Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Recombinant Adenovirus-Mediated HIF-lα Ameliorates Neurological Dysfunction by Improving Energy Metabolism in Ischemic Penumbra After Cerebral Ischemia-Reperfusion in Rats
Authors Zhou W, Tao T, Yu W, Wu W, Hui Z, Xu H, Li Y, Zhang Y, Yang X
Received 7 September 2022
Accepted for publication 29 March 2023
Published 6 April 2023 Volume 2023:19 Pages 775—784
DOI https://doi.org/10.2147/NDT.S389022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Wenmei Zhou,1 Tao Tao,1 Wenfeng Yu,2 Wanfu Wu,3 Zhirong Hui,1 Hongliang Xu,1 Yaqi Li,4 Ying Zhang,5 Xiaohui Yang1,6
1Department of Rehabilitation Medicine, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, 550002, People’s Republic of China; 2Department of Human Anatomy, Basic Medical College, Guizhou Medical University, Guiyang, Guizhou, 550004, People’s Republic of China; 3Department of Biology and Biochemistry, University of Houston, Houston, TX, 77204, USA; 4Emergency Department, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, 550002, People’s Republic of China; 5Department of Chinese Traditional Medicine, Zunyi Medical and Pharmaceutical College, Zunyi, Guizhou, 563006, People’s Republic of China; 6Department of Rehabilitation Medicine, The Affiliated Hospital Guizhou Medical University, Guiyang, Guizhou, 550001, People’s Republic of China
Correspondence: Tao Tao, Tel +86 13985162824, Email [email protected]
Background: Hypoxia inducible factor-1α (HIF-1α) regulates glucose metabolism during ischemia. This study investigated the effect of recombinant adenovirus HIF-1ɑ on neurological function and energy metabolism in a rat cerebral ischemia-reperfusion model.
Methods: Rats were divided into four groups: sham-operated (Sham) group, cerebral ischemia-reperfusion (CIR) group, recombinant adenovirus empty vector (Ad) group, and recombinant adenovirus-mediated HIF-1α (AdHIF-1α) group. The AdHIF-1α group and the Ad group were injected with AdHIF-1α and Ad in the lateral ventricle. The mNSS was performed at post-ischemia day 0 (P0) and P1, P14 and P28. At P14, the cerebral infarct volume was compared. At P28, HE staining, Nissl stains and TUNEL staining were performed. The expression of HIF-1α, GLUT1 and PFKFB3 were evaluated by Western Blot and immunohistochemistry. High performance liquid chromatography (HPLC) was used to determine the expression of GLUT1 and PFKFB3, and the level of energy metabolites: ATP, ADP and AMP.
Results: mNSS scores in the AdHIF-1α group were consistently lower than those in the CIR and Ad groups from P14 (P < 0.05) and Ad groups (P < 0.05). The cerebral infarct volume was reduced in the AdHIF-1α group compared with that in CIR group and Ad group (P < 0.05). At P28, HE showed better pathological changes in AdHIF-1α group. The number of Nissl bodies was increased in the AdHIF-1α group compared with the CIR and Ad groups (P < 0.05). The number of apoptotic cells in the AdHIF-1α group was fewer than that in the CIR and Ad groups (P < 0.05). The expression of HIF-1α, GLUT1 and PFKFB3 was significantly higher in the AdHIF-1α group compared with the CIR and Ad groups (P < 0.05). The ATP, ADP and AMP in the ischemic penumbra were also higher in the AdHIF-1α group (P < 0.05).
Conclusion: HIF-lα promoted neurological function recovery and decreased cerebral infarct volume in rats after cerebral ischemia-reperfusion injury by improving energy metabolism.
Keywords: cerebral ischemia-reperfusion injury, HIF-lα, energy metabolism, glucose transporter protein-1, GLUT1, 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3, PFKFB3
Background
To restore the cerebral blood supply to the ischemic area in the time window is one of the key treatments for cerebral ischemia. In recent years, the mortality rate of cerebral ischemia has sharply decreased with the popularization of thrombolysis and endovascular techniques. However, reperfusion after ischemia may further aggravate the damage to the ischemic brain tissue and lead to neurological dysfunction due to the sudden increase of oxygen while increasing the blood oxygen supply, which is defined as cerebral ischemia-reperfusion injury (CIRI).1 CIRI is a pathological phenomenon caused by cerebral ischemic stroke.
