Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 10
Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®)
Authors de Melo F, Nicolau P, Piovano L, Lin SL, Baptista-Fernandes T, King MI, Camporese A, Hong KK , Khattar MM, Christen MO
Received 29 June 2017
Accepted for publication 14 September 2017
Published 8 November 2017 Volume 2017:10 Pages 431—440
DOI https://doi.org/10.2147/CCID.S145195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Francisco de Melo,1 Pierre Nicolau,2 Luca Piovano,3 Shang-Li Lin,4 Tiago Baptista-Fernandes,5 Martyn I King,6 Alessandra Camporese,7 Kyungkook Hong,8 Maria M Khattar,9 Marie-Odile Christen10
1Zo Skin Centre, Dubai, United Arab Emirates; 2Clinica Dr P Nicolau, Figueres (Girona), Spain; 3Piovano Medical Center, Roma, Italy; 4Shang-Li Dermatologic & Aesthetic Clinic, New Taipei City, Taiwan, Republic of China; 5Instituto Portugues de Crurgia Plastica, Lisboa, Portugal; 6Cosmedic Skin Clinic, Tamworth, Staffordshire, UK; 7Studio Medico, Cadoneghe Padova, Italy; 8Hus-hu Dermatology Clinic, Seoul, South Korea; 9Aesthetica Clinic, Dubai, United Arab Emirates; 10Behavior, Paris, France
Background: The range of fillers currently available for soft-tissue augmentation is constantly expanding. The latest advances in filler technology include collagen biostimulators that exert their esthetic effect by promoting neocollagenesis. One such product is the next-generation collagen biostimulator (Ellansé®) that demonstrates properties as yet unseen in soft-tissue fillers. It is composed of polycaprolactone (PCL) microspheres in an aqueous carboxymethylcellulose gel carrier. Given its specific characteristics and the number of areas that can be treated with this innovative product, experts’ recommendations were deemed necessary and are therefore presented in this paper with a specific focus on the indications, treatment areas and procedures as well as injection techniques.
Methods: A multinational, multidisciplinary group of plastic surgeons and dermatologists convened to develop recommendations with a worldwide perspective. This publication provides information on the specific characteristics of the product and focuses on the recommendations on the injection techniques.
Results: Recommendations on injection techniques are provided for the upper face, mid-face and lower face and zone by zone for each of these areas, as well as hands. Based on the particular anatomy of each area, the focus is on the techniques and devices of injection and the volume and depth of injection. The information is tabulated, and photos are presented for illustration.
Conclusion: These recommendations provide a guideline for physicians who wish to perform safe and efficacious treatment with the PCL collagen stimulator for face and rejuvenation with volume augmentation.
Keywords: collagen stimulator, volumizer, polycaprolactone-Ellansé®, dermal filler, expert recommendations, injection techniques
Introduction
The use of fillers for soft-tissue augmentation has increased dramatically in recent decades, progressively supplanting surgery as a result of the improved safety and efficacy profiles, the short recovery time and the lower treatment costs.1 Different types of soft-tissue fillers can be distinguished: nonbiodegradable (eg, polymethylmethacrylate [PMMA]) and biodegradable (eg, hyaluronic acid [HA]) products.2,3 More recently, a new generation of biodegradable products has emerged: the soft-tissue fillers, calcium hydroxylapatite (CaHA) and poly-l-lactic acid (PLLA), both of which possess biostimulatory properties.4,5 However, CaHA lacks the long-lasting results6 and PLLA the immediate effect.7
A novel biodegradable collagen stimulator, Ellansé® (Sinclair Pharmaceuticals, London, UK), that combines durability and immediate outcome is now available. This unique product is composed of microspheres of a totally bioresorbable polymer, polycaprolactone (PCL), in an aqueous carboxymethyl cellulose (CMC) gel carrier. This PCL-based stimulator with a CE marking [European Conformity] was introduced to the European aesthetics market in 2009 and has since been made available in more than 80 countries. The CMC and PCL components are well known and have been classified as Generally Recognized As Safe (GRAS) by the US Food and Drug Administration (FDA). The PCL microspheres are 25–50 µm in size and are thus protected from phagocytosis.8 They are totally spherical, perfectly smooth and ideally adapted to use in esthetic treatments.9 Their biocompatibility and biodegradation have been extensively studied. PCL biodegradation and bioresorption occur via hydrolysis of the ester linkages, leading to the end products CO2 and H2O that are totally eliminated from the body.10–14
One important feature of this PCL-based stimulator is its ability to stimulate the synthesis of new collagen. While the CMC gel carrier is gradually resorbed by macrophages in 6–8 weeks, the PCL microspheres stimulate neocollagenesis.15,16 Deposition of newly synthesized collagen around the PCL microspheres was demonstrated by histological and histochemical analysis of skin biopsies from treated animals, showing that collagen type I becomes progressively predominant over collagen type III, thereby achieving earlier and superior qualitative results than other resorbable products with a long-lasting effect.15 The collagen stimulatory effect has recently been confirmed in human beings on skin biopsies from treated subjects.16
