Back to Journals » Journal of Pain Research » Volume 13
Reducing Opioid Prescriptions by Identifying Responders on Topical Analgesic Treatment Using an Individualized Medicine and Predictive Analytics Approach
Authors Gudin J, Mavroudi S , Korfiati A, Theofilatos K , Dietze D , Hurwitz P
Received 18 January 2020
Accepted for publication 9 May 2020
Published 28 May 2020 Volume 2020:13 Pages 1255—1266
DOI https://doi.org/10.2147/JPR.S246503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Jeffrey Gudin,1 Seferina Mavroudi,2,3 Aigli Korfiati,3 Konstantinos Theofilatos,3 Derek Dietze,4 Peter Hurwitz5
1Rutgers New Jersey Medical School, Newark, NJ, USA; 2Department of Nursing, School of Health Rehabilitation Sciences, University of Patras, Pátrai, Greece; 3InSyBio Ltd, Winchester, UK; 4Metrics for Learning LLC, Queen Creek, Arizona, USA; 5Clarity Science LLC, Narragansett, Rhode Island, USA
Correspondence: Peter Hurwitz
Clarity Science LLC, 750 Boston Neck Road, Suite 11, Narragansett, RI 02882, USA
Tel +1917 757 0521
Fax +1855-891-8303
Email [email protected]
Purpose: Chronic pain is a life changing condition, and non-opioid treatments have been lately introduced to overcome the addictive nature of opioid therapies and their side effects. In the present study, we explore the potential of machine learning methods to discriminate chronic pain patients into ones who will benefit from such a treatment and ones who will not, aiming to personalize their treatment.
Patients and Methods: In the current study, data from the OPERA study were used, with 631 chronic pain patients answering the Brief Pain Inventory (BPI) validated questionnaire along with supplemental questions before and after a follow-up period. A novel machine learning approach combining multi-objective optimization and support vector regression was used to build prediction models which can predict, using responses in the baseline, the four different outcomes of the study: total drugs change, total interference change, total severity change, and total complaints change. Data were split to training (504 patients) and testing (127 patients) sets and all results are measured on the independent test set.
Results: The machine learning models extracted in the present study significantly overcame other state of the art machine learning methods which were deployed for comparative purposes. The experimental results indicated that the machine learning models can predict the outcomes of this study with considerably high accuracy (AUC 73.8– 87.2%) and this allowed their incorporation in a decision support system for the selection of the treatment of chronic pain patients.
Conclusion: Results of this study revealed the potential of machine learning for an individualized medicine application for chronic pain therapies. Topical analgesics treatment were proven to be, in general, beneficial but carefully selecting with the suggested individualized medicine decision support system was able to decrease by approximately 10% the patients which would have been subscribed with topical analgesics without having benefits from it.
Keywords: individualized medicine, pain therapy, non-opioid treatment, machine learning, predictive analytics, regression, multi-objective optimization, support vector regression
Introduction
One of the most frequent appearing physical symptoms in medicine is pain. Pain weighs heavily the patient suffering, the health care system, and more broadly society.1 Chronic pain, usually described as pain persisting more than 12 weeks, is a major global issue occurring to up to 34% of the population.2 Chronic pain management is usually challenging, even for seasoned physicians, while chronic pain affects massively the quality of life and level of functioning of the patients.1 Chronic pain has been associated in the literature with the deterioration of the quality of life of the patients, with reduced performance in their work environment and with severe problems in their physical and mental health.2–9 In addition, chronic pain has been also described as one of the major comorbidities for other chronic illnesses and cardiovascular diseases contributing to an increased risk for short-term mortality and severe health-related incidents.5,10,11
Chronic pain becomes evident with both behavioral and physical aspects. Both its generation and the analgesic responsiveness have been attributed to genetic, environmental and dietary factors.12 In complex phenotypes genetics and environment interact leading to variant predisposition to pain processing and perception.13,14 There exists a significant inter-individual variability in the way pain is experienced. A number of different biological and psychosocial factors (like demographics, genetics, and psychosocial processes) explain these individual variations in pain. Recently, the prevalence of chronic pain conditions has been attributed to different sexes, ages and ethnicities group. The individual and combined effects of these factors result in a unique mosaic that shapes pain in each person. Knowledge of this mosaic is crucial in order to decide the optimal pain treatment, and more informed and personalized pain care.15
