Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Reduced plasma orexin-A levels in patients with bipolar disorder
Authors Tsuchimine S, Hattori K, Ota M, Hidese S , Teraishi T, Sasayama D, Hori H, Noda T, Yoshida S, Yoshida F, Kunugi H
Received 17 March 2019
Accepted for publication 17 May 2019
Published 6 August 2019 Volume 2019:15 Pages 2221—2230
DOI https://doi.org/10.2147/NDT.S209023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Shoko Tsuchimine,1 Kotaro Hattori,1 Miho Ota,1 Shinsuke Hidese,1 Toshiya Teraishi,1 Daimei Sasayama,1 Hiroaki Hori,1 Takamasa Noda,2 Sumiko Yoshida,2 Fuyuko Yoshida,1 Hiroshi Kunugi1
1Department of Mental Disorder Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Tokyo 187-8502, Japan; 2Department of Psychiatry, National Center Hospital, National Center of Neurology and Psychiatry, Tokyo 187-8551, Japan
Purpose: Orexins are hypothalamic neuropeptides involved in the regulation of sleep, appetite and arousal. An altered orexin system has been implicated in the pathophysiology of psychiatric disorders. This study aimed to examine whether plasma orexin-A levels differ in patients with schizophrenia, major depressive disorder (MDD), or bipolar disorder (BD) compared to in healthy controls. We also examined the possible correlations between plasma orexin-A levels and clinical variables.
Patients and methods: All participants were Japanese. The sample consisted of 80 patients with schizophrenia (42 women, 52.5%; mean age 36.8 years), 80 patients with MDD (43 women, 53.8%; 43.7 years), and 40 patients with BD (24 women, 60%; 41.1 years), as well as 80 healthy controls (48 women, 60%; 47.0 years). Plasma orexin-A levels were quantified by an enzyme-linked immunosorbent assay.
Results: Mean orexin-A levels were significantly different across the four diagnostic groups (F=4.09; df=3; p=0.007, η2=0.06). In particular, the patients with BD showed significantly lower orexin-A levels than did the controls. When the median value of the control group (109.8 pg/ml) was set as a cut-off value, subjects whose orexin-A levels were below the cut-off were more common in all psychiatric groups (schizophrenia: 73.8%, x2=9.56, df=1, p=0.003, OR=2.81, 95% CI: 1.45 to 5.45, d=0.57; MDD: 78.5%, x2=14.02, df=1, p<0.001, OR=3.65, 95% CI: 1.82 to 7.29, d=0.72; BD: 87.5%, x2=16.0, df=1, p<0.001, OR=7.00, 95% CI: 2.49 to 19.70, d=1.07). We found no association between plasma orexin-A levels and any clinical symptoms, depression severity, or medication doses.
Conclusion: Our results suggest that plasma orexin-A levels are reduced in patients with BD.
Keywords: orexin-A, plasma, schizophrenia, major depressive disorder, bipolar disorder
Introduction
Orexin A (hypocretin-1) and B (hypocretin-2) are 33- and 28-amino acid peptides, respectively, and are synthesized in a cluster of neurons in the lateral hypothalamus. Orexins selectively act on two G-protein-coupled receptors: the orexin-1 receptor (OX1R), which has higher affinity to orexin-A, and the orexin-2 receptor (OX2R), which has similar affinities to orexin-A and orexin-B.1,2 These orexin receptors are expressed throughout the brain, although they are extensively localized within the hypothalamus, hippocampus, raphe nuclei, basal ganglia, locus coeruleus and cortex.3–5 Orexin neurons are located only in the lateral hypothalamus and perifornical area.6,7 However, their projection fibers are widely distributed in the central nervous system (CNS), including the basal forebrain, thalamus, and prefrontal cortex.8
Until recently, orexin has been studied extensively in patients with narcolepsy whose cerebrospinal fluid (CSF) orexin levels were found to be very low.9,10 The orexin system plays a role not only in sleep awake status11 but also in motor control,12 feeding behavior,6 stress response14 and reward,14 and the regulation of autonomic functions and energy homeostasis.13 Preclinical and cellular studies suggest that orexin-A and/or orexin afferents modulate noradrenaline, serotonin, dopamine and gamma-aminobutyric acid (GABA) systems15–17 and that CSF orexin-A levels are correlated with corticotropin releasing hormone (CRH) levels in humans.18 These neurochemical and neuroendocrine systems have been implicated in the pathophysiology of psychiatric disorders, including mood disorders and schizophrenia.18–21 For example, orexin receptor antagonists reduced stress-induced anxiety-like behaviors in rodents.22–25 Intracerebral injection of orexin-A reduced depressive-like behaviors in mice.26 Lower orexin levels and a reduced number of orexin neurons in the hypothalamus, medial prefrontal cortex (mPFC) and ventral tegmental area (VTA) have been reported in several animal models of depression.27–29 In rodents, activation of hypothalamic orexin neurons can release orexins that in turn activate dopamine neurons in the VTA, increasing dopamine levels in the prefrontal cortex and/or the striatum.30,31 These animal studies suggest a possible role of orexin-A in the pathophysiology of psychiatric disorders.