Four versions of the PCL-based stimulator are available: Ellansé-S (short, S version), Ellansé-M (medium, M version), Ellansé-L (long, L version) and Ellansé-E (extra-long, E version) with expected in vivo longevity of 1, 2, 3 and 4 years, respectively (duration for L and E versions are based on extrapolation of clinical data with S and M versions and known PCL-degradation behavior).10,17,18 The duration of action depends on the initial polymer chain length and on the time of total bioresorption of the product.10,12–14 The long duration of action makes this product ideally suited to patients seeking long-lasting results. Based on our clinical experience as experts in the field and our knowledge of the products currently available, the sole aim of this article is to provide recommendations for use of this PCL-based stimulator with a particular focus on the main target areas, treatment modalities and injection techniques.
Methodology
A multinational, multidisciplinary group of experts, plastic surgeons and dermatologists convened to develop recommendations with a worldwide perspective on the injection techniques of a PCL-based collagen stimulator. The need was expressed in an initial experts’ meeting during which all the aspects of the product were reviewed and the treatment area to be covered was defined. All the experts contributed by giving their particular experience. Subsequently, through cross reviews of the compiled document, recommendations as reported in this publication were established.
These recommendations provide a guideline for physicians who wish to perform safe and efficacious treatment with the PCL collagen stimulator for volume augmentation and rejuvenation of the face and hands.
Clinical efficacy
The PCL-based stimulator is an injectable implant for subdermal implantation in the face for lasting correction of wrinkles and facial aging signs or conditions.
Its safety and efficacy have been demonstrated in clinical studies, some of which focused on the effect on nasolabial folds (NLFs),19,20 one of the most frequently treated facial areas in clinical investigations and in clinical practice. The first prospective, randomized, controlled 24-month-study comparing S versus M versions demonstrated the efficacy, patient satisfaction, treatment duration and safety of the PCL-based stimulator in 40 patients treated for NLFs. Results evaluated on the Global Aesthetic Improvement Scale (GAIS) were maintained with the M version up to 24 months with good safety results. With a mean satisfaction rate of 72.4% for the S version and 81.7% for the M version, this study also provides a demonstration of the tunable longevity, which is a result of the specific product characteristics.20 A second clinical study focused on the S version of the product, administered in the NLFs and using a split-face study design over a 12-month period versus HA. Results showed the superiority of the PCL-based stimulator in terms of efficacy and longevity compared to a nonanimal-stabilized HA with a significant positive effect at 6, 9 and 12 months on the Wrinkle Severity Rating Scale (WSRS).19
Recently, the efficacy of the PCL-based stimulator was also evidenced on forehead augmentation, a facial area also affected by aging with the appearance of laxity, wrinkles and volume loss. This study was performed in 56 Korean subjects using M version – 50% for uneven contours and 50% for volume augmentation. The GAIS scores increased notably from 1 to 3 months and were maintained for up to 24 months.21 Furthermore, a pilot study demonstrated that the PCL-based stimulator was safe, well tolerated and effective for hand rejuvenation throughout the 24-week follow-up period with a very high level of satisfaction measured on a Visual Analog Scale (VAS).22 In addition to the clinical studies, clinical experience has accumulated since 2009 with daily use by physicians worldwide.
Treatment modalities and procedures
Preprocedural care
Prior to administration of the PCL-based stimulator and following good medical practice and the instructions for use, a full medical history in the course of the patient interview, including a physical examination, a signed informed consent form, and details of the patient’s motivation and expectations, has to be recorded in order to identify the optimal treatment and any possible contraindications and to prevent complications. The patient must be made aware of realistically achievable results.23 Treatment and posttreatment plans as well as potential risks must be discussed in light of the patient’s expectations. Any preexisting asymmetries should be highlighted. Anesthesia can be offered (topical anesthetic cream, local infiltration), and addition of lidocaine, if not contraindicated, to the PCL-based stimulator can be safely undertaken with no damage to the physical properties of the product.24 Appropriate markings should be made on the skin to show the areas to be treated and those where injections should be avoided.
