Back to Journals » Clinical Interventions in Aging » Volume 17
Red Cell Distribution Width-to-High-Density Lipoprotein Cholesterol Ratio (RHR): A Promising Novel Predictor for Preoperative Deep Vein Thrombosis in Geriatric Patients with Hip Fracture
Authors Cheng X, Fan L, Hao J , He H , Yan J, Zhu Y
Received 30 May 2022
Accepted for publication 22 August 2022
Published 1 September 2022 Volume 2022:17 Pages 1319—1329
DOI https://doi.org/10.2147/CIA.S375762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Xinqun Cheng,1,2,* Lingjia Fan,3,* Jiabei Hao,4 Honghou He,4 Jincheng Yan,1,2 Yanbin Zhu1,2
1Department of Orthopaedic Surgery, The Third Hospital of Hebei Medical University, Shijiazhuang, 050051, People’s Republic of China; 2Hebei Orthopedic Research Institute, Key Laboratory of Biomechanics of Hebei Province, Shijiazhuang, 050051, People’s Republic of China; 3Department of Orthopadic Surgery, Shandong First Medical University, Jinan, 250000, People’s Republic of China; 4Basic Medical College, Hebei Medical University, Shijiazhuang, 050017, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jincheng Yan; Yanbin Zhu, Department of Orthopaedic Surgery, The Third Hospital of Hebei Medical University, Shijiazhuang, 050051, People’s Republic of China, Email [email protected]; [email protected]
Background: Deep vein thrombosis (DVT) is a devastating complication in geriatric patients before hip fracture surgery, and the predictive value of red cell distribution width (RDW) and high-density lipoprotein cholesterol (HDL-C) for DVTs after hip fracture remains to be established. This study aimed to assess the predictive value of RDW, HDL-C, and RDW-to-HDL-C ratio (RHR) in preoperative DVTs screening.
Methods: We retrospectively analyzed the data of geriatric patients (≥ 65 years old) admitted for hip fracture surgery between 2015 and 2020. The receiver operating characteristic (ROC) curve and related parameters were used to evaluate the predictive value of the biomarkers. Patients were divided into two groups according to the cutoff value of RHR, and propensity score matching (PSM) and subgroup analyses were performed to assess the true correlations between RHR and DVT.
Results: Among 2566 eligible patients included, we identified RDW with the area under ROC curve (AUC) of 0.532, cut-off value of 15.89, specificity of 88.2%, sensitivity of 18.2%, HDL-C with AUC of 0.574, cut-off value of 1.20, specificity of 55.6%, sensitivity of 59.3%, and RHR with AUC of 0.578, cut-off value of 13.45, specificity of 71.3%, sensitivity of 43.4%. RHR (> 13.45) was independently associated with 1.54-fold risk (95% CI: 1.11– 2.14, P=0.011) of DVTs among the post-PSM cohort. And compared with the counterparts, the relative risk of RHR associated with DVT was higher in the subgroups of aged 65– 79 years (1.61 vs 1.45), non-hypoproteinemia (2.70 vs 1.29), non-diabetic (1.58 vs 1.41), non-hypertension (2.40 vs 1.06), ASA score I-II (2.38 vs 1.04), and femoral neck fracture (1.70 vs 1.50).
Conclusion: RDW, HDL-C and RHR were valuable biomarkers in predicting preoperative DVTs in geriatric patients with hip fracture, and RHR would be more efficient in the subgroups of younger age, better medical condition or femoral neck fracture.
Keywords: geriatric patients, hip fracture, deep vein thrombosis, red cell distribution width, high-density lipoprotein cholesterol
Introduction
Deep vein thrombosis (DVT) is a prevalent (11.1–35.0%) in geriatric patients with hip fracture before surgery,1–3 which potentially causes pulmonary embolism (PE) and even leads to mortality.4 However, the vast majority (75.2–100%) of the DVTs are asymptomatic, which thus pose clinical challenges for early diagnosis and timely targeted intervention.5–7 Given that the incidence of hip fracture in geriatric patients would increase dramatically with the exponential increase of the aging population,8 improving the ability in predicting or diagnosing DVTs in newly admitted patients becomes increasingly significant and urgent.
