Back to Journals » Journal of Pain Research » Volume 13
Reducing Episodic Cluster Headaches: Focus on Galcanezumab
Authors Pellesi L , De Icco R , Al-Karagholi MA , Ashina M
Received 21 April 2020
Accepted for publication 8 June 2020
Published 2 July 2020 Volume 2020:13 Pages 1591—1599
DOI https://doi.org/10.2147/JPR.S222604
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Lanfranco Pellesi,1 Roberto De Icco,2,3 Mohammad Al-Mahdi Al-Karagholi,1 Messoud Ashina1,4
1Danish Headache Center, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; 2Headache Science Center, IRCCS Mondino Foundation, Pavia, Italy; 3Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy; 4Danish Headache Knowledge Center, Rigshospitalet Glostrup, Glostrup, Denmark
Correspondence: Lanfranco Pellesi
Danish Headache Center, Faculty of Health and Medical Sciences, University of Copenhagen, Vagtelvej 61, Copenhagen 2000, Denmark
Tel +393409383056
Email [email protected]
Abstract: The involvement of calcitonin gene-related peptide in migraine and cluster headache has led to the recent development of new therapies. Galcanezumab, a novel monoclonal antibody targeting the calcitonin gene-related peptide, is approved for the migraine prevention and has recently been tested for the prevention of cluster headache. Two clinical trials have been conducted to investigate the efficacy and safety of galcanezumab in episodic cluster headache and chronic cluster headache. While efficacy endpoints were not met in the chronic subtype, galcanezumab reduced the weekly frequency of attacks in patients with episodic cluster headaches. In both studies, the antibody was well tolerated. This review summarizes and critically reviews the available data regarding the rationale behind targeting the calcitonin gene-related peptide with galcanezumab for the prevention of cluster headache.
Keywords: cluster headache, calcitonin gene-related peptide, CGRP, antibody, LY2951742
Introduction
Cluster headache (CH) is a primary headache disorder characterized by recurrent and unilateral headache attacks lasting from 15 minutes to three hours.1 Its bouts are distinguished by a striking combination of severe pain in the periorbital area accompanied by ipsilateral autonomic symptoms, such as conjunctival injection, excessive eye tearing, ptosis, nasal congestion, facial sweating, and agitation. The prevalence is about one person per 500 and is predominant in men.2 There are two subtypes, episodic CH (eCH) and chronic CH (cCH), differentiated according to the presence and duration of periods of remission. eCH is the most common subtype, affecting up to 80% of patients3 (Table 1, panel A). It presents as repetitive daily attacks that persist for weeks or months, followed by remission periods lasting at least three months. cCH attacks occurs for one year or longer without remission, or with remission periods lasting less than three months.4 CH burdens individuals and society with lower work productivity and social functioning,5,6 as well as increased health services utilization and suicidality.7–9
 |
Table 1 Diagnostic Criteria and Current Pharmacological Treatments for Cluster Headache |
Pharmacotherapy for CH mainly focuses on terminating attacks and preventing their occurrence during recurring episodes and/or chronic periods. First-choice abortive treatments include high-flow oxygen10 and subcutaneous sumatriptan.11,12 Other strategies, such as intranasal triptans,13–15 are used only if the above are contraindicated or ineffective. Prophylactic treatments are recommended in eCH patients during the active period and cCH patients. Several options are available, but they are based on a small body of evidence.16 Of note, all prophylactic therapies are used off-label, as they are originally developed for other diseases. Suboccipital injections with corticosteroids have an established efficacy, tested in two Class I studies.17,18 Also, neuromodulatory strategies are utilized, including an external vagal nerve stimulator,19,20 and the stimulation of the sphenopalatine ganglion (SPG) with a remote-controlled device surgically placed in the pterygopalatine fossa.21,22
In recent years, the considerable progress in migraine research and the development of new treatments targeting the calcitonin gene-related peptide (CGRP), led to a new era in migraine therapy.23 Considering the overlapping pathophysiological mechanisms between migraine and CH,24 anti-CGRP therapies have also been tested in CH. Galcanezumab, a humanized monoclonal antibody targeting the CGRP peptide, has been approved as a preventive treatment for episodic and chronic migraine25,26 and more recently, as a preventive treatment in eCH.27 Here, we review the current knowledge of CGRP in CH, along with clinical trial efficacy and safety data of galcanezumab in the treatment of CH.