Patients presenting with any of the following should not be treated: autoimmune disease, pregnancy, breastfeeding, current medication with high-dose steroids, uncontrolled diabetes, metabolic syndrome, any signs of infection in the treated area, active herpes and coagulation/bleeding disorders.
Treatment area and injection techniques
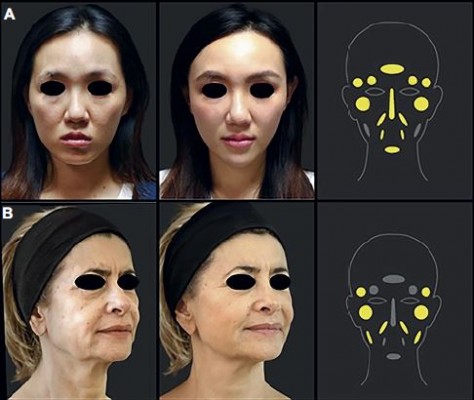
Many injection techniques have been described, and the choice depends mainly on the area to be treated, the physician’s preference and safe injection guidelines.21,25–27 Subcutaneous or deeper, supraperiosteal placement of the PCL-based stimulator is recommended for the face, and subcutaneous placement is recommended for hands. For subcutaneous injections, linear threading, fanning or cross-hatching are the recommended techniques. The bolus requires small amounts (no higher than 0.2 mL) to be injected to build a low-pressure gradient. The treated area should be gently massaged immediately afterward to ensure even distribution. The biostimulatory capacity means that there is no need for overcorrection, as the subsequent collagen synthesis will be sufficient to obtain the desired result. In areas with deficient soft-tissue coverage, such as the nose, marionette lines and pre-jowl, undercorrection is recommended, until sufficient experience with the PCL-based stimulator has been acquired. Injection techniques and a concise reminder and summary of the main anatomical features are presented by principal facial areas – upper face, mid-face and low face (Figure 1) – and for the hands.
Upper facial area
All injection modalities and techniques at the level of this area are described in Table 1 and detailed hereafter zone by zone with illustrating photographs (Figure 2).
  | Table 1 Recommended techniques and procedures for injection of the PCL-based collagen stimulator in upper face and mid-face areas Abbreviations: PCL, polycaprolactone; NLF, nasolabial fold. |
Forehead
The use of fillers in the forehead region is mainly to improve anterior projection and convexity over the supraorbital ridge rather than to reverse the signs of aging (Figure 2A). Owing to the presence of important vascular and nerve structures emerging from the supraorbital and supratrochlear foramen (terminal branches of the supraorbital artery), this area should be approached with caution and only by experienced physicians.
The optimal plane of injection is supraperiosteal, using a 22 or 25 G microcannula at three separate entry points: the first in the mid line, 4–5 cm above the orbital rim, and the others at the level of the temporal crest, 3.5–4 cm above the orbital rim (Table 1). From these points, the whole frontal area can be safely covered. To avoid any irregularities, the area should be massaged immediately after the injection to ensure an even distribution of the product.
Lateral brow
The effects of aging on the periocular region, including the lateral brow, are due to atrophy of the forehead fat pad, loss of skin elasticity and bone resorption. The main anatomical concerns in this area are the supraorbital and supratrochlear nerves (ophthalmic division of the trigeminal or fifth cranial nerve) and the different vessels (artery and veins). The supraorbital notch or foramen is generally located on the bony edge at the junction of the inner one-third and outer two-thirds of the supraorbital rim and is easily palpable.
Injection of the PCL-based stimulator to the lateral brow can give a subtle improvement in shape, position and elevation (Figure 2A). Injections that are too superficial are likely to result in a lumpy appearance and should therefore be placed in a supraperiosteal plane to avoid this risk. The product should be injected with a 25–27 G blunt cannula using a retrograde linear threading technique (0.05–0.1 mL/thread; total volume per side, 0.2–0.3 mL; Table 1).
