11 but recently increasing CLA resistance in western countries, estimated to be around 32% in North America and 22% in Europe, is alarming.12 A 25–30% CLA resistance significantly reduces the eradication rates.13,14 In a study from the US, Park et al15 reported H. pylori CLA resistance in excess of 20%, resulting in significantly higher rates of treatment failure. In the US, it is advised to utilize the regional H. pylori antibiotic resistance data to guide the selection of empiric first line therapy for H. pylori. Due to this heightened concern for CLA based therapy failure, it is recommended to use Bismuth quadruple or non-Bismuth quadruple, concomitant (Proton pump inhibitor, Amoxicillin, CLA and a nitroimidazole) therapies or Levofloxacin (LEVO) based regimens where CLA resistance is more than 15%.16
The efficacy of LEVO-based regimens as salvage therapy after failure of the first line therapy has been shown before.17,18 Increased efficacy of LEVO has been demonstrated in countries out of the US.19,21 A study conducted in Italy demonstrated superior eradication rates with LEVO-based therapy as compared with CLA containing therapy in areas with high prevalence of CLA-resistant but low prevalence of LEVO resistant H pylori strains.22 There are no US studies, evaluating the efficacy of a LEVO-based therapy for H. pylori infection. We here intend to study the efficacy of a LEVO based triple antibiotic regimen as compared to CLA based triple antibiotic therapy in our patient population.
Methods
This is a retrospective single center observational study. The period of study was 12 months between July 1st, 2017 and June 30th, 2018. The study is performed in accordance to the Declaration of Helsinki and is approved by the Institution Review Board (IRB) of Bronx Care Hospital Center. Due to the retrospective design of the study, patient consent to review their medical records was not required by the IRB. Patient data confidentiality was maintained at each and every step.
Patient Selection
The data was collected from the electronic medical records of patients and tabulated in Microsoft Excel® (Microsoft Corp, Redmond, WA, USA). Patients ≥18 years of age who presented to our gastroenterology clinics, who were diagnosed with Helicobacter pylori infection, had no prior history of treatment for Helicobacter pylori treatment, no recent antibiotic exposure within the preceding 8 weeks, and underwent treatment for H. pylori with one of the three therapies (CLA triple, Bismuth Quadruple, or LEVO triple) were included in the study.
Patients who did not complete antibiotic therapy and did not undergo a follow up testing after completion of antibiotics were excluded from the study. The prescribing physician at the index follow-up confirmed the antibiotics compliance.
A total of 177 patients were finally included and divided into three study groups based on the antibiotic regimen utilized. We also collected data on the method of diagnosing Helicobacter pylori, age, gender, comorbidities including diabetes mellitus, hypertension, and smoking status. If an upper gastrointestinal endoscopy was performed, the results of the endoscopy were reviewed and recorded, especially the presence of peptic ulcer disease.
Study Groups
In keeping with the primary objective of the study to compare the efficacy of H. pylori eradication rates among three first line therapies, patients were divided into three groups.
Group1: CLA triple therapy: Proton pump inhibitor + CLA 500 mg twice daily + Amoxicillin 1 g twice daily for 14 days.
Group 2: LEVO triple therapy: Proton pump inhibitor + LEVO 500 mg once daily + Amoxicillin 1 g twice daily for 14 days.
Group 3: Bismuth Quadruple therapy: Proton pump inhibitor + Bismuth Subsalicylate 300 mg four times daily + Tetracycline 500 mg four times daily + Metronidazole 500 mg three to four times daily for 14 days.
Diagnosis of Helicobacter pylori Infection
Patients were diagnosed with H. pylori infection by one of the following modalities:
- Upper GI endoscopy with either biopsy showing H. pylori or Campylobacter-like organism (CLO) test positive using Kimberly-Clark® kit or both.
- H. Pylori fecal antigen testing by enzyme immunoassay technique.
- Urea breath test by BreathTek®.
Confirmation of Helicobacter pylori Eradication
The confirmation of the H. pylori eradication was done 4 weeks after the completion of anti-microbial therapy. Fecal H. pylori antigen testing was the preferred modality. If the patient underwent interval follow-up gastroscopy, antral biopsy for the pathology and the CLO were performed. Two weeks of the proton pump inhibitor (PPI) abstinence was observed prior to the evaluation for H. pylori eradication.