Hypoxia-inducible factor-1α (HIF-1α) is the initiator that triggers endogenous protection pathways during hypoxia and ischemia by promoting angiogenesis, increasing erythrocytes, promoting cell migration, and inhibiting apoptosis by regulating the expression of its target genes.2 Our group has successfully constructed recombinant adenovirus-mediated hypoxia inducible factor-1 alpha (AdHIF-1α) and demonstrated that the recombinant adenovirus could be successfully introduced into the lateral ventricle. The treatment of AdHIF-1α in tMCAO rat model showed neuroprotection by decreasing apoptosis of neuronal cells.3,4 Increasing the expression of endogenous HIF-1α during cerebral ischemia can improve motor and cognitive functions in rats,5,6 but the cerebral protective mechanisms of HIF-1α need further investigation. Previous studies have shown that HIF-1α improved glucose metabolism by upregulating the expression of glucose transporter protein-1 (GLUT1) and 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3).7,8 But whether HIF-1α improves energy metabolism in the ischemic penumbra by regulating glucose metabolism and thus restoring neurological function has been little studied in cerebral ischemia-reperfusion injury.
In this study, we exogenously increased the expression of HIF-1α by adenovirus-mediated HIF-1α gene in rats with transient middle cerebral artery occlusion (tMCAO). The results demonstrated that rats treated by AdHIF-1α have better neurological recovery with smaller cerebral infarct volume and improved pathological changes. AdHIF-1α treatment decreased the number of apoptotic cells. The expression of HIF-1α, GLUT1 and PFKFB3 was significantly upregulated by AdHIF-1α treatment with higher content of ATP, ADP and AMP in the ischemic penumbra.
Materials and Methods
Adult male Sprague-Dawley (SD) rats, body weight 240–260 g (animal lot number: SCXK (Qian) (2018-0001), were used in this study. The ethical approval number for this study is 2021-009 approved by the Ethics Committee of Guizhou Provincial People’s Hospital. All the guidelines stated in Guide for the Care and Use of Laboratory Animals, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council, China (1996) have been followed. The ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) have been also followed in this study. Rats were fasted for 12 h before surgery. SD rats were randomly divided into sham-operated (Sham) group, cerebral ischemia-reperfusion (CIR) model group, recombinant adenovirus empty vector (Ad) group, and recombinant Adenovirus-mediated HIF-1α (AdHIF-1α) group (Figure 1A). Each group had 24 rats.
tMCAO Rat Model
The tMCAO model was established according to Longa’s method.9 In brief, the rat was anesthetized with 10% chloral hydrate (0.35 mL/100 g) intraperitoneally before surgery. The rat was fixed in the supine position, and the right external, common and internal carotid arteries were isolated through a median neck incision, and the proximal ends of the external and common carotid arteries were ligated in turn, and the internal carotid artery was temporarily clamped with a micro-arterial clip. Through a cut at the proximal bifurcation of the common carotid artery, a 4-0 nylon suture (fused to a hemisphere at the front end and marked at 18 mm) was inserted into the internal carotid artery until slight resistance was felt, and the insertion depth (from the bifurcation of the common carotid artery) was approximately 18 mm to block the beginning of the middle cerebral artery. After 60 min of cerebral ischemia, the nylon suture was slowly withdrawn for reperfusion to create cerebral ischemia-reperfusion injury. Rats in the Sham group underwent the same procedure but without the nylon suture insertion (Figure 1B).
Neurological function was assessed 24 h after surgery using the modified neurological severity score (mNSS). The rat with mNSS 7–12 (moderate) was taken for the experimental study. The unsuitable rats (mNSS lower than 7 or higher than 12) were excluded.