Human studies have also shown that depressed patients had lower orexin-A levels in the CSF and that their depression severity was negatively correlated with orexin-A levels in the CSF.32–34 Additionally, reduced orexin-A mRNA in peripheral blood cells was reported to be correlated with higher scores on the Hamilton Depression Rating Scale.35 Salomon et al36 reported that CSF orexin-A levels in depressive subjects tended to be higher than in control subjects, while the diurnal cycle amplitude was significantly smaller than that in controls. In contrast, Schmidt et al37,38 found no significant difference in CSF orexin-A levels in patients with either mania or unipolar depression compared to healthy controls. Regarding schizophrenia, Nishino et al39 reported a positive correlation between CSF orexin-A levels and sleep latency in patients with schizophrenia. Dalal et al40 found that mean CSF orexin levels were significantly lower in patients with schizophrenia treated with haloperidol than in unmedicated patients.
A previous report showed that orexin-A (but not orexin-B) is highly lipophilic and rapidly crosses from the brain to the periphery through the blood-brain barrier (BBB) by means of a nonsaturable mechanism.41 Indeed, plasma and CSF orexin-A levels were reported to be lower in patients with narcolepsy.45,46 Strawn et al42 reported that CSF and plasma orexin-A concentrations showed a strong correlation and that peripheral orexin-A levels were correlated with CNS serotonergic tone (5-hydroxyindolacetic acid in CSF) in patients with posttraumatic stress disorder (PTSD) and healthy subjects. One study reported that patients with schizophrenia with fewer negative and disorganized symptoms had a significantly higher mean plasma orexin-A level than healthy controls.43 To our knowledge, no study has examined plasma orexin-A levels in patients with MDD or BD, and only one study compared the plasma orexin-A levels of patients with schizophrenia with those of healthy controls.
As described above, there is a dearth of studies on orexin-A levels in psychiatric disorders. The aim of the present study was to examine whether plasma orexin-A levels are altered in patients with schizophrenia, MDD, and BD compared to in healthy controls. We further examined the possible correlation between plasma orexin-A levels and clinical variables, such as disease severity and medication doses.
Materials and methods
Subjects
The subjects included 80 patients with schizophrenia, 80 patients with MDD, 40 patients with BD, and 80 healthy controls. All subjects were biologically unrelated Japanese individuals recruited from the outpatient clinic of the National Center of Neurology and Psychiatry Hospital, Tokyo, Japan, or through advertisements in free local information magazines and on our website announcement. Consensus diagnosis by at least 2 psychiatrists was made for each patient according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria,46 on the basis of information obtained by the Japanese version of the MINI (Mini International Neuropsychiatric Interview),47,48 additional unstructured interviews and information from medical records if available. The controls were healthy volunteers with no current or past history of psychiatric disorder based on the information obtained by the M.I.N.I. Participants were excluded if they had prior medical histories of CNS diseases or severe head injury or if they met the criteria for substance abuse/dependence or mental retardation. The study protocol was approved by the ethics committee at the NCNP. The study was performed according to the Declaration of Helsinki.49 After the study was described to potential subjects, written informed consent was obtained from every subject.
The Positive and Negative Syndrome Scale (PANSS) was used to evaluate the symptoms in patients with schizophrenia.50 The 17-item version of the Hamilton Depression Rating Scale (HAMD-17) was used to assess depressive symptoms in patients with MDD or BD,51 and the cut-off score for remission was set at ≤7.52 There were no patients with BD who showed a manic state at the time of blood sampling. Daily doses of antipsychotics and antidepressants were converted to chlorpromazine and imipramine equivalent doses according to the published guidelines.53
Measurement of orexin-A
At approximately noon (before lunch), blood samples were collected into EDTA blood tubes and centrifuged to separate the plasma fraction. Plasma samples were then stored at −80 °C until they were used for the assay. Plasma orexin-A levels were measured using a fluorescent enzyme immunosorbent assay (ELISA) kit (FKE-003-30; Phoenix Pharmaceuticals, Burlingame, CA, USA). Using the results from two separate runs of standard concentrations, the interassay coefficient of validation (CV) was less than 10%.