Methods
A data search via MEDLINE for articles published up to February 29th 2020 was conducted. We used the search terms “galcanezumab” and “cluster headache”, alone and together with the terms “calcitonin gene-related peptide” or “CGRP”. Reference lists of relevant primary articles, reviews, and book chapters were also reviewed to identify any clinical and/or preclinical investigation related to the purpose of this Review that may have been missed in the search process.
Calcitonin Gene-Related Peptide (CGRP)
CGRP is a 37-amino acid peptide belonging to a family of structurally related peptides, including amylin, adrenomedullin and intermedin. It exists in two isoforms, α- and β-CGRP. The former is found in the central and peripheral nervous system, whereas β-CGRP is found mainly in the enteric nervous system.28 CGRP is a potent vasodilator, primarily released from unmyelinated C-fibers innervating meningeal and cerebral vasculature. Once released, CGRP binds to its receptor located on myelinated Aδ-fibers and vascular smooth muscle cells.29 Accordingly, CGRP dilates arteries and may activate nociceptive fibers, as well as provoking the release of other pain neurotransmitter, such as glutamate and substance P.30 CGRP receptor consists of the complex of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1). A third membrane-associated component called receptor component protein (RCP) is necessary to ensure complete functioning of the CGRP receptor, with the activation of different intracellular pathways: 1) active recruitment of adenylate cyclase, which converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), a second messenger in the cell; 2) increasing the activity of phospholipase Cβ and active recruitment of protein kinase C (PKC); 3) stimulation of protein kinase A (PKA) and activation of many transcription factors, including c-fos.31 CGRP signaling pathways have been reported in the trigeminal ganglion (TG),32 the SPG,33 the spinal trigeminal nucleus,34 and the hypothalamus,35 all largely investigated in the pathophysiology of CH. In the TG, about 50% of neurons stained positive for the CGRP peptide, while the CLR/RAMP1 complex was expressed in around 35% of TG neurons and some glial cells. Co-localization of CGRP and its receptor components suggest involvement of the CGRP signaling in both neurons and glial cells.32
CGRP and Cluster Headaches
Clinical and pre-clinical studies implicate CGRP signaling in CH pathophysiology36 (Figure 1). Sicuteri et al reported that salivary CGRP levels were increased in CH patients, compared to healthy subjects.37 Also, serum CGRP from the external jugular vein was augmented during spontaneous CH attacks.38 Additionally, CGRP level was normalized by acute treatments effective in clinical practice, but not opioids.38 Later, human provocation studies have expedited our understanding. Sublingual glyceryl trinitrate provokes CH attack in eCH patients during the active phase.39 It has been reported that CGRP levels increase at the peak of the provoked attack, and decrease after spontaneous or sumatriptan-induced remission.40 Similarly, intravenous infusion of CGRP provoked CH attacks in 89% of eCH patients during the active phase.41 CH attacks also occurred to a lesser extent (50%) in patients with cCH.41
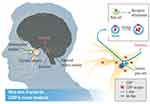 |
Figure 1 Main sites of action for CGRP in cluster headache. Reproduced from Belin AC, Ran C, Edvinsson L. Calcitonin Gene-Related Peptide (CGRP) and cluster headache. Brain Sci. 2020;10(1):30. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.36 |
Pharmacological treatments for CH displayed specific interactions with the CGRP pathway. Sumatriptan, commonly used to treat acute attacks, belongs to a group of medicines called triptans, or serotonin (5-HT) receptor agonists. It acts through 5-HT1b and 5-HT1d receptors, expressed on the surface of the cell membrane and co-localized with CGRP in the trigeminal nerve fibers and the medium-size neurons of the trigeminal ganglion.42 Their activation is thought to inhibit CGRP release from trigeminal neurons.43–45 Also, oxygen, a highly effective acute treatment for CH, blocks the release of CGRP with a similar mechanism.38 In parallel with the reduction of headache frequency, corticosteroids also decreased CGRP plasma levels in eCH patients during the active phase,46 possibly through the blockade of a cytokine-mediated trigeminal activation.47,48 More recently, the clinical development of galcanezumab and fremanezumab, monoclonal antibodies targeting the CGRP peptide, is in progress and has yielded results.