The efficient treatment of pain is important in clinical practice because uncontrolled pain can negatively affect the health condition of the patients delaying wounds healing, contributing to increased stress and sometimes even leading to anxiety and depression. For this reason, effective pain treatment is essential for ameliorating the quality of life of the patients.16 While the primary goal of pain clinicians is to achieve pain relief and thus improved function and quality of life for the patients, most patients and pain clinicians find that 30% of pain improvement is clinically important—a strikingly low success level in other fields of medicine.12 Complete pain relief is still not feasible for most chronic pain patients, regardless of the latest advancements in introducing new drugs and treatments. As an example, 38–74% of patients with cancer cannot find adequate pain relief regardless of the employed treatment.17
Existing chronic pain treatments, such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs)18 and anticonvulsants,16 are attempting to reduce the pain symptoms in order to improve the quality of life of the patients while also allowing them to be functional in their social and work-related activities.19 However, frequently, finding the most appropriate medication and its most appropriate dose for each patient can take a lot of time and even raise unpredictable dangers for specific patients. This is explained because different people present different therapeutic response and adverse effects.1 Variations in response to medications, particularly opioids, have been reported by physicians in the clinical setting by as much as 40-fold.20 Managing pain successfully means providing analgesia and at the same time minimizing adverse effects.
A significant increase of controlled substances and opioids prescriptions, as well as of their misuse has been observed over the last years21,22 and this has made the prescription of opioids a controversial topic with socioeconomic implications.23 One of the methods suggested to reduce opioid consumption is the adoption of a multimodal and multi-disciplinary approach.10
Topical agents can offer pain relief without the risks of abuse, misuse, and addiction24,25 which have been linked with the use of oral analgesics. Topical agents are administered through the skin being only concentrated at the site of application and not systemically.24 Effective topical agents are water lipophilic and soluble to reduce drug concentration, to increase local tissue absorption, and to hold the drug at the site of application. Furthermore, topical agents do not present the problems associated with orally ingested medications, such as gastric ulceration, first-pass hepatic metabolism, and problems associated with variable serum concentrations. But, even in the case of topical agents, their absorption and distribution are affected by the individual variation in both skin physiology and metabolism.12 Topical agents have limited systemic side effects and satisfactory efficacy.24 Moreover, topical medications have been proven to present additional advantages, such as a more targeted approach, since they do not present significant systemic effects, and a straightforward method to determine their dosages which was the reason of their usage by both physicians and patients.26,27 So far, there exist many successful cases of topical analgesics managing effectively various pain conditions with some of the most striking examples being treating chronic pain of patients with osteoarthritis and chronic joint-related issues.18,28–30
One of the most significant studies examining the efficacy of topical analgesics therapies for chronic pain patients was the Optimizing Patient Experience and Response to Topical Analgesics (OPERA) IRB-approved observational study.31,32 The study emphasized on assessing the effectiveness of topical analgesic based treatment and quantifying the changes in severity and interference of pain and the changes in total drugs used during the follow-up periods. This study demonstrated that topical analgesics could be a beneficial therapeutic alternative for most of chronic pain patients but there still existed some patients who did not benefit from it having an insignificant reduction of pain levels.33
The present research work attempts to utilize machine learning in the context of an individualized medicine approach for chronic pain therapies. In specific, focus is given on a topical analgesics therapy and a machine learning approach is deployed to predict the outcomes of the therapy given the demographic and clinical profile of the patients. To handle effectively the issues of high dimensionality, imbalanced datasets and non-optimal classification models of this prediction problem, in the present paper we introduce a novel hybrid approach which combines multi-objective optimization algorithms with Support Vector Regression algorithm. The proposed algorithm was shown to significantly outperform other existing state-of-the-art methods when applied to predict the outcomes of topical analgesic therapy in chronic pain patients. The sophistication of the machine learning approach allowed for an adequate accuracy (AUC 73.8–87.2%) of the predictive models which enables their incorporation to an individualized medicine approach in treating chronic pain. To the best of our knowledge, this is the first individualized medicine approach for treating chronic pain patients and it is presented in detail in the current manuscript.