In addition to rotational thromboelastometry,9–11 the hematologic parameters, used alone or in combination with other baseline characteristics to establish prediction models, were cost-effective ways to address this issue.12–14 D-dimer, as a reliable biomarker for coagulation and fibrinolysis, is generally used in clinical practice for primary screening of DVT due to its high sensitivity and convenience.15 However, low specificity limited its further application in the DVT diagnostic algorithm. Therefore, it remains a hot spot to find reliable biomarkers with high specificity for the development of DVT prediction models currently, and more emerging evidences have shown red cell distribution width (RDW) and high-density lipoprotein cholesterol (HDL-C) had potential as such. For example, for emergency admissions, high RDW (>18.9%) had a specificity of 93.4% for acute PE prediction.16 HDL can inhibit VTE through a variety of antithrombotic mechanisms,17 and low HDL-C (<1.1 mmol/L) has been identified as an independent risk factor for preoperative DVT in patients with spinal or foot fractures.18,19 Nevertheless, to our best knowledge, no studies have evaluated the role of either biomarker in predicting the incident DVTs after hip fracture; and indeed, extrapolation of the above finding available from non-hip fracture or even non-trauma patients to the geriatric hip fracture patients (characterized by elderly age, hemodynamical instability, and post-traumatic hypercoagulability, etc) might be inappropriate. Furthermore, no studies attempt to explore the predictive value of RDW-to-HDL-C ratio (RHR) in DVTs screening.
Hence, for geriatric patients with hip fracture, this study aims to assess the predictive value of RDW and HDL-C for preoperative DVTs and to further explore whether RHR could be a promising novel predictor.
Materials and Methods
Patients
All data were retrospectively collected from the database of Surgical Site Infection in Orthopaedic Surgery (SSIOS), and clinical data of 2566 geriatric patients admitted to The Third Hospital of Hebei Medical University for hip fracture surgery between October 2015 and December 2020 were included in this study. The inclusion criteria were 1) elderly (≥65 years old) patients; 2) acute hip fracture (femoral neck or intertrochanteric fracture); 3) experiencing both hematological test and duplex ultrasonography (DUS) examination preoperatively. The exclusion criteria were 1) incomplete data; 2) multiple fractures; 3) periprosthetic, pathological or open fracture; 4) old fracture (>21 days from injury to DUS); 5) history of DVT and/or PE events; 6) use of antithrombotic thromboembolism therapy (eg, aspirin, low-molecular-weight heparin) within 3 months. The retrospective study was approved by the ethics committee of The Third Hospital of Hebei Medical University, and all procedures were performed under the principles outlined in the Helsinki Declaration and complied with the guideline of Strengthening the Reporting of Cohort Studies in Surgery (STROCSS). Informed consent for the possible use of the clinical data was obtained from all participants.
Diagnosis and Management of DVT
The diagnostic criteria for DVT were incompletely compressible vein, insufficient flow augmentation to veins of foot and calf after compression, lack of respiratory vibration in the superior knee vein segment, and filling defect or obstruction of the lumen. The scanning range included common femoral vein, popliteal vein, superficial femoral vein, deep femoral vein, posterior tibial vein, anterior tibial vein, and peroneal vein. DUS examination was performed by skilled technicians to identify the presence of DVT within 24 hours of patient admission and got reviewed every 4–6 days during the preoperative waiting period. Depending on the DUS results, patients would be given prophylactic or therapeutic doses of anticoagulant agents (eg, enoxaparin sodium injection). For patients needing long-term bed rest, we usually instruct them to actively move the healthy limb, drink more water, and use the intermittent pneumatic pressure pumps to assist venous blood return for DVT prevention.