Treatments for Rats
Rats were anesthetized 10% chloral hydrate (0.35 mL/100 g) intraperitoneally and then transferred to a stereotaxic apparatus.10 Under the fixation of the brain, the skin of the head was incised medially to expose the bregma, the posterior fontanel, the sagittal suture, and the surface of the right skull. After adjusting the bregma and posterior fontanel of the skull to the same level by the localizer, the bregma was set as the origin, shifted 2 mm caudally and opened 1.5 mm parsimoniously to the right side, which was the localization point. A tiny round hole was made in the localization point with a sterilized dental drill, and the micro-injector was slowly inserted into the skull through the cranial hole to a depth of 3.5 mm (Figure 1C). 10ul AdHIF-1a (content of 108pfu AdHIF-1a) was slowly (the injection time was about 10min, and the needle remained for another 10min and then slowly withdrawn) injected into the lateral ventricle of AdHIF-1α group rats. In the same way, 10ul Ad (content of 108pfu Ad) was injected into the lateral ventricle of the Ad group. The rats in the Sham and CIR groups were injected with the equal amount of sterile normal saline. The small round hole was covered with bone wax and then the skin was sutured. At 14 d after surgery, brain tissues of three rats from the Ad group and AdHIF-1α group were harvested for evaluating the infarct volume.
Behavioral Testing
Neurological deficits were assessed by using the modified neurological severity score (mNSS)1 before modeling (0d) and at the 1st, 14th, and 28th d after tMACO. mNSS scores were performed by an investigator who was blind of the experimental groups. The score of neurological deficit was scored on a scale of 0–18 (0 for normal and 18 for most severe) on the following criteria: 1–6 for mild injury; 7–12 for moderate injury; and 13–18 for severe injury. When assessing the severity of injury, a score of 1 was given for inability to complete the test or lack of test reflexes, and the higher the neurological deficit score, the more severe the injury. Eight rats from each group were included from each group at each time point.
Cerebral Infarct Volume Assessing
At P14 three rats from each group were anesthetized. The brains were harvested by decapitation. 2-mm-thick coronal sections were prepared to be stained with 2, 3, 5-triphenyltetrazolium chloride (TTC). ImageJ was used for analysis. To minimize the error caused by cerebral edema, the indirect calculation method was used for calculating the cerebral infarct volume. The infarct volume is the percentage of the infarct volume to the non-infarcted hemisphere.
Histological Analysis
HE Staining
Brain tissues were fixed in 4% paraformaldehyde for 24 h. Four μm thickness section was cut for HE staining. Stained slides were observed under the light microscope.
Nissl Staining
Following the Nissl staining protocol, the sections were dewaxed to water, toluidine blue stained, and mounted by mounting medium. Three different fields in the ischemic penumbra were imaged under 400×. ImageJ was used to count the number of Nissl stained cells in each field.
TUNEL Staining
The sections were stained with by the TUNEL Apoptosis Detection Kit from KeyGEN Biotech Co. (KGA702). TUNEL-stained sections were observed under light microscope at 400×. The number of positive cells was collected from 3 non-overlapping fields by the researcher who was blind from experimental groups. The number of apoptotic cells was counted by ImageJ.
Western Blotting
Brain tissues from the ischemic penumbra were taken for Western blotting assay. The protein concentration was measured under UV spectrophotometer. HIF-1α (1:1000) GLUT1 (1:1000); PFKFB3 (1:3000) or β-actin rabbit monoclonal antibody (1:5000) was incubated overnight at 4°C in a shaker. After wash with PBST membrane was incubated with horseradish peroxidase (HRP)-labeled secondary antibody (goat anti-rabbit IgG, 1:3000). Membrane was developed with ECL. ImageJ was used to evaluate the corresponding band intensities.