Patients and Methods
Data
Data from the OPERA study were employed to predict the outcomes of the pain therapy under study.
The demographics, medications and clinical characteristics of the OPERA study are presented in detail.31 The four different outcomes concern the differences, as observed by the patient and measured by the physician, in 1) total severity, 2) total interference, 3) total complaints and 4) total drugs before and after undergoing a pain therapy, from day 0 to month 3. The OPERA study data included also the replies to questions of the validated questionnaire given to patients at the beginning of the study. A subset of 49 questions describing the profile of the patient before starting the treatment was used as potential features to train the predictive models.
Preprocessing of the data included arithmetic normalization to the [−1,1] interval and kNN-missing values imputation with k = 5. A random 80% of the data was used as the training set and the remaining 20% as testing set, for each of the four prediction problems, using a stratified approach to maintain the same proportion of positive and negative examples for each prediction problem. On the training dataset, 5-fold cross-validation was employed to create the prediction models.
Machine Learning Method
The four prediction problems were treated as regression problems since the outcomes of this study can be real integers. Classification was performed by binarizing outputs to class 1 if the value is bigger than or equal to 0, and to class −1 otherwise to give emphasis to the accuracy with which we classify patients who improved their measurements of interest and patients who did not increase them.
The applied machine learning method is hybrid combining dimensionality reduction, regression and classification. Specifically, it is an ensemble dimensionality reduction technique employing a heuristic optimization algorithm34,37 to a) identify the optimal feature subset to be used as input to the classifiers and to select b) the most appropriate Support Vector Regression Method35 and c) its optimal parameters.
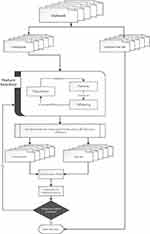
The heuristic optimization framework was inspired from the method presented in Corthésy et al36 for the optimization of the preprocessing steps for the analysis of Mass Spectrometry data. It is a pareto-optimization technique since its selection process is driven by organizing solutions to non-dominated fronts and assigning close fitness values to solutions belonging to the same front. This algorithm has been demonstrated to balance between fast convergence and good exploration of the search space while also effectively handling contradictory goals. These goals include the minimization of the number of extracted features, the maximization of the predictive accuracy and the minimization of the complexity of the classifier allowing it to achieve better generalization properties. The algorithm is described in a summary flowchart in Figure 1.
The optimization framework begins by initializing a set of solutions. Each solution consists of i) a value indicating which of the two alternative kernel types will be used in Support Vector Regression (a value greater than 0.5 indicates the selection of Radial Basis Function Kernel, otherwise the selection of linear Kernel), ii) 49 values for deciding if a feature will be used as input (values greater than 0.5 force its use) and iii) two values for parameter tuning of the gamma parameter of Radial Basis Functions Kernel and of the regularization parameter C of SVR models. The initialization of the solutions is performed randomly with values from the normal distribution of each variable. The heuristic optimization method that is being used is an evolutionary algorithm and thus uses crossover and mutation to iteratively generate new solutions and a roulette wheel selection mechanism to apply the survival of the fittest principle and apply evolutionary pressure towards the best performing solutions. Since the selected solutions should satisfy multiple goals, the multi-objective method based on Pareto frontiers and presented in36 is used. The total fitness value is the weighted sum of the different optimization goals.
Six fitness functions are used to define the optimization goals. These fitness functions include fitness functions to measure model simplicity and fitness functions to measure regression and classification performance.37
The algorithm stops when it reaches convergence (solutions become close enough for a number of iterations) or when it reaches the maximum number of generations.
The SVR models’ implementation was based on libsvm python library.38 Python programming language version 3.4 was used for all custom scripts. The rest of the analysis was performed using InSyBio Biomarkers tool39 of InSyBio Suite platform.