Statistical Methods
Statistical analysis was performed with IBM SPSS version 1.0.0–2740 (Statistical Packages for the Social Sciences). Frequencies and percentages were reported for categorical variables. Mean and standard deviations were reported for numerical continuous variables. Dichotomous variables were compared by Chi-square analysis using the Pearson test. Logistic regression analysis was used to find statistically significant predictors of H. pylori eradication therapy failure. A two-tailed value of <0.05 was considered statistically significant.
Results
A total of 177 individuals underwent the H. pylori treatment in our retrospective review. A majority, 54% (n=97), of patients were treated with the CLA based triple therapy (Group 1). Thirty-five percent (n=63) were treated with the LEVO based regimen (Group 2) and the remaining 11% (n=17) were treated with the Bismuth based Quadruple therapy (Group 3). The choice of antibiotic regimen was guided by several factors including drug interactions with other concurrent medications, patient preference, history of active alcohol use, and baseline QTc interval on the electrocardiogram. The CLA regimen was not favored in the setting of significant CYP3A based medication interaction.23 In patients with significant alcohol use, the Metronidazole based regimen was not selected due to the risk of a disulfiram like reaction and, in patients with prolonged QTc, the LEVO regimen was not prescribed.
There was no statistically significant difference in the demographic and the co-morbidities of the patient population across three groups (Table 1). The mean ages of individuals in Groups 1, 2, and 3 were 52.5 years, 50.4 years, and 56.8 years, with the female gender predominance accounting for 68%, 60%, and 75%, respectively. The patients with hypertension (59%) and diabetes mellitus (41%) were more prevalent in Group 3, but the difference, as compared to the other groups, was not significant. Active cigarette smokers were equally distributed in all three groups. Upper Gastrointestinal Endoscopy was the predominant modality utilized for H. pylori diagnosis. The gastric biopsy histopathological findings were also compared for all three groups. The lymphoid follicle finding was the one most commonly seen; however, there was no statistical significance among the three groups compared.
 |
Table 1 Baseline Demographics, Comorbidities and Methods of Helicobacter pylori Diagnosis in the Study Population |
The eradication rates were significantly higher in patients treated with CLA based triple therapy as compared to LEVO based triple therapy and Bismuth quadruple therapy (78.3% vs 49.2% vs 41.1% p=0.001). A total of 87.6% (n=155) of patients underwent an upper GI endoscopy, from which 20% (n=31) were noted to have an ulcer. Among patients with gastric ulcers on endoscopy, CLA based triple therapy had similar eradication rates as compared to LEVO based triple therapy, but higher than Bismuth quadruple therapy (75% vs 75% vs 0%, P=0.001) (Table 2).
 |
Table 2 Eradication Rates of Various Therapies |
We did a logistic regression analysis to evaluate the effect of demographics and various comorbidities on the success of various H. pylori eradication therapies. Age (OR=1.006, p=0.669), gender (OR=0.849, p=0.638), hypertension (OR=0.751, P=0.475), diabetes mellitus (OR=1.372, p=0.430), obesity (OR=0.854, p=0.616), smoking (OR=1.918, P=0.146), and peptic ulcer disease (OR=0.941, p=0.888) were not associated with failure of treatment. There was also no effect of various gastric histological changes on H. pylori eradication rates (Table 3).
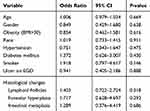 |
Table 3 Logistic Regression Analysis of Factors Associated with Successful Helicobacter pylori Eradication |
A total of 63 patients did not achieve successful eradication of H. pylori after the first antibiotic course. Among these, 33 patients underwent second therapy for H. pylori eradication. Successful eradication rates were higher in patients undergoing Bismuth quadruple therapy as compared to LEVO based triple therapy (57.9% vs 35.7%, P=0.208), although there was no significant difference that we attribute to the small sample size in the subgroup analysis.