Galcanezumab
Galcanezumab is a highly specific and potent humanized immunoglobulin G monoclonal antibody targeting the CGRP peptide.49 Currently, it is approved for the prevention of episodic and chronic migraine in the United States, Canada, United Kingdom and several European countries.25,26,50 Patients can self-inject galcanezumab as subcutaneous formulation, marketed as Emgality. More recently, the antibody has also been studied for the preventive treatment of CH. As of June 2019, it has been approved in the US for the prevention of eCH. CH dosing is different from the migraine dosing. The recommended dose of galcanezumab for CH is 300 mg at the onset of the cluster period, and once a month until the end of the active phase.
Pharmacokinetics
Galcanezumab exhibits dose-linear pharmacokinetics, with a direct proportionality between doses and exposure.51 Dosing adjusted for body weight is not warranted and patient factors, such as age, sex, ethnicity and injection-site location do not affect its pharmacokinetics.51 Due to its large size, low permeability through cell membranes and instability in the gastrointestinal tract, it is parenterally administered in the arm, thigh or abdomen, either with a pre-filled syringe or an auto-injector.52 The absorption is slow, with a time to peak concentration in the second week after administration. The apparent volume of distribution is 7.33 liters, whereas the half-life is 27 days.53 Galcanezumab is eliminated by either excretion or catabolism in smaller peptides and amino acids,54 and not in the urine. Its administration in single doses (1–600 mg) and consecutive doses (150 mg) was well tolerated in healthy male volunteers.51 There was no apparent difference between galcanezumab groups or between galcanezumab and placebo in terms of type and frequency of adverse events (AEs), or changes in vital signs, electrocardiographic parameters and laboratory values. The most common AEs were headache, nasopharyngitis, dermatitis and diarrhea. All of them were transient with no apparent relationship with the prolonged systemic drug exposure.
Mode of Action
Galcanezumab binds and neutralizes the CGRP ligand with high affinity. Thus, galcanezumab inhibits CGRP-mediated induction of cAMP and capsaicin-induced dermal blood flow (DBF).49 The latter represents a usable pharmacodynamic model in vivo to assess the “scavenging” of CGRP. The model consists of the topical application of capsaicin on the skin, which activates the transient receptor potential vanilloid 1 (TRPV1) channels expressed by primary sensory neurons. This activation results in the release of CGRP, a pivotal mediator of capsaicin-induced DBF. Moreover, neutralization of CGRP significantly reduced pain behavior in a prolonged and dose-dependent manner, independently of prostaglandins.49 In humans, blockage of CGRP in single doses and consecutive doses was also consistent with a robust, dose-dependent, and durable inhibition of capsaicin-induced DBF.51
The site of action of galcanezumab is still a matter of debate. CGRP receptors are well expressed in peripheral tissues and the central nervous system (CNS).55 However, human antibodies, including IgG, cannot easily penetrate the blood-brain barrier (BBB).56 Moreover, there is no evidence suggesting disruption of BBB in CH. In rats, galcanezumab infusion reached its highest concentration in plasma, spleen, dura and trigeminal ganglion.57 Penetration into the CNS was very low, ranging from 0.1–0.3% of the plasma concentration.57 Collectively, these data suggest that galcanezumab is likely to act in the periphery.