For comparison purposes, we generated classifiers based on SVR and Random Forests in the WEKA software (we used the default parameters suggested in WEKA documentation) with the same data and with the same cross-validation strategy.
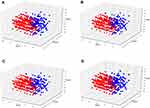
Principal component (PCA) and clustering analysis were performed to initially explore the training dataset. PCA is based on the Scree test to retain the principal components which describe at least the 95% variability of the data set. Clustering is based on the k-prototypes algorithm40 because we have both numerical and categorical inputs. The optimal number of clusters was decided (experimenting with numbers from 2 to 10) as the one which provides the optimal Calinski-Harabasz score.41 To visualize the results, all samples have been projected on the 3 most significant principal components. In the produced figures, a patient with decreased value in an outcome under study is represented with a rectangle and patients who present increased or steady values are represented with circles.
Results
PCA and Clustering Analysis
In order to explore the dynamics and the separability of the different patients’ subgroups in OPERA study, we performed Principal Component Analysis (PCA). For visualization reasons, only the first three more significant PCAs are depicted in Figure 2A–D which in addition denote the grouping of the patients based on the four examined outcomes of this study. One limitation of this simplistic analysis is that we are restricted to visualizing the 3 most significant principal components (PC) while in all cases more than 3 PCs were needed to explain 90% variability of the. Clustering analysis has revealed that with an unsupervised linear approach the patients are grouped into two main subgroups which however cannot accurately discriminate between responders and not responders for all the outcomes. Thus, a more complex non-linear supervised learning approach is required for this purpose. Enrichment analysis on the revealed subgroups using hypergeometric distribution test revealed that the red cluster is significantly enriched with patients who reduced the total number of drugs, their interference levels and their pain severity after the intervention and blue cluster is significantly enriched with patients who did not benefit from the intervention. However, no enrichment was revealed in both clusters regarding the reduction or increase in total number of complaints.
Correlation Analysis
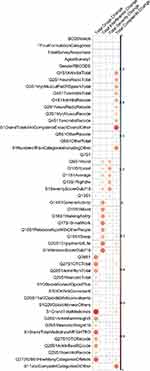
In an attempt to evaluate the features of the OPERA study, which included the responses of the participants in the baseline of the study, we associated them with the outcomes of the study using a correlation analysis approach. Spearman correlation was preferred instead of the standard Pearson correlation to account for non-linear correlation patterns. The results of this analysis are presented in Figure 3 depicting only significant correlations of p-value less than 0.05 in a red-blue color scale. Several questions were strongly associated with the outcomes of the study and this is a strong indication of the feasibility of a predictive analytics approach. Among the different features of the study, the total number of complaints, total medicines and interference score at baseline seem to be the most informative ones.
Predictive Analytics and Comparative Results
The comparative results of the proposed hybrid algorithms against the state-of-the-art regression models of the Weka package are depicted in Figure 4. This figure presents both cross-validation performance of all models as well as their performance in the test set. Since the proposed solution is based on a heuristic algorithm and not a deterministic one, we tested it in ten different runs and present the average values of all the examined regression and classification metrics.
 |
Figure 4 Predictive analytics results for the four outcomes (A–D) using proposed machine learning algorithm and comparing it with other contemporary machine learning algorithm implementations. |
This comparative analysis revealed that the newly introduced feature selection and classification model significantly improved both classification and regression metrics for all the predictive analytics tasks of the present study. The most important metric for this comparative analysis is the geometric mean of sensitivity and specificity due to the imbalanced nature of the predictive problems of the current study which make the accuracy not representative of the predictive performance of the models. The improvement of the proposed machine learning technique compared to the state-of-the-art techniques is extremely high for the metric of geometric mean of sensitivity and specificity.
Comparative results in Figure 4 were created using the default values for the parameters of the state-of-the-art ML models (SVR and Random Forests). In order to further explore whether the improvement of the proposed ML model is attributed to better parameter tuning we explored whether optimizing the most crucial parameter of RFs, the number of trees, would have a severe effect on the performance of this model. In Supplementary Table 1 we present the experimental results using 100 (default value), 250, 500 and 1000 trees. However, except of the case of total complaints the changes in the performance of the models created with different number of random trees were insignificant and the RF method was still clearly outperformed by the proposed ML technique indicating that the increase of the performance is not attributed only to better parameter tuning but mostly to the dimensionality reduction component of the method.