Statistical analysis
Statistical differences between groups were calculated using Student’s unpaired t-test, one-way analysis of variance (ANOVA), analysis of covariance (ANCOVA) adjusted for age, sex, and smoking status, and the chi-square test. Correlations were assessed using Pearson’s correlation coefficient. Differences with a two-tailed p-value of less than 0.05 were deemed statistically significant, and the Bonferroni correction was used for multiple comparisons. Statistical analyses were performed using SPSS Statistics version 23.0 (IBM Japan, Tokyo, Japan).
Results
The demographic and clinical characteristics of the patients and controls are presented in Table 1. There were no significant differences in the male-to-female ratio (x2=1.34; df=3; p=0.719) or body mass index (BMI) (F=1.98; df=3; p=0.117) across the four diagnostic groups. However, the schizophrenia group was significantly younger than the control and MDD groups (F=10.21; df=3; p<0.001) and had higher smoking status compared with the control (x2=7.864, df=3, p=0.049). There was no correlation between plasma orexin-A levels and age (r=0.168, p=0.136), and there was no significant sex difference in the healthy control group (F=0.793, df=78, p=0.156) (Figures S1 and S2).
 |
Table 1 Demographic and clinical characteristics |
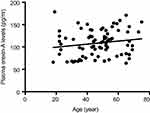 |
Figure S1 Correlation between plasma orexin-A levels and age. There was no significant correlation between plasma orexin-A levels and age in healthy controls (N=80; r=0.168, p=0.136). |
After adjusting for age, sex, and smoking status, mean orexin-A levels were significantly different across the four diagnostic groups (F=4.09; df=3; p=0.007, η2=0.06) (Table 1). When each diagnostic group was compared with the control group, there was no significant difference in plasma orexin-A levels between patients with schizophrenia and controls. The plasma orexin-A levels in patients with MDD tended to be lower than those in the control group, although the difference failed to reach statistical significance after the Bonferroni correction was applied (p=0.089). The orexin-A levels of the patients with BD were significantly lower than those of controls (p=0.010) (Figure 1). As shown in Figure 1, the number of patients with relatively lower orexin-A levels seemed to be higher for all psychiatric groups. When a cut-off point of plasma orexin-A levels was set at 109.8 pg/ml due to the median value of the control group (ie, median split), the rate of subjects below the cut-off was 73.8% in patients with schizophrenia (x2=9.56, df=1, p=0.003; OR =2.81, 95% CI: 1.45 to 5.45; d=0.57), 78.5% in patients MDD (x2=14.02, df=1, p<0.001; OR=3.65, 95% CI: 1.82 to 7.29; d=0.72), and 87.5% in patients with BD (x2=16.0, df=1, p<0.001; OR=7.00, 95% CI: 2.49 to 19.70; d=1.07) (Table 2).
 |
Table 2 Number of subjects below the cut-off point (109.8 pg/ml) and odds ratios for healthy controls |
Within each diagnostic group, there was no correlation between plasma orexin-A levels and any clinical variables, including age, BMI, severity scores, or medication doses (Table S1).
Discussion
In this study, we examined the plasma orexin-A levels in patients with schizophrenia, MDD, and BD in comparison with those of healthy controls. When each diagnostic group was compared with the control group, mean orexin-A level was significantly lower only in the patients with BD. Individuals who showed plasma orexin-A levels below the cut-off point (109.8 pg/ml; median value of controls group) were significantly more numerous in the schizophrenia, MDD, and BD groups than in the control group. However, we found no association between plasma orexin-A levels and any clinical variable, such as disease severity and medication dose.
To our knowledge, this is the first study that examined plasma orexin-A levels in the three psychiatric disorders in comparison with those of healthy controls. Several studies have reported that orexin-A is present not only in the CSF but also in the peripheral blood of healthy individuals and some narcoleptic patients.9,10,44,45 With regard to psychiatric disorders, there have been only a few reports that have examined the association between plasma orexin-A levels and mood disorder and schizophrenia. Strawn et al42 reported that the mean plasma and CSF orexin-A concentrations were significantly lower in patients with PTSD than in healthy subjects. There was a strong correlation between CSF and plasma orexin-A concentrations in both patients with PTSD and healthy subjects, and plasma orexin-A was negatively correlated with CSF 5-HIAA in healthy subjects.42 Chien et al43 showed that patients with schizophrenia (N=127) had significantly higher mean plasma orexin-A levels than did healthy controls (N=34); however, when the patients were divided into two groups consisting of high and low orexin levels, patients with normal orexin-A levels had significantly more negative symptoms than did patients with high-orexin levels.