We combined the total sum of therapies (first and second therapies in the three groups) and analyzed the overall eradication rates of all three therapies. CLA based triple therapy, as compared to LEVO based triple therapy and Bismuth quadruple therapy, had higher eradication rates (78.3% vs 46.7% vs 50%, P=0.001). Interestingly the overall eradication rate was higher for Bismuth quadruple therapy as compared to LEVO based triple therapy (Table 4).
 |
Table 4 Efficacy of Second Therapy in Patients Who Failed Their First Therapy |
We evaluated the factors associated with failure of second therapies in our patient population. Patients had a higher rate of second treatment success if they were initially treated with Bismuth quadruple therapy as compared to CLA triple therapy or LEVO triple therapy (71.4% vs 40% vs 45.5%, P=0.378) although there was no significant difference that we attribute to the small sample size in the subgroup analysis.
There was no effect of age, gender, hypertension, diabetes mellitus, or smoking status on the eradication rates of the second therapy (Table 5).
 |
Table 5 Logistic Regression Analysis Evaluating Factors Associated with Successful Second Therapy |
Discussion
Helicobacter pylori infection is a known risk factor responsible for the development of gastric ulcers and gastric carcinoma. It is reported in several studies that eradication of H. pylori infection lowers the risk of peptic ulcer recurrence and further decreases the risk of gastric cancer.24 It is also seen that delaying H. pylori eradication enhances the risk of peptic ulcer recurrence and gastric malignancies.25 Thus, it is prudent to treat and eradicate H. pylori infection promptly and appropriately to avoid peptic ulcers and ulcer-associated adverse events.
H. pylori was first discovered by Robin Warren in 1979 and first cultured by Barry Marshall in 1982. Reports of the first antibiotic treatment of H. pylori date to 1981, when it was treated with Tetracycline. Several other antibiotics were eventually tried alone or in combination but there was no consensus among physicians on the best treatment strategy.26 It was not until 1994 that the National Institute of Health brought together various experts in the field to form a consensus for H. pylori management.27 Nevertheless, considerable confusion regarding management of H. pylori persisted until the European Helicobacter pylori Study Group (EHPSG) met in Maastricht, Netherlands and formulated the European consensus on H. pylori management.30
Standard triple antibiotic therapy including CLA, Amoxicillin and a Proton pump inhibitor (PPI) has been used worldwide very commonly since this first Maastricht conference.28 Due to the increasing resistance of H. pylori infection to the commonly used CLA-based regimen, the initially set standard of achieving at least an 80% eradication rate has not been easy. Later studies have also shown that eradication rates have declined to 71% in the US and 60% in Europe.6,29 Therefore, it has been recommended to perform CLA sensitivity in populations with suspected CLA resistance prevalence of 15–20%.4,30
In recent years it has been demonstrated that the LEVO-based regimen can be used as an alternative agent for the CLA-based regimen in areas of high CLA resistance. The use of LEVO as a first-line therapy has also been studied in many trials. The eradication rates of LEVO-based triple therapy have been reported to be in the range of 72–96%.31 Interestingly all these studies have been conducted outside the US, with most being conducted in European countries.32 Cammarota et al33 conducted the first prospective trial studying the efficacy of LEVO-based triple therapy as a first-line therapy in H. pylori management. They divided one hundred patients into two groups based on Amoxicillin or tinidazole along with PPI and LEVO. It was noted that both groups achieved high eradication rates with 92% and 90% in intention to treat analysis, respectively. A German randomized trial compared LEVO, Amoxicillin, and Esomeprazole triple therapy with standard triple therapy, and found an eradication rate of 87% with LEVO triple therapy versus 84% following standard triple therapy.34 Gisbert et al35,36 conducted two prospective trials in Spain with 64 and 75 patients using LEVO-based first line therapy along with PPI or ranitidine, respectively. They reported eradication rates of 84% and 83%, respectively, in these two studies with good tolerability. A study from the Netherlands using LEVO-containing triple therapies together with Amoxicillin or CLA as a first-line against H. pylori showed high eradication rates of 96% and 93%, respectively.37 Based on the prior European studies showing high success rates with LEVO-based therapy, the American College of Gastroenterology guidelines recommended it as an option for the first line treatment of H. Pylori.38 To date there have been no studies from the US evaluating the efficacy of LEVO-based triple antibiotic therapy and comparing it with other regimens including CLA triple therapy and Bismuth quadruple therapy.39 Also, the available information from North America about H. pylori antibiotic resistance patterns is very limited.39