Immunohistochemical Staining
The sections were treated with 3% H2O2 for 15 min to block endogenous peroxidase. After wash with PBS 5% BSA containing 0.03% Triton X-100 was added for l h at room temperature. The primary antibodies (GLUT1, 1:80; PFKFB3, 1:100) was added and incubated overnight at 4°C. After incubated with biotin secondary antibodies (1:200, Vector) for 1 h at room temperature sections were washed with PBS for 3 times. Then, sections were incubated with biotin-ovalbumin-HRP complex (ABC, 1:100, Vector) for l h at room temperature. Freshly prepared 0.05% DAB chromogenic solution containing 0.03% H2O2 was used for color development and then hematoxylin counterstained. After dehydrated in gradient alcohol and xylene sections were covered with mounting medium. The negative control was performed with normal sheep serum instead of primary antibody. Five different fields were imaged under the microscope at 200× from the ischemic penumbra. The number of positive cells in each field of view was counted by Image-J.
High Performance Liquid Chromatography (HPLC)
(1) Sample preparation: 0.5g brain tissue from ischemic penumbra was rapidly made to be 10% homogenate by 0.4mol/l HCLO4 on ice. After sonication and centrifugation at low temperature (4°C, 5000rpm, 20min) the supernatant was taken.
Then, 3mol/l K2CO3 0.1mL was added to 0.2mL supernatant to neutralize HCLO4. Then, samples were stored at −80°C after centrifuged (14000rpm, 5min) and filtered by 0.45 μm filter.
(2) Chromatographic conditions: The levels of ATP, ADP and AMP were determined by high performance liquid chromatography. Chromatographic conditions: Waters Bridge R C18 column (4.6 mm×150 mm, 5 μm), mobile phase: 0.2 mmol/L phosphate buffer as mobile phase. Detection wavelength: 259 nm; flow rate: 1.0 mL/min; column temperature: 20°C; injection volume: 10 μL. Each sample was performed 3 times.
(3) Standard Preparation: 10.18mg of ATP, 10.87mg of ADP and 10.07mg of AMP were weighed precisely and dissolved in water, and then diluted to the concentrations of ATP, ADP and AMP of 48.37 μg/mL and 46.36 μg/mL, respectively. 10 μL from the gradient dilutions (2, 2.5, 5, 10, 50, and 100 times) was taken to obtain the standard curve.
(4) Sample calculation: The linear regression standard curve was made with the peak area (Y) as the vertical coordinate and the concentrations of ATP, ADP and AMP (X, μg/mL) as the horizontal coordinate. The concentrations of ATP, ADP, and AMP in samples from each group of brain tissue were calculated according to the standard curve, and then the ATP, ADP, and AMP levels per gram of brain tissue were calculated from the brain tissue homogenate concentrations, and the energy charge (EC) values were calculated 17 as EC=(ATP+0.5×ADP)/(ATP+ADP+AMP).
Statistical Analysis
Data are shown as mean ± standard error of the mean (SEM). Data were analyzed by repeated measures of analysis of variance (ANOVA) for the 4 groups or unpaired Student’s t-test two groups comparison, if they were normally distributed (Kolmogorov-Smirnov test, p > 0.05). P < 0.05 was considered as statistically significant.
Results
AdHIF-1α Treatment Improved Neurological Functional Deficit in tMCAO Rats
To investigate whether AdHIF-1α treatment could promote neurological functional recovery, we compared mNSS scores (n = 8). Before tMCAO there was no abnormal neurological function for all rats. At P1, all rats from CIR group, Ad group and AdHIF-1α group showed symptoms of neurological deficits such as leaning to one side or turning around when walking, abnormal sensation and balance. There was no significant difference in mNSS among these three groups (P > 0.05). At P14 d, the mNSS score of the AdHIF-1α group was significantly lower than that of the CIR group and the Ad group (P < 0.05). There was no statistical difference between in mNSS scores between the CIR group and the Ad group (Figure 1D). These results demonstrated that AdHIF-1α treatment promoted neurological functional recovery.
AdHIF-1α Treatment Decreased Cerebral Infarct Volume
TTC staining showed that the infarct area on the ischemic side of the brain was demonstrated with white in the CIR group, Ad group, and AdHIF-lα group at P14 after tMCAO. There was no infarct area (white) in the Sham group (Figure 2A). No significant difference was found in infarct volume between CIR group and Ad group (P > 0.05). The infarct volume was significantly reduced in the AdHIF-lα group compared with the CIR group and Ad group (P < 0.05) (Figure 2B).