Data Collection
The clinical data of interest were collected from four aspects: demographics, injury-concerned data, chronic comorbidities, and laboratory biomarkers. The demographics included age, living place, gender, and the calculated body mass index (BMI). Injury-concerned data included fracture type, time from injury to DUS, and the American Society of Anesthesiologists (ASA) score. The comorbidities comprised diabetes mellitus, hypertension, heart disease, cerebrovascular disease, hepatopathy, nephropathy, tumors, smoking, alcohol drinking, and history of allergy or operation. The laboratory biomarkers comprised prothrombin time (PT), activated partial thromboplastin time (APTT), the count of platelet (PLT), and the levels of D-dimer, sodium, hemoglobin (HGB), albumin (ALB), fasting blood glucose (FBG), antithrombin III (AT III), fibrinogen (FIB), high-sensitivity C-reactive protein (HCRP), red cell distribution width (RDW), high-density lipoprotein cholesterol (HDL-C) and RDW-to-HDL-C ratio (RHR). These biomarkers were measured using manufacturer recommended methods, where complete blood count test was performed using a hematology analyzer (UniCel DxH 800; Beckman Coulter, Brea, CA, USA), coagulation studies were conducted using an ACL TOP 750 coagulometer (Instrumentation Laboratory, Bedford, MA, USA), and biochemical tests were performed using an autoanalyzer AU5800 (Beckman Coulter). If a patient had undergone multiple hematology tests prior to DUS examination, we analyzed only the initial results. In addition to age and time to DUS, all variables were expressed as categorical variables, and the optimal cutoff values of HCRP, RDW, HDL-C, RHR, and D-dimer were determined by using the Youden’s index.
Statistical Analysis
The Kolmogorov–Smirnov test was first used to evaluate the normality of continuous variables. When normally distributed, the variables were expressed as mean ± standard deviation (SD) and analyzed by using Student’s t-test, otherwise, were presented as median [interquartile range (IQR)] and analyzed by performing the Mann–Whitney test. Categorical variables were presented as numbers and percentages (%) and tested by the chi-square test or Fisher’s exact test as appropriate. The receiver operating characteristic (ROC) curve was performed to evaluate the diagnostic yield of four indicators, including RHR, RDW, HDL-C, and D-dimer, and the diagnostic ability of which was compared via the area under the ROC curve (AUC). Comparison of AUC was performed using the test of DeLong et al20 by MedCalc software version 18.10 (MedCalc Software, Ostend, Belgium). The optimal cutoff values of these indicators were determined based on the maximum Youden’s index. Their accuracy, negative predictive value (NPV), positive predictive value (PPV), sensitivity and specificity for DVT diagnosis, and the 95% confidence interval (95% CI) were also measured.
According to the optimal cutoff value of RHR, patients were divided into two groups of “low RHR” and “high RHR”. To minimize the interference of potential confounders between groups, propensity score matching (PSM) was performed to adjust covariates (excluding RDW and HDL-C). The propensity scores for each patient were calculated by using a multivariate logistic regression model and a 1:1 nearest neighbor matching algorithm with a caliper width of 0.02 was used to determine the post-PSM datasets. Standardized mean difference (SMD) was used to measure the balance of covariates before and after PSM, in which SMD>0.1 indicates imbalance. Univariate logistic regression analysis was performed to assess the association between RHR and DVT and acquire the association magnitude.
Subgroup analysis was performed for further exploration of the diagnostic value of RHR in the post-PSM cohort. Hypoproteinemia, diabetes mellitus, and hypertension are common modifiable complications in geriatric traumatic patients on admission. Thus, we divided the post-PSM cohort into several subgroups based on age, albumin level, diabetes mellitus, hypertension, ASA score, and fracture type. Univariate Logistic regression analysis was performed to calculate the relative risk (RR) and 95% CI for incident DVT events associated with high RHR and to assess the interaction between high RHR and grouping covariates. The differences were considered statistically significant when P value was less than 0.05. All the analyses were performed by using R software 3.6.5 (R Foundation for Statistical Computing, Vienna, Austria) and the software SPSS 26.0 (IBM Corp, Armonk, New York, USA).
Results
As is shown in Figure 1, there were totally 3367 geriatric patients with surgically treated hip fracture during the study window. According to exclusion criteria, 2566 patients were finally retained for analysis, and 258 (10.1%) were diagnosed with preoperative DVT after DUS examination.