Our study illustrated significantly higher eradication rates among patients treated with CLA-based triple therapy when compared with LEVO-based triple therapy and Bismuth quadruple therapy (78.3% vs 49.2% vs 41.1%, P=0.001). A large multicenter trial from six Latin American countries, enrolling 1,463, reported that 2-week CLA-based triple therapy yielded a higher eradication rate of 82.2%.40 This is consistent with our study showing higher efficacy with CLA-based therapy. In 2007, Graham et al4 described the scoring system to rate the efficacy of H. pylori therapies. Therapies with 85% and above cure rates were described as acceptable therapies.41 In our study, even though CLA-based therapy outperformed the other two antibiotic regimens, none of the three tested antibiotic regimens achieved the acceptable outcome score described by Graham et al, nor did they achieved the first set standard of 80% eradication rate in Maastricht I. These results are possibly due to the higher prevalence of antibiotic resistant H. pylori strains in our patient population, but we lack the antibiotic resistance information in our patients due to sensitivity test not performed. It is possible to determine the resistance of H. pylori against antibiotics using molecular techniques and culturing the tissue specimen. However, it is not the widely utilized in the US.39
LEVO-based triple therapy has also been explored as a salvage therapy after failure with commonly used first line therapies and has been found by some as a useful second line treatment option for resistant H. pylori cases.42 On the contrary, in our study we observed lower eradication rates with LEVO being utilized as a second line salvage therapy. Patients who failed the initial therapy and then underwent treatment with second therapy for H. pylori with Bismuth quadruple therapy and LEVO-based triple therapy, successful eradication rates were higher in patients undergoing Bismuth quadruple therapy as compared to LEVO-based triple therapy (57.9% vs 35.7%, P=0.208). This subgroup analysis was not statistically significant, which we attribute to the small sample size.
The poor results in the LEVO-based therapy treatment group can be attributed to increasing LEVO resistance which may have reduced the efficacy of LEVO-based therapy. There is an increasing incidence of LEVO-resistant H. pylori strains in different regions of the world. This is especially true for regions where quinolones consumption is on the higher side. LEVO-resistant H. pylori strains are estimated to be around 30.3% in China,43 5% in Poland, and 5.3% in Iran.44 Yamade et al45 interestingly reported that a significantly higher number of CLA-resistant strains of H. pylori also had higher rates of quinolone resistance, particularly in patients with previous eradication failure. They included 153 patients and, overall, 55.6% of individuals had CLA-resistant strains and 38.6% had strains resistant to quinolones. A study from Colombia also reported increasing resistance, from 11.8% in 2009 to 27.3% in 2014, which was mediated by the gyrA point mutation, and N87I mutation was the most common related to LEVO resistance.46 Perna et al47 reported that eradication rates decreased significantly from 75% in LEVO-sensitive patients to 33.3% in patients who had LEVO-resistant H. pylori strains. It seems that in our patient population there is a high prevalence of quinolones resistant H. pylori strains, which led to poor treatment outcomes in the LEVO-based therapy group as compared to the CLA-based therapy group.
LEVO resistance has been reported to be higher in older patients as compared to young individuals. This difference between the older and younger population was 11.7% in Ireland and 19.1% in Italy.48,49 Other studies with Bismuth quadruple therapy have reported similar results of lower H. pylori eradication rates with increasing age.51 Some researchers have also reported lower eradication rates in females.52 In our study, eradication rates were not affected by age or gender. We also analyzed the effect of other variables including BMI and histological changes on the H. pylori eradication rates. Georgopoulos et al50 have reported that the absence of lymphoid follicles in stomach biopsies and the presence of antral and body gastritis are significantly associated with higher H. pylori eradication rates. In our analysis, the presence of lymphoid follicles did not affect the eradication rates. The association between obesity and H. pylori eradication rates is controversial.51 Studies have reported both protective and adverse effects of obesity on H. Pylori cure rates.52,53 Our study did not show any significant effect of obesity on the eradication rates.