Although the origin of plasma orexin-A has not yet been determined, orexin-A neurons are restricted to the lateral hypothalamus area,7,8 and highly lipophilic orexin-A rapidly crosses the BBB through a nonsaturable mechanism.41 Therefore, circulating orexin-A could originate from the hypothalamus via the BBB, in which case plasma orexin-A levels may at least partially reflect the production of orexin-A in the hypothalamus. Our results suggest that the observed decrease in plasma orexin-A levels reflects decreased central orexin-A in patients with psychiatric disorders.
We found that the patients in psychiatric disorder groups, particularly patients with BD, exhibited low levels of orexin-A than did the healthy controls. Because psychiatric patients frequently present sleep disorders and circadian disruption,54–56 altered orexin-A signaling may contribute to such arousal and wake-rest disruptions. Specifically, BD patients have a mismatched biological clock and sleep-wake cycle, and the rate of comorbid circadian rhythm sleep-wake disorders in BD subjects was significantly higher than that in MDD subjects.57,58 During periods of depression in patients with BD, sleep disruptions are commonly exhibited as hypersomnia or excessive sleepiness. Through the use of actigraphy data, research among patients with a current depression diagnosis suggests that patients with bipolar depression are likely to have a delayed sleep phase.59 In addition, we have previously reported that MDD and BD patients showed significant disturbances in sleep and rest-activity rhythms, along with reduced activity levels and increased sleep time each day.60 Another explanation for the lower orexin-A levels is that the genetic studies addressed the correlation between the orexin system and psychiatric disorders. The carrier of the A allele of the rs2271933 G>A polymorphism (Ile408Val) in the HCRTR1 gene was more common in unipolar depression than in controls.61 Rotter et al35 reported that orexin-A mRNA expression in peripheral blood cells was decreased in depressive patients.
We found no significant correlation between orexin-A levels and disease severity or medication dose in any diagnostic group. These findings may have been limited by the fact that patients in the present study are mostly in the chronic phase rather than in the acute phase. Additionally, our measurement of the plasma sample took place in a real-world setting; the majority of patients were taking psychotropic medication, and sampling was not performed until after a whole night of fasting (but before lunch). However, a recent investigation reported that plasma orexin-A levels are not related to circadian rhythms or feeding;62 it is not yet clear whether plasma orexin-A has any physiological significance in relation to psychiatric disorders. The role of orexin-A in the pathological states of psychiatric disorders such as schizophrenia, MDD and BD needs to be investigated in further studies.
A major limitation is that the patients were under pharmacotherapy and thus the severity of their illness was relatively mild at the time of participation in the study (mean HAM-D scores of 11.4 in MDD and 10.9 in BD; mean PANSS score of 60.6 in schizophrenia), which may have resulted in type II errors, particularly for MDD and schizophrenia groups. To address this issue, further studies in more severe subjects will be required. It is also possible that the medication may have affected the plasma orexin-A level in our patients. However, we found no significant correlation between plasma orexin-A levels and medication dose in any diagnostic group. It is therefore unlikely that medication has a major effect on plasma orexin-A levels.
Conclusion
Our results suggest that plasma orexin-A levels are reduced in patients with BD than in healthy controls, suggesting that the low plasma levels of orexin-A play a role, at least in a proportion of BD. However, the elucidation of the clinical implications of plasma orexin-A concentrations would require further clinical studies with larger sample sizes and preclinical studies to examine the mechanisms underlying the altered orexin-A levels in BD.
Acknowledgments
This study was supported by an Intramural Research Grant (30-1, 30-7) from the Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry (H.K.) and the Strategic Research Program for Brain Sciences (16dm0107100 h0001, H.K.) from Japan Agency for Medical Research and Development (AMED).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. doi:10.1146/annurev-pharmtox-010510-100528
2. Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25.