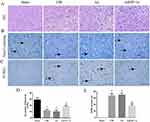
AdHIF-1α Treatment Attenuated Neuronal Loss and Reduced Apoptosis
The HE staining of Sham group showed that normal cerebral structure with regular arrangement of neuronal cells, centered nuclei and uniform nuclear and cytoplasmic staining. At P28, the HE staining of the CIR and Ad groups had obvious infarct areas with cell edema, neuronal loss or nuclear consolidation, disappearance of nucleoli, and vacuole-like arrangement. The edema, nuclear consolidation, and vacuole were attenuated by the treatment of AdHIF-1α (Figure 3A).
At P28, the number of Nissl bodies in the ischemic penumbra was reduced in the CIR group and Ad group compared with the Sham group (P < 0.05); The number of Nissl bodies in AdHIF-1α group was significantly increased compared with the CIR group and Ad group (P < 0.05) (Figure 3B and D).
No apoptotic cells were detected in Sham group by TUNEL staining. The apoptotic neuronal cells were identified in the ischemic penumbra in CIR group, Ad group and AdHIF-1α group. The number of apoptotic neuronal cells in the AdHIF-1α group was significantly decreased than that in CIR and Ad groups (P < 0.05) (Figure 3C and E).
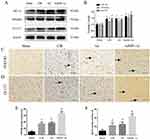
AdHIF-1α Treatment Upregulated the Expression of HIF-1α, GLUT1 and PFKFB3
The expression of HIF-1α, GLUT1 and PFKFB3 HIF-1α, PFKFB3 was significantly increased in the CIR group and Ad group compared with the Sham group (P < 0.05) (Figure 4A and B). These results confirmed that cerebral ischemia-reperfusion injury induced HIF-1α and its target gene expression. AdHIF-1α treatment further upregulated the expression of HIF-1α, PFKFB3 and GLUT1 compared with NS treatment and Ad treatment (P < 0.05) (Figure 4A and B).
The upregulated expression of PFKFB3 and GLUT1 by AdHIF-1α treatment was also confirmed with immunohistochemical staining. There was no significant difference in the number of positive cells between the CIR group and Ad group (P > 0.05) (Figure 4C–F). The number of positive cells for PFKFB3 or GLUT1 was statistically increased in the AdHIF-lα group (P < 0.05) (Figure 4C–F).
AdHIF-1α Treatment Improved Energy Metabolism in the Ischemic Penumbra
To evaluate whether AdHIF-1α treatment improved energy metabolism, the ATP, ADP and AMP levels and EC values were measured at P28 d. The results showed that the levels of ATP, ADP and AMP as well as the EC value in the ischemic penumbra were all decreased in the CIR group, Ad group and AdHIF-1α group compared with the Sham group (P < 0.05) (Table 1). AdHIF-1α treatment increased both the levels of ATP, ADP and AMP and the EC value compared with NS treatment and Ad treatment (P < 0.05) (Table 1).
 |
Table 1 ATP, ADP and AMP Levels in Each Group at P28 |
Discussion
In this study, we investigated whether AdHIF-1α ameliorates neurological dysfunction by improving energy metabolism in ischemic penumbra after cerebral ischemia-reperfusion. The results demonstrated that administration of AdHIF-1α led to a better neurological functional recovery by decreasing cerebral infarct volume, attenuating neuronal loss and reducing apoptosis. The findings of the present study further proved that AdHIF-1α treatment upregulated HIF-1α expression and its target genes: PFKFB3 or GLUT1. At last, we demonstrated that AdHIF-1α treatment improved energy metabolism in the ischemic penumbra with increased levels of ATP, ADP and AMP and the EC value as well. The results presented here provide new insights into the role of HIF-1α in the treatment for cerebral ischemia-reperfusion injury.