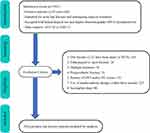 |
Figure 1 Flowchart of patient eligibility screening. |
As is shown in Table 1 and Figure 2, the AUC, PPV, NPV, accuracy, sensitivity, specificity, and their respective 95% CI were calculated for RDW, HDL-C, RHR, and D-dimer. Comparing the parameters of the four predictors, we identified that RHR has the highest AUC (0.578), RDW has the highest specificity (88.2%), accuracy (81.2%), and PPV (14.7%), and HDL-C has the highest sensitivity (59.3%) and NPV (92.4%). We noted that RDW is characterized by high specificity and low sensitivity. In the comparison with RDW, RHR got significantly improved sensitivity (%, 43.4 vs 18.2) at the cost of slightly decreased specificity (%, 71.3 vs 88.2). The AUC of RHR was higher than that of RDW (0.578 vs 0.532, P=0.024), HDL-C (0.578 vs 0.574, P=0.506), and D-dimer (0.578 vs 0.569, P=0.773). In addition, compared with D-dimer, RHR has higher specificity (%, 71.3 vs 59.6), accuracy (%, 68.5 vs 59.2), and PPV (%, 14.5 vs 13.2).
 |
Table 1 Evaluation of Characteristic Parameters in Four Biomarkers |
 |
Figure 2 ROC curves for comparisons RDW, HDL-C, RHR, and D-dimer in geriatric patients with hip fracture. |
The optimal cutoff value of RHR was 13.45 and based on which, patients were divided into two groups of “low RHR” and “high RHR”. As is shown in Table 2, for patients in the before-PSM dataset, 1790 (69.8%) were included in the low RHR group and 776 (30.2%) in the high RHR group. After univariate analyses, we found the between-group differences were statistically significant in the following covariates: age, time to DUS, BMI, fracture type, gender, living place, allergic history, HGB, PLT, ALB, PT, AT III, FIB, D-dimer and HCRP. Thirteen covariates were calculated with SMD>0.1 before PSM, including gender, time to DUS, fracture type, living place, allergic history, BMI, FIB, AT III, PT, PLT, HGB, ALB, and HCRP. After PSM, 1386 were retained with 1:1 between-group matching. The univariate logistic analyses showed no covariate statistically significant between the two groups and all covariates were calculated with SMD<0.1, indicating that all covariates got well balanced in the post-PSM cohort (Figure 3). Univariate logistic regression analysis showed that high RHR was associated with a 1.54-fold increased risk of DVT and the difference was statistically significant (OR: 1.54, 95% CI: 1.11–2.14, P=0.011).
 |
Table 2 Comparison of the Baseline Characteristics of Patients Before and After PSM According to the Propensity Score |
 |
Figure 3 Standardized mean differences (SMD) between the groups of high RHR and low RHR across baseline clinical data. |
As is shown in Figure 4, high RHR was positively associated with the risk of developing DVT among all of the subgroups (all RR>1), and the interaction between high RHR and intertrochanteric fracture was statistically significant (P<0.001). Compared with the counterparts, the relative risk of DVT associated with high RHR was higher in the subgroups of aged 65–79 years (1.61 vs 1.45, P=0.028), non-hypoproteinemia (2.70 vs 1.29, P=0.006), non-diabetic (1.58 vs 1.41, P=0.019), non-hypertension (2.40 vs 1.06, P=0.001), ASA score I-II (2.38 vs 1.04, P=0.001), and femoral neck fracture (1.70 vs 1.50, P=0.088).
Discussion
To our knowledge, this is the first study to explore the diagnostic capacities of RDW, HDL-C, and RHR in incident DVTs among geriatric patients before hip fracture surgery. When comparing the predictive performances of RDW, HDL-C, and D-dimer, we identified RDW with the highest specificity and PPV, and HDL-C with the highest sensitivity and NPV. RHR has complementary strengths of the above two biomarkers. Compared with D-dimer, RHR, a novel biomarker, has higher AUC and specificity in predicting DVTs. We identified that high RHR (>13.45) was independently associated with 1.54-fold risks of incident DVT in the post-PSM cohort. Compared with the counterparts, the relative risk of DVT associated with high RHR was higher in the subgroups of aged 65–79 years, non-diabetic, non-hypertension, non-hypoproteinemia, ASA score I-II, and femoral neck fracture.