In addition to the classification metrics of Figure 4, ROC analysis was conducted for the performance of the proposed models in the test set calculating the Area Under the Curve metric (Figure 5). In Supplementary Figure 2, we present the ROC analysis using the best performing RF model selecting the number of trees based on the cross-validation performance presented in Supplementary Table 1. The proposed ML model clearly outperforms the RF approach in this ROC analysis.
 |
Figure 5 ROC analysis on the test set for the prediction of the four different outcomes of the OPERA study. |
In Supplementary Figure 1, we present the number of selected features from each one of the prediction models for the four outcomes of interest. From this figure, it is noteworthy that 8/49 questions were not selected as inputs to any of the prediction models implying that the overall questionnaire can be further simplified. The most significant features were according to the selection frequency were the ones associated total complaints, total use of narcotics and the ability of the participants to perform normal work-related tasks in the baseline of the study. The final trained predictive models for each one of the outcomes are accessible as trained libsvm python models in https://www.insybio.com/pain_research/.
Intelligent System for the Individualized Medicine Application of Topical Analgesics
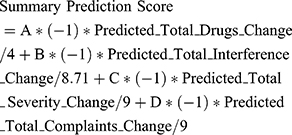
As a next step, the machine learning models which were trained and validated to predict outcomes of a topical analgesics therapy in the previous subsections were incorporated in an intelligent system which was designed and implemented to allow the individualized medicine application of this chronic pain therapy (Figure 6). In specific, the prediction scores for the four outcomes were combined with the weighted normalized score to allow it to act as a decision support system for clinicians to support their decision in subscribing or not topical analgesics therapy in chronic patient patients. In order to incorporate clinicians’ opinion in this score the overall method is parametrizable and the clinicians can state the weights/significance of each one of the outcomes according to the needs of each individual. The overall prediction score of the intelligent decision support system is calculated with the following equation:
 |
Figure 6 Application of intelligent decision support system for the individualized medicine pipeline of topical analgesics therapy. |
where A, B, C, D are the positive weights that the clinician should assign according to the needs of each patient and the variables with the prefix predicted are the predicted outcomes for this patient based on the answers to their OPERA questionnaire and the results of the trained machine learning models. In the above equation, the predicted scores are divided with their maximum value present in the OPERA study dataset to scale their values to the interval [−1,1]. The overall summary prediction score reflects how sure we are that the topical analgesics therapy will be beneficial with positive values meaning that it would be in general beneficial and the bigger the values the bigger the expected benefits in the considered outcomes.
Considering weights equal to 1 and a threshold of zero for the summary prediction score, in our test set the topical analgesics therapy was in general beneficial for the 83.4% (83.73% in training set) of the participants. When the intelligent decision support system was applied 92.38% (94.16% when using 5-fold cross-validation results) of the participants selected for topical analgesics therapy were found to be in general benefited from this therapy.
Discussion - Conclusions
Chronic pain condition has been proven to have a severe negative effect in the quality of life of patients, their performance in the work environment and their health. However, until now there does not exist a treatment that can completely alleviate chronic pain without having side effects. On the one hand, the use of opioids can be effective, but they have been linked among other with severe side effects, addiction and misuse and have raised a debate about their actual benefits for the patients. On the other hand, topical analgesics can be extremely beneficial for some chronic pain patients minimizing the side effects but are not effective for all patients. For this reason in the present research work we attempt to introduce an individualized medicine approach which will alleviate this problem by using a novel machine learning approach to prioritize patients who will benefit from topical analgesics.
In order to examine the feasibility of an individualized medicine approach for chronic pain treatment, we conducted PCA, clustering and correlation analysis using the data from OPERA study. This analysis revealed that even though some of the study’s features are strongly correlated with the outcomes of the study, linear unsupervised learning methods were not enough to accurately cluster patients into responders and non-responders of the topical analgesic treatment.