Galcanezumab in Cluster Headaches
Assessment of Primary and Secondary Outcomes
Efficacy of galcanezumab in eCH patients has been investigated in a single clinical trial.58 An 8-week, double-blind, placebo-controlled study was conducted at 35 sites in Europe and North America (Table 2). 106 patients were randomized to receive a single injection of subcutaneous galcanezumab at a dose of 300 mg or placebo, at baseline and at 1 month. All patients were allowed to use traditional abortive treatments, including triptans, oxygen, paracetamol and nonsteroidal anti-inflammatory drugs. At the baseline, patients complained 17.8 attacks per week in the galcanezumab group, compared with 17.3 in the placebo group. Across weeks 1 through 3, the galcanezumab group benefited 8.7 fewer weekly attacks compared to baseline, respect to 5.2 fewer weekly attacks in the placebo group. Also, the proportion of patients with ≥ 50% reduction in weekly attacks at week 3 was 71% in the galcanezumab group, significantly higher when compared to placebo (53%). For other secondary endpoints, the findings were consistent with the direction of the effect for the primary endpoint measure, with a larger effect in the initial weeks of the double-blind phase. After week 4, the reduction of the weekly attacks in the galcanezumab group and the placebo group converged. Spontaneous improvement or remission may have occurred, according to the natural course of the disease. In cCH patients, galcanezumab failed to meet both primary and secondary endpoints.59 A total of 237 patients were randomized to monthly subcutaneous injection of galcanezumab (300 mg) or placebo, for 12 weeks (Table 2). The mean reduction in weekly attack frequency, as well as the mean percentage of patients with ≥ 50% reduction in weekly attacks across weeks 1–12 was not different between groups. Also, there was no difference in acute treatment use between galcanezumab and placebo.60 An open-label extension study (NCT02797951) evaluating the long-term safety and tolerability of galcanezumab administered in participants with eCH and cCH is expected to end in 2020.
 |
Table 2 Galcanezumab Trials for eCH and cCH Prevention |
Assessment of Adverse Events
The safety of galcanezumab was consistent among patients with migraine, eCH and cCH patients.58,59,61–63 Most AEs were rated mild to moderate in intensity, without any relationship with the prolonged half-life of the antibody. The most frequently treatment-related AEs were injection-site pain, nasopharyngitis and other injection-site reactions. Injection-site pain was the most common,58 reported from 8% of the patients who received galcanezumab compared with none in the placebo group. Discontinuation due to AEs resulted in 4% of the patients treated with galcanezumab, compared with 2% who received placebo. Vital signs, laboratory analyses and electrocardiographic variables were not different between the two groups. There is no evidence that the safety profile of galcanezumab is similar in more vulnerable patients, including those with cardiovascular diseases and other risk factors, including smoking. CH is prevalent in middle-aged and older men, when the risk of cardiovascular disease is increased.64 Also, some risk factors, such as smoking, are more prevalent in CH.65 Unfortunately, current results from clinical trials are inconclusive, CH patients with serious diseases or significant cardiovascular risks were excluded on account of the study criteria.58,59 Data from sources other than traditional trials, including real-world data, are accordingly required to clarify matters. Another concern for the safety of galcanezumab is the emergence of anti-drug antibodies (ADAs). Such emergence is correlated with possible allergic reactions, and low efficacy in several disorders.66 As far as we know, no patient had a positive result for ADAs during the double-blind period of the single study conducted in eCH patients.58 In the cCH study, treatment-emergent ADAs appeared only in one galcanezumab-treated patient, with no further complications.59 The limited use and the discontinuation of the antibody shortly after patients are out of the cluster period may be helpful in limiting the emergence of neutralizing antibodies and their negative implications.