RDW reflects the heterogeneity of circulating red blood cell volume, which is a convenient and cost-effective measurement of the variation in size of red blood cells (RBCs).21 Zöller et al22 studied 27,042 subjects (60.6% women, aged 45–73 years) and found that those with the top 5% RDW values had a 2.51-fold higher risk of developing venous thrombus embolism (VTE) than those of the bottom quartile. Increased RDW indicates significant deregulation of erythrocyte homeostasis, including both abnormal RBC survival and impaired erythropoiesis, and was strongly associated with a higher risk of DVT, which can be explained by the following underlying mechanisms. For one thing, increased RDW is significantly associated with reduced RBC deformability, which can cause elevated blood viscosity and impair microcirculatory blood flow; furthermore, enhanced RBC aggregation promotes greater margination of leukocytes and platelets.23–25 For another, high RDW is closely related to oxidative stress. Increased reactive oxygen species (ROS) can stimulate coagulation by inhibiting tissue factor pathway inhibitor (TFPI) and upregulating the tissue factor (TF) expression; in addition, ROS can make fibrinogen more readily translatable to fibrin, and decrease the interaction between thrombin and anticoagulants, such as the antithrombin III-heparin complex.26
Low HDL-C is a common feature of dyslipidemia, and its predictive role in thrombus formation has been verified by numerous studies. HDL-C has been identified as an independent predictor of DVT in both the trauma and oncology fields,18,19,27 and HDL-C levels were found lower in thromboembolic patients among researches providing evidence of the direct link between dyslipidemia and VTE.28,29 The inverse relationship between HDL-C levels and DVT risks could be attributed to the antithrombotic effect of HDL. Firstly, HDL has antiplatelet actions, which not only can decrease platelet aggregation by downregulating the production of thromboxane A2 but also inhibit platelet activation by upregulating biosynthesis and release of nitric oxide (NO).17,30 Secondly, HDL can help maintain the integrity of endothelial cells.31 Dysfunctional and apoptotic endothelial cells can promote thrombosis by releasing membrane microparticles and promoting the platelets–leukocytes adhesion reaction.32 Thirdly, the selectins interaction promotes platelet–leukocyte adherence and aggregation to vessel walls in the post-traumatic inflammatory response period, and thus the anti-inflammatory effects of HDL could contribute to the inhibition of thrombosis.33
RDW and HDL-C in combination with other biomarkers had been consistently the hot topic in risk factor analyses of clinical diseases in recent years. For RDW, RDW-to-lymphocyte ratio was confirmed to be independently associated with the prognosis of cutaneous malignant melanoma, and RDW-to-platelet ratio can be used for the early prediction of cirrhosis and hepatic fibrosis in patients with chronic hepatitis B.34,35 For HDL-C, neutrophils-to-HDL-C ratio had been confirmed as a significant predictor of severe coronary artery stenosis, and triglyceride-to-HDL-C ratio was employed to identify insulin resistance and cardiometabolic risk among children with obesity.36,37 Different from previous studies, the current one was the first attempt to use RDW-to-HDL-C ratio and fully demonstrate its excellent thrombotic predictive value in the field of trauma.
The different distribution of characteristics in geriatric hip fracture patients increased the heterogeneity and may lead to differences in the predictive efficacy of RHR for DVTs. Based on the subgroup analyses, we found that the predictive value of RHR was efficient in most subgroups, especially in those of younger age, better medical conditions, or femoral neck fracture. The underlying mechanism is presumably that high RHR and pathological conditions, including advanced age, poor medical conditions and intertrochanteric fracture, collectively contributed to the development of DVT, and the correlation between high RHR and DVT got highlighted after excluding the influential effects of these pathological conditions.38 Thus, it is rational to speculate that RHR would be more effective in predicting DVT in traumatic patients of middle age and relatively good health status, which warrants further investigation. In addition, we noted that albumin is a potentially modifiable nutritional indicator in the subgroup analyses, and recent studies have confirmed that hypoproteinemia is closely associated with the development of pneumonia in geriatric patients before or after hip fracture surgery.39,40 Therefore, it is worth emphasizing that targeting correction of hypoproteinemia is a feasible and cost-effective way to prevent or reduce preoperative DVTs and improve prognosis in geriatric patients with hip fracture.
