Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Recent trends in the development of nanophytobioactive compounds and delivery systems for their possible role in reducing oxidative stress in Parkinson’s disease models
Authors Ganesan P , Ko H, Kim I, Choi DK
Received 6 August 2015
Accepted for publication 19 September 2015
Published 29 October 2015 Volume 2015:10(1) Pages 6757—6772
DOI https://doi.org/10.2147/IJN.S93918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Palanivel Ganesan,1,2 Hyun-Myung Ko,2 In-Su Kim,2 Dong-Kug Choi1,2
1Nanotechnology Research Center, Department of Applied Life Science, 2Department of Biotechnology, College of Biomedical and Health Science, Konkuk University, Chungju, Republic of Korea
Abstract: Oxidative stress plays a very critical role in neurodegenerative diseases, such as Parkinson’s disease (PD), which is the second most common neurodegenerative disease among elderly people worldwide. Increasing evidence has suggested that phytobioactive compounds show enhanced benefits in cell and animal models of PD. Curcumin, resveratrol, ginsenosides, quercetin, and catechin are phyto-derived bioactive compounds with important roles in the prevention and treatment of PD. However, in vivo studies suggest that their concentrations are very low to cross blood–brain barrier thereby it limits bioavailability, stability, and dissolution at target sites in the brain. To overcome these problems, nanophytomedicine with the controlled size of 1–100 nm is used to maximize efficiency in the treatment of PD. Nanosizing of phytobioactive compounds enhances the permeability into the brain with maximized efficiency and stability. Several nanodelivery techniques, including solid lipid nanoparticles, nanostructured lipid carriers, nanoliposomes, and nanoniosomes can be used for controlled delivery of nanobioactive compounds to brain. Nanocompounds, such as ginsenosides (19.9 nm) synthesized using a nanoemulsion technique, showed enhanced bioavailability in the rat brain. Here, we discuss the most recent trends and applications in PD, including 1) the role of phytobioactive compounds in reducing oxidative stress and their bioavailability; 2) the role of nanotechnology in reducing oxidative stress during PD; 3) nanodelivery systems; and 4) various nanophytobioactive compounds and their role in PD.
Keywords: Parkinson’s disease, phytobioactive compounds, nanotechnology delivery systems, nanocurcumin, nanoresveratrol
Introduction
The increase in the aging population in many countries is threatened by the second most common neurodegenerative disease, namely Parkinson’s disease (PD).1–3 Oxidative stress plays a key role in the development of PD, including several degenerative reactions, such as nitric oxide toxicity, mitochondrial toxicity, and development of several toxic components, leading to impaired neuronal function.2,4,5 Synthetic bioactive compounds are extensively used to reduce oxidative stress but have toxicity limitations. Phytobioactive compounds serve as natural antioxidants to reduce toxicity, and are extensively used to reduce oxidative stress, repair the central nervous system, and prevent PD.1,2 The phenolic compounds are the most beneficial, such as phenolic acids and flavonoids, which reduce disease by scavenging free radicals and limiting oxidative stress.2,6 In addition, flavonoids chelate metal ions, preventing formation of free radicals and limits limiting the onset of PD.6–8 Oral administration is the most convenient for the repeated and routine delivery of bioactive compounds.9–11 However, it is most challenging due to the protection of brain by blood–brain barrier with the narrow diameter of approximately less than 20 nm that limits the entry of most bioactive molecules. Nanotechnology research has been utilized to enhance the permeability, solubility, and stability of bioactive compounds and to enhance delivery of phytobioactive compounds12,13 to the various target sites including brain.
Natural polymer-based delivery systems have been used to deliver a variety of nanoscaled proteins and carbohydrates, including gelatin, whey proteins, zein, gum arabic, and maltodextrin.14 These polymer-based nanoparticles are highly beneficial for delivering hydrophilic bioactive compounds, which bind to the membranes and increase the life of the bioactive compounds. In addition, nanosized bioactive compounds can be delivered to the plasma through transcellular or paracellular pathways or receptor-mediated endocytosis. Lipid-based delivery systems have been used to enhance delivery of a variety of digestible lipids, such as tocopherols, flavonoids, polyphenols, and oil soluble vitamins.15,16 These digestible lipids greatly enhance the delivery of bioactive compounds in the small intestine by increasing the number of mixed micelles, which generally enhance solubility and transport of hydrophobic bioactive compounds.17–23
Many studies have focused on the health beneficial aspects of nanophytobioactive compounds to reduce oxidative stress and treat neurological disorders and PD.24–28 Nanocurcumin shows a higher mean residential time in the mice brain than that of natural curcumin.29 In addition, co-delivery of bioactive compounds greatly enhances the delivery rate of curcumin in the plasma.30–32 Similarly, nanoresveratrol greatly reduces the oxidative stress of various cell and animal models of PD.33–35 Bioactive nanoparticles enhance release of antioxidants to the brain with physical carrier properties of high biodegradability and lower toxicity. This review focuses on three main objectives: 1) the role of phytobioactive compounds in PD and their limitations; 2) nanotechnologies involved in the development of bioactive nanoparticles; and 3) the role of bioactive nanocompounds in reducing the rates of neurodegenerative diseases.