For this reason, we deployed a set of non-linear supervised learning regression techniques to solve the predictive analytics problems which were stated for predicting the outcomes of the study using information available in the baseline of the study. However, existing state-of-the-art regression models presented mediocre performance because of not efficiently handling the feature selection problem, the imbalanced nature of the datasets as well as not tuning effectively the parameters of the models. In order to handle these limitations, in the present paper we introduced a new hybrid machine learning method combining a multi-objective optimization algorithm with SVR models. The multi-objective optimization technique was used to select the optimal feature subset and regression algorithm’s parameters for each one of the predictive analytics problems, while being able to balance the trade-off of multiple contradictory performance objectives. In specific, the ultimate goals of the algorithm were to identify models which are simple enough to allow for better generalization properties but also performed equally good in the regression and classification metrics. The comparative results revealed that the proposed algorithm significantly outperformed existing machine learning methods solving with adequately high-performance metrics all the predictive analytics problems stated.
To the best of our knowledge, this is the first time that machine learning methods have been used to prioritize chronic pain patients for a specific therapy. The individualized medicine framework which was designed allowed for an efficient solution which can be used to select the patients which should be treated with a topical analgesics therapy and the patients for whom a different therapeutic approach should be followed. Moreover, the feature selection component of the machine learning analysis revealed potential methods to simplify the questionnaires used for this study and provided insight into which features are the most important for the prediction of each one of the outcomes of interest. The latter is a significant step into a more transparent stratification of the chronic pain patients.
The trained models are not specific for one topical analgesic treatment. In specific the study that the present paper was based on utilized 4 different setups of the topical analgesic treatments.31,32 These suggested predictive models can also be applicable to other topical analgesic treatments and setups however, this remains to be validated. Even if the performance of these models in predicting the outcomes of other topical analgesic treatments not examined in the present study is not expected to be optimal, they can act as a basis to identify the optimal predictors using the technique of transfer learning.
The present study presents several limitations considering the availability of data and the limitations of the analysis techniques. In particular, due to the nature of OPERA study (observational study) patients history and detailed annotation of the diseases that each participant suffered from were not available and thus it was not feasible in the present study to match responders and non-responders to topical analgesic therapies to specific diseases and other clinical characteristics of the patients. Another limitation of the present study is the lack of genetics and epigenetics data which can be used to further understand the genetic and epigenetic profile of responders and non-responders to topical analgesics therapies. Finally, from a methods point of view, the present study is based on the utilization of machine learning methods to train predictive models and this specific category of algorithms is difficult to interpret and understand because of their complex and non-linear nature. The proposed ML method provides as outcome the features subset which gives the best predictive performance for each one of the models revealed allowing for an interpretation of the final models but still a lot of effort is required in the field of ML to end up with fully explainable machine learning models.
For future research, the suggested intelligent decision support system for the individualized medicine application of topical analgesics therapies should be further validated in bigger cohorts of patients and its threshold should be optimized to better fit the clinicians’ goals. Moreover, the suggested intelligent decision support system should be made accessible to physicians though a user-friendly interface to support them in taking the optimal decisions for treating their chronic pain patients.
Data Sharing Statement
The OPERA dataset is available upon request to our paper’s primary author (Peter Hurwitz). The analysis conducted in the present research work was within the scope of the data analysis described in the study’s ethics approval (approved by INTEGREVIEW IRB) and thus no further approval was required. Regarding the rest of the results and predictive models, they are freely available from (www.insybio.com/pain_research/).
Acknowledgments
The authors acknowledge the contribution of Labros Digonis (CEO of InSyBio) and Mackenzie Hastings (Business Development Manager of InSyBio) for facilitating the collaboration of the authoring group and organizing meetings. This work is an expanded (more than 50%) version of the work presented by the same authoring group at The 10th International Conference on Information, Intelligence, Systems and Applications, 15–17 July 2019 (IISA 2019).