Temporal area
A combination of fat and muscle atrophy and underlying bone loss are the main explanations for hollowed out temples. The temporal area is limited by the temporal crest above, the zygomatic arch below, and the frontal process anteriorly. The superficial temporal artery runs along the posterior border of the temporal area in the preauricular region. On the lower margin, close to the zygomatic arch and midway from the tragus to the lateral canthus, the frontal branch of the facial nerve becomes more superficial and the sentinel vein sits at the same level. It is for these reasons that the inferior approach over the zygoma is to be avoided.28
Collagen biostimulators have an important role in this area as restoring the lost volume has a dramatic effect on the overall appearance of the face (Figure 2A–C). The temporal area should be approached from the hairline or from the temporal crest. The optimum plane of injection is a relatively avascular area between the superficial temporal fascia and the superficial layer of the deep temporal fascia using a 22 or 25 G blunt cannula to cover the whole area, fanning from the posterior limit to the orbital rim and zygomatic arch (Table 1). A needle can only be used for small corrections. Alternatively, deep, preperiosteal injections are another valid option using a low-pressure bolus injection technique. This will however require more product to achieve the same result.
Mid-facial area
All injection modalities and techniques at the level of this area are described in Table 1 and detailed hereafter zone by zone with illustrating photographs (Figure 2).
Malar area
Aging in this area is mainly associated with atrophy of the deep fat compartment and bone loss. Although superficial volume loss plays a minimal role in the aging process,29 the subcutaneous injection can be used to reverse a number of these effects. Restoring volume is the main approach to rejuvenating this area (Figure 2A).
A cannula (22 G or 25 G) is preferable for this indication, although a 27 G needle will provide a greater accuracy if required. The retrograde injection should be in the deep plane, supraperiosteally 0.05–0.1 mL/line or small boluses, and, in more experienced hands, in the subcutaneous plane (Table 1). The volume of PCL-based stimulator can vary considerably in this area but generally does not exceed 1 mL/side. This treatment can be extended to the zygomatic arch, supraperiosteally, to provide a more uniform contour as long as attention is paid to the frontal branch of the facial nerve.
Nose
The PCL-based stimulator is ideal for adding volume to depressed areas of the nose, lifting the tip or smoothing the appearance of irregularities along the bridge of the nose.30 M, E and L versions with their long-lasting effects are particularly indicated for treatment of this area (Figure 2A).
The product should only be injected in the supraperiosteal or suprachondral plane using a retrograde linear threading technique, close to the midline to avoid injury to the dorsal or lateral nasal arteries, using a cannula or a needle (0.05-0.1 mL/thread; total volume, 0.5–1 mL; Table 1). Attention is drawn to the use of soft-tissue fillers in general for nose reshaping after rhinoplasty due to anatomic changes resulting from surgery and scar-tissue formation, which are more prone to additional complications. This treatment should be reserved for experienced physicians with the ability to manage such potential adverse events (AEs).
NLFs
NLFs are skin folds starting at either side of the nose and ending at the corners of the mouth. Correction of NLFs with the PCL-based stimulator has been shown to be safe and well tolerated with a long-lasting effect (Figure 2A–D).19,20
Injections should be supraperiosteal in the upper third (piriform fossae) and subcutaneous in the lower two-thirds, with multiple injection threads medial to the NLFs (retrograde fanning injection) using a 27 G needle (0.1 mL maximum/bolus) or a 25 G cannula (Table 1). In deeper folds, the product can be placed in multiple layers as long as care is taken to avoid damaging the facial artery (0.5–1.5 cm lateral to the alar sulcus, ~5 mm deep).
Submalar area
The submalar region is prone to volume loss of the buccal fat pad but is an area that is often overlooked in soft-tissue filler treatments. Submalar augmentation should however only be considered after the malar region has been addressed first as esthetically unpleasant results may arise when this area is not treated concomitantly with others.
Treatment with the PCL-based biostimulator requires injections with a 25–27 G blunt cannula to be placed in the subcutaneous plane using retrograde linear threading with fanning and cross-hatching (0.05–0.1 mL/thread; total volume, 0.5–1 mL; Table 1). However, if preferred, a 27 G needle may be used, provided special attention is paid to avoid injections that are either too superficial (risk of a lumpy appearance) or too deep (risk of deposits being felt in the buccal mucosa).
Lower facial area
All injection modalities and techniques at the level of this area are described in Table 2 and detailed hereafter zone by zone with illustrating photographs (Figure 2).
  | Table 2 Recommended techniques and procedures for injection of the PCL-based collagen stimulator in lower face areas Abbreviations: PCL, polycaprolactone; PJS, prejowl sulcus. |
Marionette lines (melomental fold) and oral commissures
The oral commissures are located where the upper and lower lips join at the corner of the mouth. With time, they take on a depressed appearance, highlighted by the formation of a fold (melomental fold or marionette lines), caused by the downward sliding of a small fat compartment, the anterior border of which follows the anterior border of the depressor angulis oris muscle. This is a difficult area to treat due to the lack of structural support and the dynamic role played by the major muscles in the perioral area.