The advantages of the current study included identification of RHR as a high specificity novel predictor for preoperative DVT and also confirmation and validation of their true association and association magnitude by using large size samples. However, several limitations ought to be mentioned. First, some variables that might have caused differences between groups were not collected, such as the mechanism of injury. Second, recall bias is unavoidable for that some variables, especially the potential complications, were self-reported by patients at admission. Third, although the clinical data were drawn from a prospectively collected database, the potential selection bias of this single-center study would limit the generalizability of the findings. Fourth, we did not further follow up the influence of RHR on the recurrence of DVT and occurrence of postoperative DVT, which would be valuable for improving patient prognosis.
Conclusion
In this study, we identified that RDW, HDL-C and RHR are valuable biomarkers in predicting preoperative DVTs in geriatric patients with hip fracture. In the post-PSM cohort, RHR, as a novel predictor with high specificity (71.3%), was independently associated with 1.54-fold risk of DVT before hip fracture surgery, and would be more efficient in the subgroups of younger age, better medical condition and femoral neck fracture.
Abbreviations
DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thrombus embolism; SSIOS, Surgical Site Infection in Orthopaedic Surgery; STROCSS, Strengthening the Reporting of Cohort Studies in Surgery; DUS, duplex ultrasonography; ROC, receiver operating characteristic; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; PSM, propensity score matching; IQR, interquartile range; RR, relative risk; CI, confidence interval; RDW, red cell distribution width; HDL-C, high-density lipoprotein cholesterol; RHR, red cell distribution width-to-high-density lipoprotein cholesterol ratio; BMI, body mass index; ASA, American Society of Anesthesiologists; HGB, hemoglobin, reference range: Females, 110–150g/L; males, 120–160g/L; PLT, platelet; ALB, albumin; FBG, fasting blood glucose; AT III, antithrombin III; PT, prothrombin time; APTT, activated partial thromboplastin time; FIB, fibrinogen; HCRP, high-sensitivity C-reactive protein; RBCs, red blood cells; TFPI, tissue factor pathway inhibitor; ROS, reactive oxygen species; TF, tissue factor; NO, nitric oxide.
Data Sharing Statement
All the data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of The Third Hospital of Hebei Medical University, and the informed consent was waived for its retrospective nature. All the data were analyzed anonymously to safeguard patient privacy.
Consent for Publication
We have obtained the consent for publication from all participants.
Acknowledgments
The Key Laboratory of Biomechanics of Hebei Province provided the site for querying data. Xiang Lei and Haifeng Wu approved the final version of the manuscript.
Author Contributions
Xinqun Cheng and Lingjia Fan are considered first co-authors. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The authors have received no external funding in order to support this project.
Disclosure
All the authors declare that they do not have a conflict of interest
References
1. Shin WC, Woo SH, Lee SJ, Lee JS, Kim C, Suh KT. Preoperative prevalence of and risk factors for venous thromboembolism in patients with a hip fracture: an indirect multidetector CT venography study. J Bone Joint Surg Am. 2016;98(24):2089–2095. doi:10.2106/JBJS.15.01329
2. Song K, Yao Y, Rong Z, Shen Y, Zheng M, Jiang Q. The preoperative incidence of deep vein thrombosis (DVT) and its correlation with postoperative DVT in patients undergoing elective surgery for femoral neck fractures. Arch Orthop Trauma Surg. 2016;136(10):1459–1464. doi:10.1007/s00402-016-2535-4