The results of our study for the first time demonstrate the dismal cure rates associated with the LEVO-based triple antibiotic regimen in a US population. LEVO-based therapy not only performed poorly as a salvage therapy, but also performed significantly below par as a first line therapy. These results underscore the inferiority of LEVO-based triple therapy in our patient population as compared to legacy CLA triple therapy. Though our results pertain to a US population, these cannot be generalized to other US individuals infected with H. pylori without the results being validated in further larger studies involving other ethnic groups. Nevertheless, we hope that our study will serve as the first step among US researchers to explore the role of LEVO-based therapy in the US population. The LEVO-based regimen may still have a place as a last resort in patients where side-effects to other antibiotic regimens limit tolerability.
Our study does have certain limitations. First and foremost, it includes the inherent limitations related to the retrospective design of our study. Secondly, we did not perform antimicrobial sensitivity before treating the patients as it is not a common clinical practice. But still our results indicate towards the prevalence of high resistance patterns in a US population. Lastly, patient compliance was checked by calling their pharmacy to confirm the medication delivery and by inquiring the patients, but we did not perform a pill count.
Conclusion
In conclusion, our study shows a poor H. pylori eradication rate of 49% with LEVO-containing triple therapy, arguing against its use as a first-line therapy. Our results also showed that the standard triple therapy with CLA may still be used successfully in our population. Clinicians must follow their local antibiogram to assess resistance patterns before selecting an optimal therapy for H. pylori.
Abbreviations
H. pylori, Helicobacter pylori; CLA, Clarithromycin; USA, United States of America; LEVO, Levofloxacin.
Data Sharing Statement
Data are available from the authors upon reasonable request of the editors and reviewers.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi:10.1172/JCI20925
2. Kamboj AK, Cotter TG, Oxentenko AS. Helicobacter pylori: the past, present, and future in management. Mayo Clin Proc. 2017;92:599–604. doi:10.1016/j.mayocp.2016.11.017
3. Romano M, Ricci V, Zarrilli R. Mechanisms of disease: helicobacter pylori-related gastric carcinogenesis—implications for chemoprevention. Nat Rev Gastroenterol Hepatol. 2006;3:622.
4. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–278. doi:10.1111/hel.2007.12.issue-4
5. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi:10.1056/NEJMra020542
6. Malfertheiner P, Bazzoli F, Delchier J. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, Phase 3 trial (vol 377, pg 905, 2011). Lancet. 2011;378:1778.
7. Zhao F, Wang J, Yang Y, et al. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor‐based triple therapy for Helicobacter pylori eradication: a meta‐analysis. Helicobacter. 2008;13:532–541. doi:10.1111/hel.2008.13.issue-6
8. Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi:10.1136/gutjnl-2012-302254
9. Cosme A, Montes M, Ibarra B, et al. Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World j Gastroenterol. 2017;23:3367–3373. doi:10.3748/wjg.v23.i18.3367
10. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2008;5:321.
11. Zagari RM, Bianchi-Porro G, Fiocca R, Gasbarrini G, Roda E, Bazzoli F. Comparison of 1 and 2 weeks of omeprazole, amoxicillin and clarithromycin treatment for Helicobacter pylori eradication: the HYPER Study. Gut. 2007;56:475–479. doi:10.1136/gut.2006.102269
12. Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in Helicobacter pylori: a recent literature review. World J Methodol. 2015;5:164–174. doi:10.5662/wjm.v5.i3.164
13. Fischbach L, Evans E. Meta‐analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first‐line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi:10.1111/apt.2007.26.issue-3
14. Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi:10.1136/gut.2006.101634
15. Park JY, Dunbar KB, Mitui M, et al. Helicobacter pylori clarithromycin resistance and treatment failure are common in the USA. Dig Dis Sci. 2016;61:2373–2380. doi:10.1007/s10620-016-4091-8