Correction by the PCL-based stimulator can be obtained by retrograde fanning, cross-hatching injection or microbolus (0.05–0.1 mL per two to three lines) injection with a 27 G needle but should only be undertaken by experienced injectors (Table 2 and Figure 2A, C and D). Full correction is difficult, even with a combination of other methods (botulin toxin, peelings, laser resurfacing). For these reasons, the biostimulatory effect of the PCL-based stimulator plays a major role in achieving progressive long-lasting correction of these irregularities.
Mental crease
The mental crease is the horizontal anatomical groove formed near the top of the chin and below the lower lip in the gingivolabial sulcus above the mentalis muscle. A deep mental crease is generally associated with aging and also with dental disorders, including malocclusions or missing teeth, an overshot or undershot jaw, bone resorption of the lower jaw or genetic conditions (pseudoxanthoma elasticum). Injections directly into the mental foramen should be avoided as pressure can affect the mental nerve. An injection that is too deep may result in intraoral lumps within the buccal sulcus, whereas an injection that is too superficial or within the orbicularis oris muscle is likely to cause lumpiness. Superficial lines surrounding the mental crease may not disappear after treatment. In the event of a hyperactive mentalis muscle and prominent dimpling of the chin with contraction of this muscle, prior treatment with botulinum toxin is preferable.
The injection should be performed with a 27 G or 25 G needle in the subcutaneous plane up to the midline and laterally to each end of the mental crease using a linear threading technique and retrograde injection (0.05 mL/thread; total volume, 0.3–0.4 mL) (Table 2). A line superior and inferior to the mental crease is recommended to provide optimum, more natural results.
Chin
Augmentation can be performed on a small, hypoplastic, disproportionate chin and should be considered when the nose is being treated. In certain patients with mandibula senilis (atrophic age-related jaws and chin), expansion of the jowl tip can provide a more esthetic outcome (Figure 2A).
Retrograde linear threading or several small boluses with the PCL-based stimulator (<0.2 mL) can be used in the supraperiosteal plane on both sides, near the tuberculum mentale, using a 27 G needle or a 25 G/22 G cannula (Table 2). It is also worth considering extending the treatment to the pre-jowl area, along the same anatomical plane, as increasing the chin length and/or projection can create a more noticeable deformity in these areas.
Prejowl sulcus (PJS) and jaw line
The PJS is a soft-tissue depression on either side of the chin, medial to the jowl, created by atrophy of the prejowl fat compartment and descent of the inferior jowl fat compartment, resulting in an irregular-looking outline. The facial artery and vein emerge anteriorly to the anterior border of the masseter, and the marginal branch of the facial nerve passes around the mandibular.
The PJS, which is anterior to these structures, can be approached directly over the defect or from the top with a 27 G needle at an oblique angle from the lower one-third of the melomental fold. A 22 G/25 G cannula can also be used, usually with an insertion point over the jowl or from the tuberculum mentale, parallel to the jaw line (Table 2 and Figure 2B). The PCL-based stimulator should be placed deep, supraperiosteally, limited by the mandibular border and the digastric muscle, using a fanning technique (0.3–0.6 mL). For the posterior part of the jaw line, in the parotid area, a “lifting-like” effect can be achieved by placing the PCL-based stimulator subcutaneously over the anterior border of the masseter, close to the jowl, and upward into the preauricular area using a 27 G needle or a 25 G cannula. Two injection entry points are generally needed, one over the angle of the mandible and a second one more medial, overlapping the vectors. Great care should be paid to avoid injecting the PCL-based stimulator into the parotid gland. The mandible angle can be improved by adding volume, deep in the supraperiosteal plane, using a 27 G needle or a 25–27 G cannula in a retrograde manner (0.02–0.03 mL/vector). This treatment is particularly well suited to male patients, as it increases definition of the jaw line in this area.
Hands
The aging hand is characterized by atrophy of the subcutaneous tissue, loss of elasticity and thinning of the dermis. The dorsum of the hand is an area characterized by great mobility to accommodate for the strong gripping capacity of the palmar skin. Below the dermis, three distinct fibrofatty laminae from superficial to deep locations are separated from one another by a thin fibrous sheet and linked by thin fibrous walls. The deepest and most protected structures are the tendons, followed by the intermediate layer containing the veins and the nerves. There are no structures in the upper layer.