3. Zhang BF, Wei X, Huang H, et al. Deep vein thrombosis in bilateral lower extremities after Hip fracture: a retrospective study of 463 patients. Clin Interv Aging. 2018;13:681–689. doi:10.2147/CIA.S161191
4. Durand WM, Goodman AD, Johnson JP, Daniels AH. Assessment of 30-day mortality and complication rates associated with extended deep vein thrombosis prophylaxis following Hip fracture surgery. Injury. 2018;49(6):1141–1148. doi:10.1016/j.injury.2018.03.019
5. Sun Y, Chen D, Xu Z, et al. Incidence of symptomatic and asymptomatic venous thromboembolism after elective knee arthroscopic surgery: a retrospective study with routinely applied venography. Arthroscopy. 2014;30(7):818–822. doi:10.1016/j.arthro.2014.02.043
6. Froehlich JA, Dorfman GS, Cronan JJ, Urbanek PJ, Herndon JH, Aaron RK. Compression ultrasonography for the detection of deep venous thrombosis in patients who have a fracture of the Hip. A prospective study. J Bone Joint Surg Am. 1989;71(2):249–256. PMID: 2645291. doi:10.2106/00004623-198971020-00012
7. Smith EB, Parvizi J, Purtill JJ. Delayed surgery for patients with femur and hip fractures-risk of deep venous thrombosis. J Trauma. 2011;70(6):E113–E116. doi:10.1097/TA.0b013e31821b8768
8. Zhang C, Feng J, Wang S, et al. Incidence of and trends in Hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med. 2020;17(8):e1003180. doi:10.1371/journal.pmed.1003180
9. Tsantes AG, Papadopoulos DV, Trikoupis IG, et al. The procoagulant effect of COVID-19 on the thrombotic risk of patients with hip fractures due to enhanced clot strength and fibrinolysis shutdown. J Clin Med. 2021;10(15):3397. doi:10.3390/jcm10153397
10. Tsantes AG, Papadopoulos DV, Trikoupis IG, et al. Rotational thromboelastometry findings are associated with symptomatic venous thromboembolic complications after hip fracture surgery. Clin Orthop Relat Res. 2021;479(11):2457–2467. doi:10.1097/CORR.0000000000001832
11. Tsantes AG, Trikoupis IG, Papadopoulos DV, et al. Higher coagulation activity in hip fracture patients: a case-control study using rotational thromboelastometry. Int J Lab Hematol. 2021;43(3):477–484. doi:10.1111/ijlh.13409
12. Lin Z, Mi B, Liu X, et al. Nomogram for predicting deep venous thrombosis in lower extremity fractures. Biomed Res Int. 2021;2021:9930524. doi:10.1155/2021/9930524
13. Peng G, Wang Q, Sun H, et al. Development and prospective validation of a novel risk score for predicting the risk of lower extremity deep vein thrombosis among multiple trauma patients. Thromb Res. 2021;201:116–122. doi:10.1016/j.thromres.2021.02.020
14. Cheng X, Lei X, Wu H, et al. Development and validation of a predictive nomogram for preoperative deep vein thrombosis (DVT) in isolated calcaneal fracture. Sci Rep. 2022;12(1):5923. doi:10.1038/s41598-022-10002-8
15. Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1–46. doi:10.1016/bs.acc.2014.12.001
16. Celik A, Ozcan IT, Gündes A, et al. Usefulness of admission hematologic parameters as diagnostic tools in acute pulmonary embolism. Kaohsiung J Med Sci. 2015;31(3):145–149. doi:10.1016/j.kjms.2014.12.004
17. Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98(11):1352–1364. doi:10.1161/01.RES.0000225982.01988.93
18. Ma J, Du P, Qin J, et al. Incidence and risk factors predicting deep venous thrombosis of lower extremity following spinal fractures. Sci Rep. 2021;11(1):2441. doi:10.1038/s41598-021-82147-x
19. Ma J, Qin J, Hu J, et al. Incidence and hematological biomarkers associated with preoperative deep venous thrombosis following foot fractures. Foot Ankle Int. 2020;41(12):1563–1570. doi:10.1177/1071100720943844
20. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi:10.2307/2531595
21. Simel DL, DeLong ER, Feussner JR, Weinberg JB, Crawford J. Erythrocyte anisocytosis. Visual inspection of blood films vs automated analysis of red blood cell distribution width. Arch Intern Med. 1988;148(4):822–824. doi:10.1001/archinte.148.4.822