Future Perspectives
CH is an extremely painful and debilitating headache disorder. Only a few preventive therapies are currently available, in many cases with limited efficacy or poor tolerability (Table 1, panel B). Galcanezumab is the first FDA-approved drug for the reduction of CH attacks in patients with eCH in the active phase.67 The approval was issued in US and in some other countries, whereas the Committee for Medicinal Products for Human Use of European Medicines Agency considered a single study not robust enough for regulatory endorsement in Europe. In patients with cCH, galcanezumab was not effective. Interestingly, CGRP infusion provoked CH attacks very frequently in eCH patients during the active phase, but CGRP-provoked attacks were less frequent in patients with cCH.41 Accordingly, the lack of efficacy of anti-CGRP antibodies in cCH patients might be related to a less hypersensitivity to CGRP-induced attacks. Of interest, no headache attacks were reported after CGRP infusion in patients with eCH, in remission.41 Compared to the other subgroups of CH patients, eCH patients in remission displayed higher baseline levels of CGRP.68 Also, the lack of effect of galcanezumab parallels the poor treatment response in patients with cCH,69 that are more treatment resistant than patients with eCH.70
A separate investigation in patients with CH has also been conducted with fremanezumab, a different antibody targeting the CGRP peptide. Its clinical development programme, which also included a long-term safety study, was suspended after futility analyses found that the primary endpoint would not be met in either patients with eCH (NCT02945046) and cCH (NCT02964338). To date, no data are published and therefore cannot be discussed.
New data on galcanezumab and CH are coming in, including the 52-week open-label extension study in chronic patients.59 Further information is also pending from the clinical practice in patients with eCH, in terms of effectiveness, safety and tolerability. As example, any new adverse event associated with the blockade of physiological functions of CGRP and/or the repeated, albeit short-term, use of galcanezumab in eCH. Monitoring for cardiovascular function and immunogenicity is also desirable, as is safety in certain vulnerable populations, including individuals over 65 years, pregnant and/or breastfeeding women.
Abbreviations
5-HT, serotonin; 5-HT1b, serotonin receptor 1B; 5-HT1d, serotonin receptor 1D; ADAs, antidrug antibodies; AEs, adverse events; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; cCH, chronic cluster headache; eCH, episodic cluster headache; CGRP, calcitonin gene-related peptide; CH, cluster headache; CLR, calcitonin receptor-like receptor; DBF, dermal blood flow; FDA, Food and Drug Administration; PKC, protein kinase C; RAMP1, receptor activity-modifying protein 1; RCP, receptor component protein; SPG, sphenopalatine ganglion; TRPV1, transient receptor potential vanilloid 1; US, United States.
Author Contributions
Each author made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; as well as agree to be accountable for all aspects of the work.
Disclosure
MMK has acted as an invited speaker for Novartis and received travel grant from ElectroCore, LLC. MA is a consultant, speaker or scientific advisor for Allergan, Amgen, Alder, ATI, Eli Lilly, Novartis, and Teva, primary investigator for Alder, Amgen, Allergan, Eli Lilly, Novartis and Teva trials. MA has no ownership interest and does not own stocks of any pharmaceutical company. MA serves as associate editor of Cephalalgia, associate editor of Headache, co-editor of the Journal of Headache and Pain. MA is President of the International Headache Society. The authors report no other conflicts of interest in this work.