Injections of the PCL-based stimulator should be performed in the most superficial layer using either a 25 G or 22 G cannula. The injection can be inserted either through a single-entry point, distal to the wrist, or through each interdigital zone. The thin walls may interfere with insertion, but by injecting a very small amount of product at this point and waiting for a few minutes, tissue distension due to edema will allow the cannula to glide easily. Bolus injection is not recommended.
Postprocedural care
Following treatment, patients should be told to keep their face clean, use no make-up and avoid exposure to heat and radiation (sauna, sun), swimming/bathing and alcohol consumption for the first 24 hours. This is in line with the global recommendations for dermal fillers as described very recently by Urdiales-Gálvez et al,23 among many other authors.
Safety
The safety of the PCL-based stimulator was evaluated throughout its development by investigating the tolerability of its components and the finished ready-to-use product. Biocompatibility, biodegradation and bioresorption were extensively demonstrated.8,9 The good safety profile was also demonstrated in clinical efficacy and safety studies.19,20,22 Since receiving the marketing license, a pharmacovigilance system has been set up to record AEs worldwide. More than 490,000 syringes have been used since the launch in 2009 to December 2016, and the AE rate is low, at 0.049% (one event per 2.055 syringes), indicating that the PCL-based stimulator is well tolerated. The majority of the AEs reported are considered as minor (Table 3). With the PCL-based stimulator, no trend of a specific side effect has been reported.
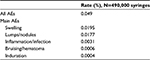  | Table 3 Safety from PMS Abbreviations: PMS, post market survey; AE, adverse event. |
Several cases reported are related to the injection procedure itself, such as edema that disappears spontaneously after a few days. Nodules or indurations appear to be generally linked to technical errors, such as injections that are too superficial or bolus injections. Health authorities (FDA and local country authorities) and many experts have described different types of side effects observed with dermal fillers.19,20,22
Adequate training of physicians is mandatory and should include recommendations on injection techniques, with a particular focus on the strictly regulated volumes to be injected in different areas and where not to inject (ie, the lips, glabella and eyelids). Several publications also provide recommendations on how to avoid complications with dermal fillers in general.23,27,31–35 With regard to the management of AEs related to dermal fillers, numerous experts have described potential complications and proposed different treatment modalities.35–38 Detailed information on the contraindications, warnings, precautions and directions for use is provided in the instructions for use of the product.
Discussion
Volume replacement is nowadays widely recognized as an integral step toward achieving satisfactory results in facial rejuvenation.39–41 The ease of use and adjustable longevity of the completely bioresorbable PCL-based stimulator (Ellansé) make this product ideally suited for patients seeking stable long-lasting results. It offers noteworthy advantages over PLLA-based fillers in as far as the results are immediately visible, and over HA- and CaHA-based fillers, thanks to the stability and duration of the results.42 The efficacy and the safety of this PCL-based stimulator have been demonstrated in clinical trials19,20,22and throughout the extensive clinical experience that has been acquired over many years worldwide. Carruthers et al,17 in their introduction to dermal fillers, mentioned the PCL collagen stimulator and referred to the study of Moers-Carpi and Sherwood,20 indicating that this product was particularly efficient when used in the NLFs, with no serious adverse effects.
This biostimulatory effect generates type I collagen,15,16 thus explaining the sustained results and very low rate of adverse effects in the authors’ experience. The PCL-based stimulator exerts an immediate effect, which is subsequently prolonged by the production of collagen that is visible 5–8 weeks posttreatment.15,16
This effect makes it unsuitable for use in the eyelids and vermilion border of the lips, in the same way as other resorbable biostimulators (CaHA and PLLA).43,44 For the same reasons, overcorrection should be avoided in superficial areas such as the melomental folds and the nose.26,30 There are some other precautions that should be taken into consideration. These are of particular importance as the learning curve is longer when compared to HA-based fillers, and the PCL-based collagen stimulator is more technically demanding. For this reason, the authors emphasize the need for appropriate training in establishing the correct diagnoses and developing the necessary skills to reach the desired cosmetic improvement when using PCL, avoiding overcorrection. In the authors’ opinion, PCL should not be used by inexperienced physicians. For those physicians, an HA-based filler will offer an advantage; the same applies to eyelid rejuvenation and lips enhancement or augmentation or glabella treatment for which toxin is the product of choice; PCL, as the other biostimulatory fillers should not be used in these areas due to the lack of subcutaneous fat and a subsequent increased risk of product visibility and nodule formation.