22. Zöller B, Melander O, Svensson P, Engström G. Red cell distribution width and risk for venous thromboembolism: a population-based cohort study. Thromb Res. 2014;133(3):334–339. doi:10.1016/j.thromres.2013.12.013
23. Dai X, Wang X, Huang Z, Wang K, Ding W. Exact association between preoperative blood viscosity and postoperative deep venous thrombosis risk in knee osteoarthritis patients: a 10-year retrospective study. Clin Appl Thromb Hemost. 2021;27:10760296211048896. doi:10.1177/10760296211048896
24. Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013;765:211–216. doi:10.1007/978-1-4614-4989-8_29
25. Yu FT, Armstrong JK, Tripette J, Meiselman HJ, Cloutier G. A local increase in red blood cell aggregation can trigger deep vein thrombosis: evidence based on quantitative cellular ultrasound imaging. J Thromb Haemost. 2011;9(3):481–488. doi:10.1111/j.1538-7836.2010.04164.x
26. Gutmann C, Siow R, Gwozdz AM, Saha P, Smith A. Reactive oxygen species in venous thrombosis. Int J Mol Sci. 2020;21(6):1918. doi:10.3390/ijms21061918
27. Ferroni P, Roselli M, Riondino S, Guadagni F. Predictive value of HDL cholesterol for cancer-associated venous thromboembolism during chemotherapy. J Thromb Haemost. 2014;12(12):2049–2053. doi:10.1111/jth.12737
28. Deguchi H, Pecheniuk NM, Elias DJ, Averell PM, Griffin JH. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation. 2005;112(6):893–899. doi:10.1161/CIRCULATIONAHA.104.521344
29. Di Minno MN, Tufano A, Guida A, et al. Abnormally high prevalence of major components of the metabolic syndrome in subjects with early-onset idiopathic venous thromboembolism. Thromb Res. 2011;127(3):193–197. doi:10.1016/j.thromres.2010.12.005
30. Florentin M, Liberopoulos EN, Wierzbicki AS, Mikhailidis DP. Multiple actions of high-density lipoprotein. Curr Opin Cardiol. 2008;23(4):370–378. doi:10.1097/HCO.0b013e3283043806
31. Soran H, Hama S, Yadav R, Durrington PN. HDL functionality. Curr Opin Lipidol. 2012;23(4):353–366. doi:10.1097/MOL.0b013e328355ca25
32. Martínez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005;288(3):H1004–H1009. doi:10.1152/ajpheart.00842.2004
33. Culmer DL, Dunbar ML, Hawley AE, et al. E-selectin inhibition with GMI-1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model. Thromb Haemost. 2017;117(6):1171–1181. doi:10.1160/TH16-04-0323
34. Hannarici Z, Yilmaz A, Buyukbayram ME, Tekin SB, Bilici M. A novel prognostic biomarker for cutaneous malignant melanoma: red cell distribution width (RDW) to lymphocyte ratio. Melanoma Res. 2021;31(6):566–574. doi:10.1097/CMR.0000000000000785
35. Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One. 2013;8(7):e68780. doi:10.1371/journal.pone.0068780
36. Kou T, Luo H, Yin L. Relationship between neutrophils to HDL-C ratio and severity of coronary stenosis. BMC Cardiovasc Disord. 2021;21(1):127. doi:10.1186/s12872-020-01771-z
37. NurZati Iwani AK, Jalaludin MY, Wan Mohd Zin RM, et al. TG: HDL-C ratio is a good marker to identify children affected by obesity with increased cardiometabolic risk and insulin resistance. Int J Endocrinol. 2019;2019:8586167. doi:10.1155/2019/8586167
38. Wang T, Guo J, Long Y, Yin Y, Hou Z. Risk factors for preoperative deep venous thrombosis in Hip fracture patients: a meta-analysis. J Orthop Traumatol. 2022;23(1):19. doi:10.1186/s10195-022-00639-6
39. Tian Y, Zhu Y, Zhang K, Tian M, Qin S, Li X. Relationship between preoperative hypoalbuminemia and postoperative pneumonia following geriatric hip fracture surgery: a propensity-score matched and conditional logistic regression analysis. Clin Interv Aging. 2022;17:495–503. doi:10.2147/CIA.S352736
40. Wang X, Dai L, Zhang Y, Lv Y. Gender and low albumin and oxygen levels are risk factors for perioperative pneumonia in geriatric hip fracture patients. Clin Interv Aging. 2020;15:419–424. doi:10.2147/CIA.S241592
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