Disclosure
A. Korfiati, K. Theofilatos and S. Mavroudi are affiliated to InSyBio Ltd and the named inventors of the provisional patent (Theofilatos, K., Alexakos, C., Korfiati, A., Dimitrakopoulos, C., & Mavroudi, S. (2018). US Patent Application No. 15/837,407) submitted to the US Patent Office by InSyBio Ltd which includes the description of the computational framework for predictive biomarkers and building predictive models for diagnosis, prognosis and treatment. D. Dietze is affiliated with Metrics for Learning LLC and reports personal fees from Clarity Science, during the conduct of the study and outside the submitted work. P. Hurwitz is affiliated with Clarity Science LLC. The authors report no other conflicts of interest in this work.
References
1. Ting S, Schug S. The pharmacogenomics of pain management: prospects for personalized medicine. J Pain Res. 2016;9:49.
2. Nielsen LM, Olesen AE, Branford R, Christrup LL, Sato H, Drewes AM. Association between human pain-related genotypes and variability in opioid analgesia: an updated review. Pain Pract. 2015;15(6):580–594. doi:10.1111/papr.12232
3. Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–462. doi:10.1185/03007995.2010.545813
4. Breivik H. A major challenge for a generous welfare system: a heavy socio-economic burden of chronic pain conditions in Sweden–and how to meet this challenge. Eur J Pain. 2012;16(2):167–169. doi:10.1002/j.1532-2149.2011.00025.x
5. Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10-year mortality. a cohort record linkage study. Eur J Pain. 2010;14(4):380–386. doi:10.1016/j.ejpain.2009.07.006
6. de Sola H, Salazar A, Dueñas M, Ojeda B, Failde I. Nationwide cross-sectional study of the impact of chronic pain on an individual’s employment: relationship with the family and the social support. BMJ Open. 2016;6(12):e012246. doi:10.1136/bmjopen-2016-012246
7. Gerdle B, Björk J, Cöster L, Henriksson K, Henriksson C, Bengtsson A. Prevalence of widespread pain and associations with work status: a population study. BMC Musculoskelet Disord. 2008;9:102. doi:10.1186/1471-2474-9-102
8. Liedgens H, Obradovic M, De Courcy J, Holbrook T, Jakubanis R. A burden of illness study for neuropathic pain in Europe. Clinicoecon Outcomes Res. 2016;8:113–126. doi:10.2147/CEOR.S81396
9. Langley P, Müller-Schwefe G, Nicolaou A, Liedgens H, Pergolizzi J, Varrassi G. The impact of pain on labor force participation, absenteeism and presenteeism in the European Union. J Med Econ. 2010;13(4):662–672. doi:10.3111/13696998.2010.529379
10. Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011;12(7):996–1004. doi:10.1111/j.1526-4637.2011.01187.x
11. Grimby-Ekman A, Gerdle B, Björk J, Larsson B. Comorbidities, intensity, frequency and duration of pain, daily functioning and health care seeking in local, regional, and widespread pain—a descriptive population-based survey (SwePain). BMC Musculoskelet Disord. 2015;16(1):165. doi:10.1186/s12891-015-0631-1
12. Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part one: skin physiology and delivery systems. Pain Ther. 2015;4(1):17–32. doi:10.1007/s40122-015-0031-0
13. Webster LR, Belfer I. Pharmacogenetics and personalized medicine in pain management. Clin Lab Med. 2016;36(3):493–506. doi:10.1016/j.cll.2016.05.007
14. McClearn GE. Nature and nurture: interaction and coaction. Am J Med Genet B Neuropsychiatr Genet. 2004;124B(1):124–130. doi:10.1002/ajmg.b.20044
15. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158(Suppl 1):S11. doi:10.1097/j.pain.0000000000000775
16. Owusu Obeng A, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy. 2017;37(9):1105–1121. doi:10.1002/phar.1986
17. Davis MP, Walsh D. Epidemiology of cancer pain and factors influencing poor pain control. Am J Hosp Palliat Care. 2004;21(2):137–142. doi:10.1177/104990910402100213
18. Argoff CE, Albrecht P, Irving G, Rice F. Multimodal analgesia for chronic pain: rationale and future directions. Pain Med. 2009;10(Suppl 2):S53–S66. doi:10.1111/j.1526-4637.2009.00669.x
19. Mills S, Torrance N, Smith BH. Identification and management of chronic pain in primary care: a review. Curr Psychiatry Rep. 2016;18(2):22. doi:10.1007/s11920-015-0659-9
20. Trescot AM, Faynboym S. A review of the role of genetic testing in pain medicine. Pain Physician. 2014;17(5):425–445.