3. Blanco M, López M, GarcIa-Caballero T, et al. Cellular localization of orexin receptors in human pituitary. J Clin Endocrinol Metab. 2001;86:1616–1619. doi:10.1210/jcem.86.7.7433
4. Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG. Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J Clin Endocrinol Metab. 2001;86:778–782. doi:10.1210/jcem.86.2.7233
5. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16:1785–1797. doi:10.1517/13543784.16.11.1785
6. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585.
7. Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260.
8. Peyron C, Tighe DK, van Den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015.
9. Ripley B, Overeem S, Fujiki N, et al. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57:2253–2258. doi:10.1212/wnl.57.12.2253
10. Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562.
11. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. Review. doi:10.1038/nature04284
12. Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–5345. doi:10.1523/JNEUROSCI.4812-03.2004
13. Plazzi G, Moghadam KK, Maggi LS, et al. Autonomic disturbances in narcolepsy. Sleep Med Rev. 2011;15:187–196. doi:10.1016/j.smrv.2010.05.002
14. Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. doi:10.3389/fnbeh.2013.00028
15. Singareddy R, Uhde T, Commissaris R. Differential effects of hypocretins on noise-alone versus potentiated startle responses. Physiol Behav. 2006;89:650–655. doi:10.1016/j.physbeh.2006.08.004
16. Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi:10.1111/j.1460-9568.2006.04792.x
17. Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi:10.1111/j.1460-9568.2005.04121.x
18. Sarchielli P, Rainero I, Coppola F, et al. Involvement of corticotrophin-releasing factor and orexin-A in chronic migraine and medication-overuse headache: findings from cerebrospinal fluid. Cephalalgia. 2008;28:714–722. doi:10.1111/j.1468-2982.2008.01566.x
19. Cowen P. Neuroendocrine and neurochemical processes in depression. Psychopathol Rev. 2015;3:3–15.
20. Lieberman JA, Koreen AR. Neurochemistry and neuroendocrinology of schizophrenia: a selective review. Schizophr Bull. 1993;19:371–429. Review. doi:10.1093/schbul/19.2.371
21. Kunugi H, Hori H, Ogawa S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin Neurosci. 2015;69:597–608. doi:10.1111/pcn.12299
22. Johnson PL, Truitt W, Fitz SD, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi:10.1038/nm.2075
23. Plaza-Zabala A, Martín-García E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi:10.1523/JNEUROSCI.5724-09.2010
24. Heydendael W, Sharma K, Iyer V, et al. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology. 2011;152:4738–4752. doi:10.1210/en.2011-1652
25. Staples LG, Cornish JL. The orexin-1 receptor antagonist SB-334867 attenuates anxiety in rats exposed to cat odor but not the elevated plus maze: an investigation of trial 1 and trial 2 effects. Horm Behav. 2014;65:294–300. doi:10.1016/j.yhbeh.2013.12.014
26. Ito N, Yabe T, Gamo Y, et al. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 2008;157:720–732. doi:10.1016/j.neuroscience.2008.09.042
27. Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38:311–315. doi:10.1016/j.npep.2004.06.004
28. Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–3075. doi:10.1523/JNEUROSCI.5584-07.2008
29. Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi:10.1016/j.neuroscience.2012.05.033
30. Rasmussen K, Hsu MA, Noone S, Johnson BG, Thompson LK, Hemrick-Luecke SK. The orexin-1 antagonist SB-334867 blocks antipsychotic treatment emergent catalepsy: implications for the treatment of extrapyramidal symptoms. Schizophr Bull. 2007;33:1291–1297. doi:10.1093/schbul/sbm087
31. Rasmussen K, Hsu MA, Yang Y. The orexin-1 receptor antagonist SB-334867 blocks the effects of antipsychotics on the activity of A9 and A10 dopamine neurons: implications for antipsychotic therapy. Neuropsychopharmacology. 2007;32:786–792. doi:10.1038/sj.npp.1301239
32. Brundin L, Björkqvist M, Petersén A, Träskman-Bendz L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol. 2007;17:573–579. doi:10.1016/j.euroneuro.2007.01.005
33. Brundin L, Petersén A, Björkqvist M, Träskman-Bendz L. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord. 2007;100:259–263. doi:10.1016/j.jad.2006.10.019
34. Brundin L, Björkqvist M, Träskman-Bendz L, Petersén A. Increased orexin levels in the cerebrospinal fluid the first year after a suicide attempt. J Affect Disord. 2009;113:179–182. doi:10.1016/j.jad.2008.04.011
35. Rotter A, Asemann R, Decker A, Kornhuber J, Biermann T. Orexin expression and promoter-methylation in peripheral blood of patients suffering from major depressive disorder. J Affect Disord. 2011;131:186–192. doi:10.1016/j.jad.2010.12.004