The product should be injected in small gradual amounts at each pass, with care being taken to avoid high-pressure gradients and bolus injections. Good injection technique and detailed knowledge of the anatomy are paramount to achieving sustained improvements with minimal adverse effects or complications.
In our experience, the minor adverse effects observed are similar to those encountered with other fillers but are observed at a low rate.32,33,45 With the PCL-based stimulator, only low rates of edema and nodules are reported, and they are essential due to deficient injection techniques and overcorrection. To date, in excess of 490,000 patients have been treated with the PCL-based stimulator, and no reports of granuloma or other nonspecific immune responses have been documented.
Our recommendations as experts, on target areas and injection techniques detailed in this paper, can be considered as a guide for safe optimal use by physicians who want their patients to benefit from this unique PCL-based collagen stimulator to restore volume, redefine contours and reduce wrinkles while at the same time improving the quality of their skin.
Conclusion
In the authors’ experience, the new-generation PCL-based collagen stimulator (Ellansé) provides safe long-lasting correction of the volume losses related to aging and can be used in several indications. The primary esthetic results with the PCL-based stimulator are restored volume, redefined contours and reduced wrinkles. Its positive effect on skin quality has also been widely reported. The safety profile, ease of injection and tunable longevity are determining factors for choosing the PCL-based collagen stimulator in our practice to ensure high levels of patient satisfaction.
Acknowledgments
The authors were fully responsible for the content, editorial decisions, and opinions expressed in this review. They thank Sharon King from Cosmedic Skin Clinic, UK, for her contribution, Dr Sophie Converset from the Clinique du Parc, Lyon, France, for the before and after pictures published in this paper and Potentiel d’action (France) for medical writing assistance.
Disclosure
Drs. de Melo, Piovano, Baptista-Fernandes, Camporese, Hong, Khattar and Mrs Christen are consultants to Sinclair Pharmaceuticals. Dr Nicolau is a medical advisor at Sinclair Pharmaceuticals. Dr Poivano is a member of the IBSA academy. Dr Camporese is a consultant to Anteis, and Dr King is a consultant to Teoxane and a member of the medical esthetic group. Dr Lin has no conflicts of interest in this work.
References
Plasticsurgery. 2015 Plastic Surgery Statistics. American Society of Plastic Surgeons; 2017. Available from: https://www.plasticsurgery.org/news/plastic-surgery-statistics?sub=2015+Plastic+Surgery+Statistics. Accessed April 21, 2017. | ||
Johl SS, Burgett RA. Dermal filler agents: a practical review. Curr Opin Ophthalmol. 2006;17(5):471–479. | ||
Eppley BL, Dadvand B. Injectable soft-tissue fillers: clinical overview. Plast Reconstr Surg. 2006;118(4):98e–106e. | ||
Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014;13(9):1047–1052. | ||
Goldberg D, Guana A, Volk A, Daro-Kaftan E. Single-arm study for the characterization of human tissue response to injectable poly-L-lactic acid. Dermatol Surg. 2013;39(6):915–922. | ||
Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging. 2008;3(1):161–174. | ||
Redaelli A, Rzany B, Eve L, et al. European expert recommendations on the use of injectable poly-L-lactic acid for facial rejuvenation. J Drugs Dermatol. 2014;13(9):1057–1066. | ||
Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg. 2002;28(6):484–490. | ||
Laeschke K. Biocompatibility of microparticles into soft tissue fillers. Semin Cutan Med Surg. 2004;23(4):214–217. | ||
Pitt CG. Poly-epsilon caprolactone and its polymers. In: Chassain M, Langer R, editors. Biodegradable Polymers as Drug Delivery Systems. Vol. 45. New York, USA: Marcel Dekker; 1990:71–119. | ||
Pitt CG, Gratzl MM, Kimmel GL, Surles J, Schindler A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2(4):215–220. | ||
Taylor MS, Daniels AU, Andriano KP, Heller J. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater. 1994;5(2):151–157. | ||
Ma G, Song C, Sun H, Yang J, Leng X. A biodegradable levonorgestrel-releasing implant made of PCL/F68 compound as tested in rats and dogs. Contraception. 2006;74(2):141–147. | ||
Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27(9):1735–1740. | ||
Nicolau PJ, Marijnissen-Hofsté J. Neocollagenesis after injection of a polycaprolactone based dermal filler in a rabbit. Eur Cell Mater. 2013;3(1):19–26. | ||
Kim JA, Van Abel D. Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J Cosmet Laser Ther. 2015;17(2):99–101. | ||
Carruthers J, Carruthers A, Humphrey S. Introduction to fillers. Plast Reconstr Surg. 2015;136(5 suppl):120S–131S. | ||
Gritzalas K. Preliminary results in using a new dermal filler based on poly-caprolactone. Eur J Aesthetic Med Dermatol. 2011;1(1):22–26. | ||
Galadari H, van Abel D, Al Nuami K, Al Faresi F, Galadari I. A randomized, prospective, blinded, split-face, single-center study comparing polycaprolactone to hyaluronic acid for treatment of nasolabial folds. J Cosmet Dermatol. 2015;14(1):27–32. | ||
Moers-Carpi MM, Sherwood S. Polycaprolactone for the correction of nasolabial folds: a 24-month, prospective, randomized, controlled clinical trial. Dermatol Surg. 2013;39(3 pt 1):457–463. | ||
Bae B, Lee G, Oh S, Hong K. Safety and long-term efficacy of forehead contouring with a polycaprolactone-based dermal filler. Dermatol Surg. 2016;42(11):1256–1260. | ||
Figueiredo VM. A five-patient prospective pilot study of a polycaprolactone based dermal filler for hand rejuvenation. J Cosmet Dermatol. 2013;12(1):73–77. | ||
Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Preventing the complications associated with the use of dermal fillers in facial aesthetic procedures: an expert group consensus report. Aesthetic Plast Surg. 2017;41(3):667–677. | ||
de Melo F, Marijnissen-Hofsté J. Investigation of physical properties of a polycaprolactone dermal filler when mixed with lidocaine and lidocaine/epinephrine. Dermatol Ther. 2012;2(1):13. | ||
Moradi A, Watson J. Current concepts in filler injection. Facial Plast Surg Clin North Am. 2015;23(4):489–494. | ||
Sykes JM, Cotofana S, Trevidic P, et al. Upper face: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(5 suppl):204S–218S. | ||
Vedamurthy M, Vedamurthy A, Nischal K. Dermal fillers: do’s and dont’s. J Cutan Aesthet Surg. 2010;3(1):11–15. | ||
Breithaupt AD, Jones DH, Braz A, Narins R, Weinkle S. Anatomical basis for safe and effective volumization of the temple. Dermatol Surg. 2015;41(suppl 1):S278–S283. | ||
Baroni ERV, Biondo-Simões MLP, Auersvald A, et al. Influence of aging on the quality of the skin of white women: the role of collagen. Acta Cir Bras. 2012;27(10):736–740. | ||
Wang LL, Friedman O. Update on injectables in the nose. Curr Opin Otolaryngol Head Neck Surg. 2017;25(4):307–313. | ||
Sherman RN. Avoiding dermal filler complications. Clin Dermatol. 2009;27:S23–S32. | ||
DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33(4):561–575. | ||
DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34(4):584–600. | ||
Wagner RD, Fakhro A, Cox JA, Izaddoost SA. Etiology, prevention, and management of infectious complications of dermal fillers. Semin Plast Surg. 2016;30(2):83–86. | ||
Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31(1):110–121. | ||
Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(6):1842–1863. | ||
Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. Treatment options. Plast Reconstr Surg. 2009;123(6):1864–1873. | ||
Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33(6):862–877. | ||
Wollina U. Facial rejuvenation starts in the midface: three-dimensional volumetric facial rejuvenation has beneficial effects on nontreated neighboring esthetic units. J Cosmet Dermatol. 2016;15(1):82–88. | ||
Jones D. Volumizing the face with soft tissue fillers. Clin Plast Surg. 2011;38(3):379–390. | ||
Rose AE, Day D. Esthetic rejuvenation of the temple. Clin Plast Surg. 2013;40(1):77–89. | ||
Kontis TC. Contemporary review of injectable facial fillers. JAMA Facial Plast Surg. 2013;15(1):58–64. | ||
Pavicic T. Complete biodegradable nature of calcium hydroxylapatite after injection for malar enhancement: an MRI study. Clin Cosmet Investig Dermatol. 2015;8:19–25. | ||
Byun S-Y, Seo K-I, Shin J-W, et al. Objective analysis of poly-L-lactic acid injection efficacy in different settings. Dermatol Surg. 2015;41(suppl 1):S314–S320. | ||
Lucey P, Goldberg DJ. Complications of collagen fillers. Facial Plast Surg. 2014;30(6):615–622. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