16. Malfertheiner P, Megraud F, O’morain C, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66:6–30.
17. Nista EC, Candelli M, Zocco MA, et al. Levofloxacin-based triple therapy in first-line treatment for Helicobacter pylori eradication. Am J Gastroenterol. 2006;101:1985. doi:10.1111/j.1572-0241.2006.00716.x
18. Gisbert J, De La Morena F. Systematic review and meta‐analysis: levofloxacin‐based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35–44. doi:10.1111/apt.2006.23.issue-1
19. Kahramanoglu Aksoy E, Pirincci Sapmaz F, Goktas Z, Uzman M, Nazligul Y. Comparison of Helicobacter pylori eradication rates of 2-week levofloxacin-containing triple therapy, levofloxacin-containing bismuth quadruple therapy, and standard bismuth quadruple therapy as a first-line regimen. Med Princ Pract. 2017;26:523–529. doi:10.1159/000484930
20. Su J, Zhou X, Chen H, Hao B, Zhang W, Zhang G. Efficacy of 1st-line bismuth-containing quadruple therapies with levofloxacin or clarithromycin for the eradication of Helicobacter pylori infection: a 1-week, open-label, randomized trial. Medicine. 2017;96:e5859. doi:10.1097/MD.0000000000005859
21. Yee Y, Cheung T, Chu KM, et al. Clinical trial: levofloxacin‐based quadruple therapy was inferior to traditional quadruple therapy in the treatment of resistant Helicobacter pylori infection. Aliment Pharmacol Ther. 2007;26:1063–1067. doi:10.1111/j.1365-2036.2007.03452.x
22. Romano M, Cuomo A, Gravina AG, et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010;59:1465–1470. doi:10.1136/gut.2010.215350
23. Zhou Q, Zhu LL, Yan XF, Pan WS, Zeng S. Drug utilization of clarithromycin for gastrointestinal disease treatment. World j Gastroenterol. 2008;14:6065–6071. doi:10.3748/wjg.14.6065
24. Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi:10.1136/bmj.g3174
25. Sverden E, Brusselaers N, Wahlin K, Lagergren J. Time latencies of Helicobacter pylori eradication after peptic ulcer and risk of recurrent ulcer, ulcer adverse events, and gastric cancer: a population-based cohort study. Gastrointest Endosc. 2018;88:242–250.e1. doi:10.1016/j.gie.2017.11.035
26. Pajares JM, Gisbert JP. Helicobacter pylori: its discovery and relevance for medicine. Rev Esp Enferm Dig. 2006;98:770–785. doi:10.4321/s1130-01082006001000007
27. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. doi:10.1001/jama.272.1.65
28. Malfertheiner P, Megraud F, O’morain C, et al. Current European concepts in the management of Helicobacter pylori infection-the Maastricht Consensus Report. Eur J Gastroenterol Hepatol. 1997;9:1–2. doi:10.1097/00042737-199701000-00002
29. Gisbert JP, Calvet X, O’Connor A, Megraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 2010;44:313–325. doi:10.1097/MCG.0b013e3181c8a1a3
30. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi:10.1136/gut.2009.192757
31. Seven G, Cinar K, Yakut M, Idilman R, Ozden A. Assessment of Helicobacter pylori eradication rate of triple combination therapy containing levofloxacin. Turkish J Gastroenterol. 2011;22:582–586. doi:10.4318/tjg.2011
32. Telaku S, Manxhuka-Kerliu S, Kraja B, Qirjako G, Prifti S, Fejza H. The efficacy of levofloxacin-based triple therapy for first-line helicobacter pylori eradication. Med Archiv. 2013;67:348–350. doi:10.5455/medarh.2013.67.348-350
33. Cammarota G, Cianci R, Cannizzaro O, et al. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:1339–1343. doi:10.1046/j.1365-2036.2000.00846.x
34. Antos D, Schneider‐Brachert W, Bästlein E, et al. 7‐day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high‐dose esomeprazole in patients with known antimicrobial sensitivity. Helicobacter. 2006;11:39–45. doi:10.1111/j.0083-8703.2006.00375.x
35. Gisbert J, Fernandez‐Bermejo M, Molina‐Infante J, et al. First‐line triple therapy with levofloxacin for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2007;26:495–500. doi:10.1111/j.1365-2036.2007.03384.x
36. Gisbert JP, Bermejo MF, Infante JM, et al. Levofloxacin, amoxicillin, and omeprazole as first-line triple therapy for Helicobacter pylori eradication. J Clin Gastroenterol. 2009;43:384–385. doi:10.1097/MCG.0b013e31816d921c
37. Schrauwen R, Janssen M, De Boer W. Seven-day PPI-triple therapy with levofloxacin is very effective for Helicobacter pylori eradication. Neth J Med. 2009;67:96–101.
38. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212. doi:10.1038/ajg.2016.563
39. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi:10.1038/ajg.2016.563
40. Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378:507–514. doi:10.1016/S0140-6736(11)60825-8
41. Fraser A, Delaney BC, Ford AC, Qume M, Moayyedi P. The Short-Form Leeds Dyspepsia Questionnaire validation study. Aliment Pharmacol Ther. 2007;25:477–486. doi:10.1111/j.1365-2036.2006.03233.x
42. Gisbert JP, Perez-Aisa A, Bermejo F, et al. Second-line therapy with levofloxacin after failure of treatment to eradicate helicobacter pylori infection: time trends in a Spanish Multicenter Study of 1000 patients. J Clin Gastroenterol. 2013;47:130–135. doi:10.1097/MCG.0b013e318254ebdd
43. Liao J, Zheng Q, Liang X, et al. Effect of fluoroquinolone resistance on 14‐day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter. 2013;18:373–377. doi:10.1111/hel.2013.18.issue-5
44. Abadi ATB, Ghasemzadeh A, Taghvaei T, Mobarez AM. Primary resistance of Helicobacter pylori to levofloxacin and moxifloxacine in Iran. Intern Emerg Med. 2012;7:447–452. doi:10.1007/s11739-011-0563-1
45. Yamade M, Sugimoto M, Uotani T, Nishino M, Kodaira C, Furuta T. Resistance of Helicobacter pylori to quinolones and clarithromycin assessed by genetic testing in Japan. J Gastroenterol Hepatol. 2011;26:1457–1461. doi:10.1111/j.1440-1746.2011.06815.x
46. Trespalacios-Rangél AA, Otero W, Arévalo-Galvis A, Poutou-Piñales RA, Rimbara E, Graham DY. Surveillance of levofloxacin resistance in Helicobacter pylori isolates in Bogotá-Colombia (2009-2014). PLoS One. 2016;11:e0160007. doi:10.1371/journal.pone.0160007
47. Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Digest Liver Dis. 2007;39:1001–1005. doi:10.1016/j.dld.2007.06.016
48. O’Connor A, Taneike I, Nami A, et al. Helicobacter pylori resistance rates for levofloxacin, tetracycline and rifabutin among Irish isolates at a reference centre. Ir J Med Sci. 2013;182:693–695. doi:10.1007/s11845-013-0957-3
49. Zullo A, Perna F, Hassan C, et al. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment Pharmacol Ther. 2007;25:1429–1434. doi:10.1111/(ISSN)1365-2036
50. Georgopoulos SD, Ladas SD, Karatapanis S, et al. Factors that may affect treatment outcome of triple Helicobacter pylori eradication therapy with omeprazole, amoxicillin, and clarithromycin. Dig Dis Sci. 2000;45:63–67. doi:10.1023/A:1005405209503
51. Carabotti M, D’Ercole C, Iossa A, Corazziari E, Silecchia G, Severi C. Helicobacter pylori infection in obesity and its clinical outcome after bariatric surgery. World j Gastroenterol. 2014;20:647–653. doi:10.3748/wjg.v20.i3.647
52. Osawa H. Ghrelin and Helicobacter pylori infection. World j Gastroenterol. 2008;14:6327–6333. doi:10.3748/wjg.14.6327
53. Arslan E, Atilgan H, Yavasoglu I. The prevalence of Helicobacter pylori in obese subjects. Eur J Intern Med. 2009;20:695–697. doi:10.1016/j.ejim.2009.07.013
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