Ischemic stroke caused by thrombotic or embolic occlusion of a cerebral artery leads to cell death including necrosis or apoptosis by the disturbances in energy metabolism in neuronal cells.11 In recent years, studies have shown that neurological functions such as motor and cognition are directly or indirectly affected by brain energy metabolism.12 During cerebral ischemia-reperfusion, the cells in the ischemic penumbra can still maintain certain level of energy metabolism due to the establishment of collateral circulation.13 Therefore, it is critical to save the ischemic penumbra by preventing infarct core expansion. Improving the energy metabolism of the ischemic penumbra may prevent or reverse the cerebral ischemia-reperfusion injury, thus ameliorating neurological dysfunction caused by ischemic stroke.
HIF-1α, a key transcription factor in the body’s response to oxygen deficiency, plays a very important role in cellular adaptation to hypoxia during cerebral ischemia-reperfusion.14 It has been shown that HIF-1α promotes a metabolic shift favoring anaerobic glycolysis and reduced mitochondrial respiration by upregulating GLUTs and by inducing glycolytic enzymes during ischemia.15 Under hypoxia, HIF-1α plays an important role in regulating energy metabolism by upregulating glucose transporters and by enhancing glycolytic enzyme activity to maintain energy supply.16,17 Previous studies have shown that HIF-1α plays a protective role in anti-ischemic brain injury by improving neuronal survival.4,18–21 In the present study, we confirmed that the treatment of AdHIF-1α ameliorated the neurological function deficits. We then demonstrated that AdHIF-1α administration reduced cerebral infarct volume, attenuated neuronal loss and decreased apoptosis. We further showed that treatment of AdHIF-1α was able to upregulate HIF-1α expression and its regulated genes: PFKFB3 and GLUT1. At last, we proved that AdHIF-1α treatment increased levels of ATP, ADP and AMP and the EC value in the ischemic penumbra. These results suggest that exogenous upregulation of the HIF-1α by recombinant adenovirus promotes neurological recovery by improving energy metabolism in the ischemic penumbra of tMCAO rat model.
During cerebral ischemia, glycolysis becomes the main source of energy in the ischemic area of the brain and the hypoxic cells tend to consume more glucose in order to adapt increased energy requirements. After cerebral ischemia, the upregulation of GLUT1, a key protein for glucose transport, represents this adaptive shift.22,23 The inhibition of GLUTs function led to abnormal brain function and neuronal death, and the upregulation of GLUT1 and GLUT3 demonstrated neuroprotective effect on cerebral ischemia.23 PFKFB3, the target of HIF1α, is a critical regulatory molecule in glycolysis control and has high kinase activity.24 The activation of HIF1α-PFKFB3 promoted β-cell survival at the expense of β-cell function in type 1 and type 2 diabetes.25,26 HIF1α-PFKFB3 signaling has also been implicated in the control of neovascular formation and neurodegeneration.27,28 The present study showed that heat treatment of AdHIF-1α upregulated HIF-1α expression, which in turn induced the increased expression of PFKFB3 and GLUT1. Other studies have also shown that hypoxia leads to increased expression of HIF-1α and its target genes, GLUT1 and GLUT3, which in turn improved the supply of glucose for hypoxic neuronal glycolysis.23,29
In the present study, the HIF-1α gene was cloned into a recombinant adenovirus, which is widely used in gene therapy study because of its high efficiency in infecting cells, wide host range, and low cytotoxicity.30 Previous studies from our group have demonstrated that the recombinant adenovirus carrying HIF-1α could reduce apoptotic neurons in ischemic penumbra by upregulating astrocytic erythropoietin (EPO) expression.20 HIF-1α controls cellular survival, glucose metabolism and transport, and metabolic adaptation. More than 30 target genes are reported regulated by HIF-1α, including vascular endothelial growth factor (VEGF), EPO, glycolytic enzymes and glucose transporter 1, insulin-like growth factor 2, etc.31 Therefore, more studies are needed to investigate other mechanisms involving in HIF-1α mediated neuroprotection during the cerebral ischemia-reperfusion.
Conclusion
This study demonstrated that AdHIF-1α treatment ameliorates neurological dysfunction by improving energy metabolism in the ischemic penumbra with increased levels of ATP, ADP and AMP and the EC value after tMCAO in rat. The data presented by this study provide new insights into the role of HIF-1α for the treatment of cerebral ischemia-reperfusion injury. Thus, HIF-1α could be a potential therapeutic target for the treatment of ischemic stroke.