Phytobioactive compounds and PD
PD is a multifactorial neurological disorder characterized by loss of dopaminergic neurons leading to subsequent loss of dopamine in the midbrain region.36 This causes an imbalance in neurotransmitters, such as dopamine and acetylcholine, which leads to various symptoms of PD. The major symptoms of PD include tremor, speech and writing changes, slowed movement, and rigid muscles.37–39 Bioactive compounds play a major role in sustained protection against loss of dopaminergic neuron due to oxidative stress, among the various treatments to improve these symptoms in patients with PD.3,36,40–43 Extensive animal model studies have been conducted about the sustained protective role of different synthetic and natural bioactive compounds against dopaminergic neuron loss in PD.44–49 Based on limitations for using synthetic compounds,50–52 natural phytobioactive compounds play an important role in preventing PD.38,48 Phytobioactive compounds from various medicinal plants show neuroprotective effects in various animal models.8,26
Phytobioactive compounds are secondary metabolites with higher health beneficial activity that occurs in smaller amounts in various plant parts, such as leaves, fruits, seeds, nuts, and roots.42,53–57 These include polyphenols, flavonoids, and triterpenoids, which contain one or more hydroxyl groups in their phenolic ring that scavenge free radicals and act as strong antioxidants. A diet rich in these bioactive compounds has a greater protective effect against neurodegenerative disorders.3,58,59 Consuming tea rich in flavonoids reduces the risk of PD in human trials. Similarly, older rats fed a diet rich in fruits, such as blueberries and strawberries, and vegetables, such as spinach, showed had better cognitive function.60 Figure 1 shows the possible preventive role of nanobioactive compounds in reducing oxidative stress and the onset of PD. Most polyphenols occur as methoxylated, hydroxylated, or glycoxylated derivatives and the linking sugars are glucose, galactose, or rhamnose.61 The polyphenol is absorbed either in the small intestine or in the colon depending on the sugar linked to the polyphenolic group.61,62 The activities of most polyphenols are linked with the number of hydroxyl groups present at the active site. For example, the hydroxyl groups present in the third and sixth positions determine the antioxidant potential of bioactive compounds. However, some hydroxyl groups present in the fifth and seventh dihydroxyl and fourth hydroxyl positions readily undergo degradation. Some acetylated flavonoids, such as epicatechin and epigalocatechin, are readily absorbed without hydrolysis.63,64 A diet rich in plant foods with more bioactive compounds has a greater potential neuroprotective effect.38,65
Bioavailability of phytobioactive compounds
Most of the health benefits of bioactive phytobioactive compounds in vitro are associated with their capacity to scavenge free radicals, quench nitrogen species, and chelate metal ions.9,58,63 Different concentrations of various bioactive compounds that exert health beneficial activities in vitro are unlikely to be beneficial in vivo.9 Individuals prefer the oral route for consuming bioactive compounds with higher health beneficial activities. Bioactive compounds undergo breakdown and antioxidant activity in the intestinal system, which limits their bioavailability to the brain.9,62 Resveratrol-rich foods have higher absorption rates in humans but lower bioavailability in its active form in plasma.66 Unlike other organs, the brain is well protected by the blood–brain barrier, which selectively filters molecules in and out of the brain. Oral administration of 100 mg/kg curcumin to mice results in only 0.4 μg curcumin/g brain.67 Nanotechnology is an alternative approach to overcome these bioavailability challenges. Modifying phytobioactive compounds to a nanosize of 1–1,000 nm enhances their availability to cells, thereby enhancing activity. Trans-resveratrol loaded nanoparticle systems and optimized self nanoemulsifying systems enhance bioavailability fivefold to various target sites because of the optimum formulation.68,69 Recently, nanotechnology-based approach of treatment gained more importance for the enhanced crossing blood–brain barrier through its unique nanosize to various brain diseases, such as PD, brain cancer, and Alzheimer’s disease.70
The role of nanotechnology in reducing oxidative stress in PD