36. Salomon RM, Ripley B, Kennedy JS, et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104.
37. Schmidt FM, Brügel M, Kratzsch J, et al. Cerebrospinal fluid hypocretin-1 (orexin A) levels in mania compared to unipolar depression and healthy controls. Neurosci Lett. 2010;483:20–22. doi:10.1016/j.neulet.2010.07.038
38. Schmidt FM, Arendt E, Steinmetzer A, et al. CSF-hypocretin-1 levels in patients with major depressive disorder compared to healthy controls. Psychiatry Res. 2011;190:240–243. doi:10.1016/j.psychres.2011.06.004
39. Nishino S, Ripley B, Mignot E, Benson KL, Zarcone VP. CSF hypocretin-1 levels in schizophrenics and controls: relationship to sleep architecture. Psychiatry Res. 2002;110:1–7.
40. Dalal MA, Schuld A, Pollmächer T. Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol Psychiatry. 2003;8:836–837. doi:10.1038/sj.mp.4001363
41. Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–223.
42. Strawn JR, Pyne-Geithman GJ, Ekhator NN, et al. Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology. 2010;35:1001–1007. doi:10.1016/j.psyneuen.2010.01.001
43. Chien YL, Liu CM, Shan JC, et al. Elevated plasma orexin A levels in a subgroup of patients with schizophrenia associated with fewer negative and disorganized symptoms. Psychoneuroendocrinology. 2015;53:1–9. doi:10.1016/j.psyneuen.2014.12.012
44. Nishino S, Mignot E. Article reviewed: plasma orexin-A is lower in patients with narcolepsy. Sleep Med. 2002;3(4):377–378.
45. Higuchi S, Usui A, Murasaki M, et al. Plasma orexin-A is lower in patients with narcolepsy. Neurosci Lett. 2002;318:61–64.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
47. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33.
48. Otsubo T, Tanaka K, Koda R, et al. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59:517–526. doi:10.1111/j.1440-1819.2005.01408.x
49. World Medical Assocition. World Medical Association Declaration of Helsinki: ethical principles for medical reseach involving humansubjects. Jama. 2000;284:3043–3045.
50. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi:10.1093/schbul/13.2.261
51. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;56–62. doi:10.1136/jnnp.23.1.56
52. Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. J Affect Disord. 2013;150:384–388. doi:10.1016/j.jad.2013.04.028
53. Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69:440–447. doi:10.1111/pcn.12275
54. Kaskie RE, Graziano B, Ferrarelli F. Schizophrenia and sleep disorders: links, risks, and management challenges. Nat Sci Sleep. 2017;9:227–239. doi:10.2147/NSS.S121076
55. Abreu T, Bragança M. The bipolarity of light and dark: a review on bipolar disorder and circadian cycles. J Affect Disord. 2015;185:219–229. doi:10.1016/j.jad.2015.07.017
56. Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–585. doi:10.1002/hup.964
57. Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi:10.1176/appi.ajp.2008.08010098
58. Takaesu Y, Inoue Y, Ono K, et al. Circadian rhythm sleep-wake disorders as predictors for bipolar disorder in patients with remitted mood disorders. J Affect Disord. 2017;220:57–61. doi:10.1016/j.jad.2017.05.041
59. Robillard R, Naismith SL, Rogers NL, et al. Delayed sleep phase in young people with unipolar or bipolar affective disorders. J Affect Disord. 2013;145:260–263. doi:10.1016/j.jad.2012.06.006
60. Hori H, Koga N, Hidese S, et al. 24-h activity rhythm and sleep in depressed outpatients. J Psychiatr Res. 2016;77:27–34. doi:10.1016/j.jpsychires.2016.02.022
61. Rainero I, Ostacoli L, Rubino E, et al. Association between major mood disorders and the hypocretin receptor 1 gene. J Affect Disord. 2011;130:487–491. doi:10.1016/j.jad.2010.10.033
62. Mäkelä KA, Karhu T, Jurado Acosta A, Vakkuri O, Leppäluoto J, Herzig KH. Plasma orexin-A levels do not undergo circadian rhythm in young healthy male subjects. Front Endocrinol (Lausanne). 2018;9:710. doi:10.3389/fendo.2018.00420
Supplementary materials
 |
Table S1 Simple correlations between plasma orexin-A levels and clinical variables |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