Acknowledgments
All authors have read and agreed the submission and publication. All experiments were approved by the Ethics Committee of Guizhou Provincial People’s Hospital, Guizhou, China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by: Projects supported by the National Natural Science Foundation of China (82160265); Science and Technology Program of Guizhou province (2019-1207); Project of Science and Technology Foundation of Guizhou Provincial Health Commission (2021-141).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Panisello-Roselló A, Roselló-Catafau J. Molecular mechanisms and pathophysiology of ischemia-reperfusion injury. Int J Mol Sci. 2018;19(12):4093. doi:10.3390/ijms19124093
2. Singh N, Sharma G, Mishra V, et al. Hypoxia inducible factor-1: its potential role in cerebral ischemia. Cell Mol Neurobiol. 2012;32(4):491–507. doi:10.1007/s10571-012-9803-9
3. Yang ML, Tao T, Xu J, Liu Z, Xu D. Antiapoptotic effect of gene therapy with recombinant adenovirus vector containing hypoxia-inducible factor-1α after cerebral ischemia and reperfusion in rats. Chin Med J. 2017;130(14):1700–1706. doi:10.4103/0366-6999.209909
4. Li J, Tao T, Xu J, Liu Z, Zou Z, Jin M. HIF-1α attenuates neuronal apoptosis by upregulating EPO expression following cerebral ischemia-reperfusion injury in a rat MCAO model. Int J Mol Med. 2020;45(4):1027–1036. doi:10.3892/ijmm.2020.4480
5. Gruneberg D, Montellano FA, Plaschke K, et al. Neuronal prolyl-4-hydroxylase 2 deficiency improves cognitive abilities in a murine model of cerebral hypoperfusion. Exp Neurol. 2016;286:93–106. doi:10.1016/j.expneurol.2016.10.001
6. Balamurugan K. HIF-1 at the crossroAds of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138(5):1058–1066. doi:10.1002/ijc.29519
7. Hu L, Zeng Z, Xia Q, et al. Metformin attenuates hepatoma cell proliferation by decreasing glycolytic flux through the HIF-1α/PFKFB3/PFK1 pathway. Life Sci. 2019;239:116966. doi:10.1016/j.lfs.2019.116966
8. Yan RY, Wang SJ, Yao GT, Liu ZG, Xiao N. The protective effect and its mechanism of 3-n-butylphthalide pretreatment on cerebral ischemia reperfusion injury in rats. Eur Rev Med Pharmacol Sci. 2017;21(22):5275–5282. doi:10.26355/eurrev_201711_13852
9. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates.
10. Zhu Y, Deng L, Tang H, et al. Electroacupuncture improves neurobehavioral function and brain injury in rat model of intracerebral hemorrhage. Brain Res Bull. 2017;131:123–132. doi:10.1016/j.brainresbull.2017.04.003
11. Wang D, Yuan X, Liu T, et al. Neuroprotective activity of lavender oil on transient focal cerebral ischemia in mice. Molecules. 2012;17(8):9803–9817. doi:10.3390/molecules17089803
12. Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 2018;9(5):924–937. doi:10.14336/AD.2017.1126
13. Dunn LL, Kong SMY, Tumanov S, et al. Hmox1 (Heme oxygenase-1) protects against ischemia-mediated injury via stabilization of HIF-1α (Hypoxia-Inducible Factor-1α). Arterioscler Thromb Vasc Biol. 2021;41(1):317–330. PMID: 33207934. doi:10.1161/ATVBAHA.120.315393
14. Sarkar S, Chakraborty D, Bhowmik A, Ghosh MK. Cerebral ischemic stroke: cellular fate and therapeutic opportunities. Front Biosci. 2019;24:435–450. doi:10.2741/4727
15. Cerychova R, Pavlinkova G. HIF-1, metabolism, and diabetes in the embryonic and adult heart. Front Endocrinol. 2018;9:460. PMID: 30158902; PMCID: PMC6104135. doi:10.3389/fendo.2018.00460
16. Karshovska E, Schober A. Hypoxia-inducible factor (HIF)-1alpha regulates macrophage energy metabolism by mediating miRNAs. Cardiovasc Res. 2016;111:S29–S30.