Nanotechnology plays a very significant role in reducing oxidative stress that occurs in various diseases, including cancers, Alzheimer’s disease, and PD.23,24,29,34 However, the role of this technology in various other diseases has not been elucidated. Among various ways of developing nanobioactive compounds, nanoparticles play a very significant role in reducing disease by reducing oxidative stress through their antioxidant mechanism.71 The most common nanoparticle antioxidant mechanism involves reduction of the natural bioactive molecule (curcumin, resveratrol, or vitamin E) to a nanosize that can be readily absorbed and reach the target site without much loss in activity.16,29,35 Nanosized bioactive compounds vary in the size from 10 to 1,000 nm, which increases bioactivity and target specificity, reduces toxicity, and enhances safety.17,29,33,34,68,72,73 The most important characteristics of nanoparticles delivered to the PD brain include the size of the bioactive compound, surface activity, and carrier toxicity.17,33,73 Smaller bioactive nanoparticles release faster to the brain target compared with larger bioactive nanoparticles.17 Hydrophilic coatings on nanobioactive compounds protect against phagocytosis. The carrier should also be highly biodegradable and nontoxic.20 Nanoparticles or nanobioactive compounds can be placed in the core or on the surface, which depends on the method used to prepare the nanobioactive compound. The oxidization or hydroxylation of curcumin in the body can be prevented using nanocapsules in which curcumin is the core material.13 Some nanobioactive molecules are designed on the surface, such as thiamine-coated nanoparticles, which enhances delivery of the antioxidant to the brain.74,75
Nanotechnological delivery systems used to develop nanobioactive compounds
Careful design of the delivery method is important for various neurodegenerative disorders.13,22–24 The best nanotechnological methods deliver the bioactive compound efficiently to the target site without any side effects.22,73 The activity of the bioactive compound also depends on the physicochemical properties at the target site. Numerous methods have been developed, such as solid lipid nanoparticles, liposomes, polymeric nanoparticles, nanoemulsions, and nanoniosomes.16,24,76 The method is classified based on whether the compound is a solid or liquid, and each has distinct advantages and disadvantages based on the activity of the bioactive molecule. A few of these methods are shown in Figure 2.
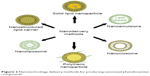  | Figure 2 Nanotechnology delivery methods for producing nanosized phytobioactive compounds. |
Bioactive nanoparticle delivery systems
Solid lipid nanoparticles
Solid lipid nanoparticles contain solid lipid as triglycerides, which incorporate the bioactive compounds in a lipophilic and hydrophilic shell surrounded by a phospholipid layer for controlled delivery of the bioactive compound to the target site.72,77–81 The mobility of the bioactive compound is greatly reduced with a solid lipid core, so it remains in the gut, which enhances the sustained release of the compound for a prolonged period of time. Various methods have been used to develop solid lipid nanoparticles, including multiple emulsion, high-pressure homogenization, and ultrasonication.82–86 Lipophilic bioactive compounds are highly dispersed in the lipid matrix, whereas the hydrophilic bioactive compounds are outside the lipid matrix. Dispersing the bioactive compounds in lipid involves the appropriate solvent or mechanical force. A polyethylene glycol (PEG) coating is used to stabilize nanoparticles incorporated in lipid. This coating enhances the stability of the bioactive compound in blood plasma by minimizing phagocytic uptake.87 Several synthetic drugs are used to prepare solid lipid nanoparticles to prevent various conditions in PD.88–91 Bromocriptine-loaded solid lipid nanoparticles have been developed and studied in patients with PD and are highly effective in reducing dyskenesia.88 However, nanophytobioactive compounds developed using a solid lipid nanoparticle delivery system showed higher bioavailability.92,93 Curcumin-loaded solid lipid nanocarriers achieved approximately 155 times higher curcumin delivery than that of natural curcumin in cancer cells.94 Curcumin-loaded solid lipid nanoparticles are highly efficient and delivery to the brain delivery was approximately 16.5 and 30 times higher than that of natural curcumin treatment in rats via oral and intravenous routes, respectively.95 The bioavailabilities of quercetin are also increased significantly in a formulation using solid lipid nanoparticles.96–98 Similarly, resveratrol-loaded solid lipid nanoparticles also enhance bioavailability eightfold during oral delivery.92,93