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd Edition. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
2. Russell MB. Epidemiology and genetics of cluster headache. Lancet Neurol. 2004;3(5):279–283. doi:10.1016/S1474-4422(04)00735-5
3. Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28(6):614–618. doi:10.1111/j.1468-2982.2008.01592.x
4. Hoffmann J, May A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol. 2018;17(1):75–83. doi:10.1016/S1474-4422(17)30405-2
5. Jürgens TP, Gaul C, Lindwurm A, et al. Impairment in episodic and chronic cluster headache. Cephalalgia. 2010;31(6):671–682. doi:10.1177/0333102410391489
6. Choi YJ, Kim PW, Chung PW, et al. Impact of cluster headache on employment status and job burden: a prospective cross-sectional multicenter study. J Headache Pain. 2018;19(1):78. doi:10.1186/s10194-018-0911-x
7. Jensen RM, Lyndberg A, Jensen RH. Burden of cluster headache. Cephalalgia. 2007;27(6):535–541. doi:10.1111/j.1468-2982.2007.01330.x
8. Choong CK, Ford JH, Nyhuis AW, Robinson RL, Aurora SK. Health care utilization and direct costs among patients diagnosed with cluster headache in U.S. health care claims data. J Manag Care Spec Pharm. 2018;24(9):921–928. doi:10.18553/jmcp.2018.24.9.921
9. Ji Lee M, Cho SJ, Wook Park J, et al. Increased suicidality in patients with cluster headache. Cephalalgia. 2019;39(10):1249–1256. doi:10.1177/0333102419845660
10. Cohen AS, Burns B, Goadsby PJ. High-flow oxygen for treatment of cluster headache: a randomized trial. JAMA. 2009;302(22):2451–2457. doi:10.1001/jama.2009.1855
11. The Sumatriptan cluster headache study group. Treatment of acute cluster headache with Sumatriptan. The Sumatriptan cluster headache study group. N Engl J Med. 1991;325(5):322–326. doi:10.1056/NEJM199108013250505
12. Ekbom K, Monstad I, Prusinski A, et al. Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. The Sumatriptan cluster headache study group. Acta Neurol Scand. 1993;88(1):63–69. doi:10.1111/j.1600-0404.1993.tb04189.x
13. Van Vliet JA, Bahra A, Martin V, et al. Intranasal sumatriptan in cluster headache: randomized placebo-controlled double-blind study. Neurology. 2003;60(4):630–633. doi:10.1212/01.WNL.0000046589.45855.30
14. Cittadini E, May A, Straube A, et al. Effectiveness of intranasal zolmitriptan in acute cluster headache: a randomized, placebo-controlled, double-blind crossover study. Arch Neurol. 2006;63(11):1537–1542. doi:10.1001/archneur.63.11.nct60002
15. Rapoport AM, Mathew NT, Silberstein SD, et al. Zolmitriptan nasal spray in the acute treatment of cluster headache: a double-blind study. Neurology. 2007;69(9):821–826. doi:10.1212/01.wnl.0000267886.85210.37
16. Robbins MS, Starling AJ, Pringsheim TM, Becker WJ, Schwedt TJ. Treatment of cluster headache: the American headache society evidence-based guidelines. Headache. 2016;56(7):1093–1106. doi:10.1111/head.12866
17. Ambrosini A, Vandenheede M, Rossi P, et al. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: a double-blind placebo-controlled study. Pain. 2005;118(1–2):92–96. doi:10.1016/j.pain.2005.07.015
18. Leroux E, Valade D, Taifas I, et al. Suboccipital steroid injections for transitional treatment of patients with more than two cluster headache attacks per day: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2011;10(10):891–897. doi:10.1016/S1474-4422(11)70186-7
19. Goadsby PJ, de Coo IF, Silver N, et al. Noninvasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38(5):959–969.
20. Gaul C, Magis D, Liebler E, Straube A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post hoc analysis of the randomised, controlled PREVA study. J Headache Pain. 2017;18(1):22. doi:10.1186/s10194-017-0731-4
21. Schoenen J, Jensen RH, Lanteri-Minet M, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816–830. doi:10.1177/0333102412473667
22. Goadsby PJ, Sahai-Srivastava S, Kezirian EJ, et al. Safety and efficacy of sphenopalatine ganglion stimulation for chronic cluster headache: a double-blind, randomised controlled trial. Lancet Neurol. 2019;18(12):1081–1090. doi:10.1016/S1474-4422(19)30322-9
23. Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394(10210):1765–1774. doi:10.1016/S0140-6736(19)32504-8
24. Vollesen AL, Benemei S, Cortese F, et al. Migraine and cluster headache - the common link. J Headache Pain. 2018;19(1):89. doi:10.1186/s10194-018-0909-4
25. Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75(2):187–193. doi:10.1001/jamaneurol.2017.3859
26. Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, Placebo-Controlled REGAIN study. Neurology. 2018;91(24):e2211–e2221. doi:10.1212/WNL.0000000000006640
27. FDA news release: FDA approves first treatment for episodic cluster headache that reduces the frequency of attacks; 2019. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-episodic-cluster-headache-reduces-frequency-attacks.
28. Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142. doi:10.1152/physrev.00034.2013
29. Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing cgrp and cgrp receptors in the peripheral trigeminovascular system. J Pain. 2013;14(11):1289–1303. doi:10.1016/j.jpain.2013.03.010
30. Durham PL. Calcitonin Gene-Related Peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1):S3–S8. doi:10.1111/j.1526-4610.2006.00483.x
31. Cottrell GS. CGRP receptor signalling pathways. Handb Exp Pharmacol. 2019;255:37–64.
32. Eftekhari S, Salvatore CA, Calamari A, et al. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169(2):683–696. doi:10.1016/j.neuroscience.2010.05.016
33. Csati A, Tajti J, Tuka B, Edvinsson L, Warfvinge K. Calcitonin gene-related peptide and its receptor components in the human sphenopalatine ganglion - Interaction with the sensory system. Brain Res. 2012;1435:29–39. doi:10.1016/j.brainres.2011.11.058
34. Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci. 2011;12(1):112. doi:10.1186/1471-2202-12-112
35. Takahashi K, Mouri T, Sone M, et al. Calcitonin gene-related peptide in the human hypothalamus. Endocrinol Jpn. 1989;36(3):409–415. doi:10.1507/endocrj1954.36.409
36. Belin AC, Ran C, Edvinsson L. Calcitonin Gene-Related Peptide (CGRP) and cluster headache. Brain Sci. 2020;10(1):30. doi:10.3390/brainsci10010030
37. Sicuteri F, Fanciullacci M, Nicolodi M, et al. Substance P theory: a unique focus on the painful and painless phenomena of cluster headache. Headache. 1990;30(2):69–79. doi:10.1111/j.1526-4610.1990.hed3002069.x
38. Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117(Pt 3):427–434. doi:10.1093/brain/117.3.427
39. Ekbom K. Nitroglycerin as a provocative agent in cluster headache. Arch Neurol. 1968;19(5):487–493. doi:10.1001/archneur.1968.00480050057005
40. Fanciullacci M, Alessandri M, Sicuteri R, Marabini S. Responsiveness of the trigeminovascular system to nitroglycerine in cluster headache patients. Brain. 1997;120(Pt 2):283–288. doi:10.1093/brain/120.2.283
41. Vollesen ALH, Snoer A, Beske RP, et al. Effect of Infusion of calcitonin gene-related peptide on cluster headache attacks: a randomized clinical trial. JAMA Neurol. 2018;75(10):1187–1197. doi:10.1001/jamaneurol.2018.1675
42. Hou M, Kanje M, Longmore J, et al. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001;909(1–2):112–120. doi:10.1016/S0006-8993(01)02645-2
43. Goadsby PJ, Edvinsson L. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache. 1994;34(7):394–399. doi:10.1111/j.1526-4610.1994.hed3407394.x
44. Durham PL, Russo AF. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J Neurosci. 1999;19(9):3423–3429. doi:10.1523/JNEUROSCI.19-09-03423.1999
45. Durham PL, Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci. 2003;23(3):807–815. doi:10.1523/JNEUROSCI.23-03-00807.2003
46. Neeb L, Anders L, Euskirchen P, et al. Corticosteroids alter CGRP and melatonin release in cluster headache episodes. Cephalalgia. 2015;35(4):317–326. doi:10.1177/0333102414539057
47. Lee SW, Tsou AP, Chan H, et al. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gen and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988;85(4):1204–1208. doi:10.1073/pnas.85.4.1204
48. Neeb L, Hellen P, Hoffmann J, Dirnagl U, Reuter U. Methylprednisolone blocks interleukin 1 beta induced calcitonin gene related peptide release in trigeminal ganglia cells. J Headache Pain. 2016;17(1):19. doi:10.1186/s10194-016-0609-x
49. Benschop RJ, Collins EC, Darling RJ, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthr Cartil. 2014;22(4):578–585. doi:10.1016/j.joca.2014.01.009
50. Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–1088. doi:10.1001/jamaneurol.2018.1212
51. Monteith D, Collins EC, Vandermeulen C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the CGRP binding monoclonal antibody LY2951742 (Galcanezumab) in healthy volunteers. Front Pharmacol. 2017;8:740. doi:10.3389/fphar.2017.00740
52. Stauffer VL, Sides R, Lanteri-Minet M, et al. Comparison between prefilled syringe and autoinjector devices on patient-reported experiences and pharmacokinetics in galcanezumab studies. Patient Prefer Adherence. 2018;12:1785–1795.