17. Wang H, Niu F, Fan W, Shi J, Zhang J, Li B. Modulating effects of preconditioning exercise in the expression of ET-1 and BNP via HIF-1α in ischemically injured brain. Metab Brain Dis. 2019;34(5):1299–1311. doi:10.1007/s11011-019-00450-z
18. Gao Y, Yin H, Zhang Y, et al. Dexmedetomidine protects hippocampal neurons against hypoxia/reoxygenation-induced apoptosis through activation HIF-1α/p53 signaling. Life Sci. 2019;232:116611. doi:10.1016/j.lfs.2019.116611
19. Li C, Zhang B, Zhu Y, et al. Post-stroke constraint-induced movement therapy increases functional recovery, angiogenesis, and neurogenesis with enhanced expression of HIF-1α and VEGF. Curr Neurovasc Res. 2017;14(4):368–377. doi:10.2174/1567202614666171128120558
20. Amin N, Chen S, Ren Q, et al. Hypoxia inducible factor-1α attenuates ischemic brain damage by modulating inflammatory response and glial activity. Cells. 2021;10(6):1359. PMID: 34205911; PMCID: PMC8229365. doi:10.3390/cells10061359
21. Dienel GA. Brain glucose metabolism: integration of energetics with function. Physiol Rev. 2019;99(1):949–1045. doi:10.1152/physrev.00062.2017
22. Sharma V, Singh TG, Mannan A. Therapeutic implications of glucose transporters (GLUT) in cerebral ischemia. Neurochem Res. 2022;47(8):2173–2186. doi:10.1007/s11064-022-03620-1
23. Wu X, Wang C, Wang J, Zhu M, Yao Y, Liu J. Hypoxia preconditioning protects neuronal cells against traumatic brain injury through stimulation of glucose transport mediated by HIF-1α/GLUTs signaling pathway in rat. Neurosurg Rev. 2021;44(1):411–422. doi:10.1007/s10143-019-01228-8
24. Lu L, Chen Y, Zhu Y. The molecular basis of targeting PFKFB3 as a therapeutic strategy against cancer. Oncotarget. 2017;8(37):62793–62802. doi:10.18632/oncotarget.19513
25. Montemurro C, Nomoto H, Pei L, et al. IAPP toxicity activates HIF1α/PFKFB3 signaling delaying β-cell loss at the expense of β-cell function. Nat Commun. 2019;10(1):2679. doi:10.1038/s41467-019-10444-1
26. Nomoto H, Pei L, Montemurro C, et al. Activation of the HIF1α/PFKFB3 stress response pathway in beta cells in type 1 diabetes. Diabetologia. 2020;63(1):149–161. doi:10.1007/s00125-019-05030-5
27. De Bock K, Georgiadou M, Schoors S, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154(3):651–663. PMID: 23911327. doi:10.1016/j.cell.2013.06.037
28. Lv Y, Zhang B, Zhai C, et al. PFKFB3-mediated glycolysis is involved in reactive astrocyte proliferation after oxygen-glucose deprivation/reperfusion and is regulated by Cdh1. Neurochem Int. 2015;91:26–33. doi:10.1016/j.neuint.2015.10.006
29. Yu J, Li J, Zhang S, et al. IGF-1 induces hypoxia-inducible factor 1α-mediated GLUT3 expression through PI3K/Akt/mTOR dependent pathways in PC12 cells. Brain Res. 2012;1430:18–24. doi:10.1016/j.brainres.2011.10.046
30. Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the central nervous system. Front Cell Neurosci. 2015;9:304. doi:10.3389/fncel.2015.00304
31. Cheng L, Yu H, Yan N, Lai K, Xiang M. Hypoxia-inducible factor-1α target genes contribute to retinal neuroprotection. Front Cell Neurosci. 2017;11:20. doi:10.3389/fncel.2017.00020.eCollection
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.