Nanostructured lipid carriers
Nanostructured lipid carriers are prepared with a mixture of solid and liquid phase lipids in which the bioactive compound is incorporated.92,99–104 Approximately 70% of the bioactive molecules incorporated into the mixture are well encapsulated into the carrier system and effectively reach the target site without much drop in bioactivity.99,104,105 Solid phase lipids generally used to prepare nanostructured lipid carriers include acetyl alcohol, glycerol monosterate, and stearic acid and the liquid phase lipid includes caprylic triglycerides, oleic acid, and cupric triglycerides.106 The type of lipid also determines the stability of the bioactive compound in the bioactive compound-loaded nanolipid particles. The liquid lipid concentrations determine the size of the nanolipid particles.92,104,107 Higher concentrations of the liquid lipid particles make a smaller sized nanolipid particle but a higher release rate of the bioactive particles.107 Based on the structure of the matrix lipids, nanolipid carrier particles are subdivided into three types, such as the imperfect type, which contains less oil, leading to lower stability of the bioactive molecules. The imperfect type of nanostructured lipid carrier has significant advantages compared with solid lipid nanocarriers. The second type is the multiple nanostructured lipid carrier, which contains more oil, and can be loaded with more bioactive compound in their nanocompartments to enhance drug release.92 The third type is the amorphous type of lipid, which lacks the crystalline structure of a solid lipid, and expels the bioactive compound during cooling. Baicalin-loaded nanostructured lipid carriers show enhanced bioavailability and sustained baicalin release.105 Similarly, poorly soluble bioactive molecules, such as curcumin and genistein, have enhanced bioavailability in nanostructured lipid carriers and have a stronger effect inhibiting prostate cancer.108,109
Nanoliposomes
Nanoliposomes are phospholipids with a hydrophilic head and two hydrophobic tails. They range in size from 30 nm to a few microns and are formed by high-energy dispersion.110–113 When the phospholipid bilayer is exposed to water it forms a continuous closed bilayer that encapsulates hydrophilic and hydrophobic bioactive compounds. Further aggregation of the nanoliposomes can be prevented by repulsion of the charged lipids in the membrane.114–116 Many bioactive compounds encapsulated in nanoliposomes have prolonged antioxidant activity with more surface area exposed.113,117,118 Extended circulation of bioactive compounds encapsulated in nanoliposomes in plasma is achieved through a modified surface. Several nanosynthetic compounds have been designed to effectively deliver drugs to the brain.119,120 Similarly, phtobioactive nanoliposomes, such as Orthosiphon stamineus extract nanoliposomes, have higher bioavailability and in vitro antioxidant activity.121 Curcumin encapsulated nanoliposomes show higher bioavailability after oral treatment in rats with enhanced antioxidant activity.122 In vitro studies of multifunctional curcumin nanoliposomes proved their ability to cross the blood–brain barrier and were effective against Alzheimer’s disease.123
Nanoniosomes
Nanoniosomes are liposomes made of nonionic surfactant type vesicles at a nanosize ranging from 10 to 1,000 nm. These niosomes can bind both hydrophilic and hydrophobic bioactive compounds for enhanced delivery.124–127 They have advantages over other liposomes due to their higher chemical stability, enhanced protection of bioactive compounds, lower toxicity due to their nonionic nature, non-immunogenicity, and enhanced oral bioavailability.126 Niosomes can leak their bioactive compound contents during dispersion and aggregation but this quite negligible. Furthermore, coating niosomes with PEG prevents their detection by Kupfer cells in blood plasma; thereby, enhancing delivery to the target site. In vitro and in vivo studies have confirmed that smaller sized niosomes are better able