21. Volkow ND, McLellan AT. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253–1263. doi:10.1056/NEJMra1507771
22. Campbell G, Nielsen S, Larance B, et al. Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the pain and opioids in treatment (POINT) cohort. Pain Med. 2015;16(9):1745–1758. doi:10.1111/pme.12773
23. Grosen K, Olesen AE, Gram M, et al. Predictors of opioid efficacy in patients with chronic pain: a prospective multicenter observational cohort study. PLoS One. 2017;12(2):e0171723. doi:10.1371/journal.pone.0171723
24. Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21(3):15. doi:10.1007/s11916-017-0615-y
25. Flores MP, Castro AP, Nascimento Jdos S. Topical analgesics. Rev Bras Anestesiol. 2012;62(2):244–252. doi:10.1016/S0034-7094(12)70122-8
26. McCarberg BH, D’Arcy Y. Target pain with topical peripheral analgesics. Nurse Pract. 2007;32(7):44–49. doi:10.1097/01.NPR.0000279572.01195.84
27. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27(5):263–271. doi:10.1097/DER.0000000000000216
28. Massey T, Derry S, Moore RA, et al. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev. 2010;6:CD007402.
29. Mason L, Moore RA, Edwards JE, et al. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Disord. 2004;5(1):28. doi:10.1186/1471-2474-5-28
30. Argoff CE. Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013;88(2):195–205. doi:10.1016/j.mayocp.2012.11.015
31. Gudin JA, Brennan MJ, Harris ED, et al. Changes in pain and concurrent pain medication use following compounded topical analgesic treatment for chronic pain: 3- and 6-month follow-up results from the prospective, observational optimizing patient experience and response to topical analgesics study. J Pain Res. 2017;10:2341. doi:10.2147/JPR.S134133
32. Gudin JA, Brennan MJ, Harris ED, Hurwitz PL, Dietze DT, Strader JD. Reduction of opioid use and improvement in chronic pain in opioid-experienced patients after topical analgesic treatment: an exploratory analysis. Postgrad Med. 2018;130(1):42–51. doi:10.1080/00325481.2018.1414551
33. Kopsky DJ, Hesselink JM. Phenytoin in topical formulations augments pain reduction of other analgesics in the treatment of neuropathic pain. Int J Anesthetic Anesthesiol. 2018;5:061. doi:10.23937/2377-4630/1410061
34. Mishra KK, Harit S. A fast algorithm for finding the non dominated set in multi objective optimization. Int J Comput Appl. 2010;1(25):35–39.
35. Smola AJ, Schölkopf B. A tutorial on support vector regression. Stat Comput. 2004;14(3):199–222. doi:10.1023/B:STCO.0000035301.49549.88
36. Corthésy J, Theofilatos K, Mavroudi S, et al. An adaptive pipeline to maximize isobaric tagging data in large-scale MS-based proteomics. J Proteome Res. 2018;17(6):2165–2173. doi:10.1021/acs.jproteome.8b00110
37. Gudin J, Mavroudi S, Korfiati A, Theofilatos K, Dietze D, Hurwitz P. A precision medicine approach for non-opioid pain therapy using a combination of multi-objective optimization and support vector regression.
38. Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2011;2(3):27. doi:10.1145/1961189.1961199
39. InSyBio. InSyBio Biomarkers. Available from: https://www.insybio.com/biomarkers.html. Accessed May 1, 2019.
40. Ji J, Bai T, Zhou C, Ma C, Wang Z. An improved k-prototypes clustering algorithm for mixed numeric and categorical data. Neurocomputing. 2013;120:590–596. doi:10.1016/j.neucom.2013.04.011
41. Caliński T, Harabasz J. A dendrite method for cluster analysis. Commun Stat Theory Methods. 1974;3(1):1–27. doi:10.1080/03610927408827101
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.