53. Kielbasa W, Quinlan T. Population pharmacokinetics of galcanezumab, an anti-CGRP antibody, following subcutaneous dosing to healthy individuals and patients with migraine. J Clin Pharmacol. 2020;60(2):229–239. doi:10.1002/jcph.1511
54. Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–588. doi:10.1002/psp4.12224
55. Hay DL, Walker CS. CGRP and its receptors. Headache. 2017;57(4):625–636. doi:10.1111/head.13064
56. Tumani H, Huss A, Bachhuber F. The cerebrospinal fluid and barriers - anatomic and physiologic considerations. Handb Clin Neurol. 2017;146:21–32.
57. Johnson KW, Morin SM, Wroblewski VJ, Johnson MP. Peripheral and central nervous system distribution of the CGRP neutralizing antibody [125 I] galcanezumab in male rats. Cephalalgia. 2019;39(10):1241–1248. doi:10.1177/0333102419844711
58. Goadsby PJ, Dodick DW, Leone M, et al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med. 2019;381(2):132–141. doi:10.1056/NEJMoa1813440
59. Dodick DW, Goadsby PJ, Lucas C, et al. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: results from 3-month double-blind treatment. Cephalalgia. 2020;333102420905321.
60. Lanteri-Minet M, Kalidas K, Oakes TM, et al. Acute and preventive treatment use in a phase 3 randomized trial of galcanezumab in chronic cluster headache. J Neurol Sci. 2019;405S:103996.
61. Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine. BMC Neurol. 2018;18(1):188. doi:10.1186/s12883-018-1193-2
62. Bangs ME, Kudrow D, Wang S, et al. Safety and tolerability of monthly galcanezumab injections in patients with migraine: integrated results from migraine clinical studies. BMC Neurol. 2020;20(1):25. doi:10.1186/s12883-020-1609-7
63. Zhao X, Xu X, Li Q. Efficacy and safety of galcanezumab for preventive treatment of migraine: a systematic review and meta-analysis. J Neurol. 2020. doi:10.1007/s00415-020-09707-5
64. Lasaosa SS, Diago EB, Calzada JN, Benito AV. Cardiovascular risk factors in cluster headache. Pain Med. 2017;8(6):1161–1167.
65. Manzoni GC. Cluster headache and lifestyle: remarks on a population of 374 male patients. Cephalalgia. 1999;19(2):88–94. doi:10.1046/j.1468-2982.1999.019002088.x
66. Bloem K, Hernández-Breijo B, Martínez-Feito A, Rispens T. Immunogenicity of therapeutic antibodies: monitoring antidrug antibodies in a clinical context. Ther Drug Monit. 2017;39(4):327–332. doi:10.1097/FTD.0000000000000404
67. Sancar F. Episodic cluster headache antibody therapy. JAMA. 2019;322(3):199.
68. Snoer A, Vollesen ALH, Beske RP, et al. Calcitonin gene-related peptide and disease activity in cluster headache. Cephalalgia. 2019;39(5):575–584. doi:10.1177/0333102419837154
69. Goadsby PJ, de Coo IF, Silver N, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38(5):959–969.
70. Mitsikostas DD, Edvinsson L, Jensen RH, et al. Refractory chronic cluster headache: A consensus statement on clinical definition from the European Headache Federation. J Headache Pain. 2014;15(1):79. doi:10.1186/1129-2377-15-79
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