to retain a bioactive compound at the target site, regardless of the administration route.128,129 Some bioactive compounds encapsulated in nanoniosomes have beneficial activities, including antioxidant, antimalarial, antifungal, and anti-Alzheimer’s disease.126,130 Nanoniosomes are frequently used to deliver bioactive compounds to the central nervous system with high efficiency and bioactivity. Ellagic acid-loaded nanoniosomes have been developed for optimal delivery of bioactive compounds to human dermal cells.129 Synthetic compounds with diameters of 200 nm, such as doxorubicin, have been developed using the nanoniosome technique.124 Similarly, nanosized ganciclovir niosomes were developed to enhance bioavailability of ganciclovir in plasma for at least 8 hours after administration.131
Polymeric nanoparticles
Polymeric nanoparticles are widely used as a carrier for phytobioactive compounds, such as curcumin and resveratrol, which are incorporated into the polymer or adsorbed on the surface by nanoprecipitation or emulsion-diffusion methods to form polymeric nanoparticles.132 These nanosized particles are used to deliver phytobioactive compounds with minimal toxicity to the target site.133 Polymeric nanoparticle such as polylactic-co-glycolic acid (PLGA) particles can be hydrolyzed into lactic and glycolic acids, which are readily excreted without much toxicity.133 Quercetin and voglibose coated with poly-D,L-lactide-co-glycolide nanoparticles with a mean size of 41.3 nm have been developed using a solvent evaporation technique and showed good efficiency for treating diabetes through controlled trans-delivery systems.134 Similarly, quercetin nanoparticles showed 20-fold increased efficiency and controlled ethanol-induced gastric ulcers in rats.135 Synthetic PLGA-coated nanoparticles, such as loperamide-loaded g7 and Pep TGN, were designed for controlled delivery to the brain.136,137 Curcumin nanoparticles of 80 nm stabilized using poly ethylene glycol were highly stable in an in vitro blood brain mice model of Alzheimer’s disease.29 Similarly, curcumin-conjugated magnetic nanoparticles were used to detect Alzheimer’s disease in mice.138 Curcumin-loaded PLGA nanoparticles of 163 nm were highly bioavailable in liver, heart, spleen, kidney, and brain. In addition, these curcumin-loaded PLGA nanoparticles were effectively retained in brain.13,139
Nanoemulsions
Nanoemulsions are a mixture of two immiscible liquids to form a clear stable emulsion of particles <100 nm with higher optical clarity and greater bioavailability of the encapsulated functional compounds.140 These emulsions are prepared by high-energy and low-energy methods. The high-energy method uses physical force, such as a homogenizer, to obtain the emulsion, and the low-energy method involves spontaneous formation of the nanoemulsion with a suitable surfactant, water, and oil under specified conditions.141–143 A nanoemulsion is effective for encapsulating various bioactive compounds that are unstable under in vivo conditions for effective delivery to the brain.144 Oral administration of nanoemulsified curcumin enhances bioavailability of curcumin in mice with reduced inflammation.144 Similarly, a vitamin E-loaded resveratrol nanoemulsion with 102 nm particles was produced using the spontaneous emulsification technique and reduced brain-induced oxidative stress to treat PD.145 A pomegranate seed oil nanoemulsion with 135 nm particles was produced using the sonication technique and reduced lipid peroxidation and neuronal loss with strong protective effects.146 Several other plant bioactive compounds have been studied using nanoemulsion delivery methods, such as a betlunic acid nanoemulsion with 200 nm particles produced by sonication and enhanced bioavailability.147 A resveratrol nanoemulsion with 128 nm particles produced by high-speed homogenization enhanced bioavailability.148
Nanophytobioactive compounds and their role in PD
Plant bioactive compounds are a large group that readily undergoes degradation during oral intake, leading to lower bioavailability to the brain.9,11,60,66,149–151 Nanosizing of phytobioactive compounds along with suitable protective agents enhances the bioavailability of the compound to the brain.24,72,95,145,152 A few of the bioactive nanosize compounds with enhanced bioactivity and less toxicity are discussed in this section. Some of these nanobioactive compounds are listed in Table 1.
Nanocurcumin
Curcumin is a highly hydrophobic water insoluble compound widely used in medicines and the pharmaceutical and food industries.30,122,153,154 Curcumin has multiple health benefits, including antioxidant, antimicrobial, anti-inflammatory, anti-aging, anti-Alzheimer, anti-Parkinson, and anticancer activities.30,32,94,144,153–155 A lower retention time in circulation leads to the lower therapeutic potential of this compound.139,144 Reducing the size of the curcumin compound to the nanolevel and formulating it with polyesters leads to higher bioavailability in systemic circulation.122,123,144,155 Many studies have confirmed that nanosizing curcumin enhances bioavailability and therapeutic efficiency for many diseases including PD.6,13,29,31,67,94,108,122,138,139 Nanocurcumin greatly reduces the oxidative stress and apoptosis in the brain of PD flies.156 Similarly, an alginate curcumin nanocomposite has a neuroprotective effect in a transgenic Drosophila PD model with reduced oxidative stress and brain cell death.156 Choice of the delivery systems is more important to enhance the bioavailability of nanocurcumin in the circulatory system and for crossing the blood–brain barrier.157 For example, curcumin-loaded PLGA nanoparticles show enhanced bioavailability compared with other nanodelivery systems.158 The enhanced bioavailability of nanocurcumin in circulation systems has been studied but studies related to the distribution of those compounds in organs are limited. A few studies have confirmed that nanocurcumin is bioavailable in blood plasma and can readily cross the blood–brain barrier into the brain.13,139 The bioavailability of solid lipid nanocurcumin is greatly enhanced in the mouse brain with significant pharmacological activity.159,160 Similarly, the bioavailability of nanocurcumin is higher in mouse brain and has a protective effect against the oxidative stress in mice brain.161 The bioavailability of nanosize curcumin is higher in various PD models, which will lead to the development of more nanodelivery techniques for curcumin treatments.
Nanoginsenosides
Ginsenosides are active compounds predominantly found in ginseng. The type of ginsenoside varies with ginseng variety.162–164 Ginsenosides are broadly classified into 20(S) glycosides called protopanaxadiol and protopanaxatriol.165,166 These compounds reduce oxidative stress in the liver, brain, and other organs by scavenging hydrogen peroxide radicals. In addition, ginsenosides also play a critical role in reducing the oxidative stress of PD. Ginsenoside Rg1 protects cells against H2O2 induced oxidative stress and increases cell survival of a PD model in vitro.167 Similarly, ginsenoside Rg1 protects neurons against 6-hydroxydopamine-induced death and iron-induced neuronal toxicity.168,169 Although these compounds play a critical role in reducing oxidative stress, their activities are lower than those of some other compounds in several in vivo studies.170,171 To increase the activity and bioavailability of these compounds or crude extracts, nanosizing the formulation is an alternative for an enhanced protective effect against PD. The nanoginsenosides Rg1 and Rb1 with 19.9 nm particles synthesized using a nanoemulsion technique have enhanced bioavailability in the brain. Intranasal delivery of these compounds results in better bioavailability in the brain with an enhanced protective effect compared with those of the intragastric administration.165 In addition to the individual compounds, crude nanoextracts also have a beneficial effect against oxidative stress-related disease. Nanoliposomes of approximately 150 nm containing a ginseng crude extract rich in ginsenosides have been studied for their effect against hydrogen peroxide-induced oxidative stress in L929 cells. That study confirmed that liposomal nanovesicles effectively suppress hydrogen peroxide-induced oxidative stress.172 Similarly, fabricated nanoginseng extracted powder with 300 nm particles has been synthesized using a ball mill technique and has enhanced bioavailability and antioxidant activity.173 Similarly, nanoliposomal vesicles loaded with panax notoginsenoside have a protective effect against cerebral ischemia and myocardial ischemia in rats.174
Nanoresveratrol
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenolic compound found widely in grapes, peanut, peanut sprouts, blueberry, cranberry, and mulberry.34,152,162 Resveratrol has multiple health benefits, including antiaging, anticancer, cardioprotective, and PD protective effects.66,68,145 Resveratrol exists in cis and trans forms, in which trans-resveratrol is more stable than cis-resveratrol which is pharmacologically less active.66 Trans-resveratrol is readily converted to cis-resveratrol when exposed to sunlight for 1 hour; therefore, protecting these compounds is biologically more important for a sustained effect. Nanoencapsulation protects trans-resveratrol from this rapid conversion and enhances its bioavailability in systematic circulation for prolonged activity.68,175 PLGA-coated resveratrol nanoparticles enhance the bioavailability of resveratrol for up to 4 days in a rat model.176 Their research group also studied sustained release of trans-resveratrol in vitro and found higher solubility and dissolubility of trans-resveratrol.68,175,176 In addition, a combination of one or two nanosized bioactive compounds has multiple health beneficial effects for certain diseases, which further reduces the multiple drug load. Curcumin and resveratrol encapsulated nanoliposomes have an antitumor effect against prostate cancer. The role of nanoresveratrol in preventing PD and enhancing neuronal survival against oxidative stress has been shown certain study.145 A vitamin E-loaded nanoresveratrol emulsion prepared with by self-emulsification followed by high-pressure homogenization with particles of 102 nm makes resveratrol available to the brain, thereby reducing the oxidative stress of PD.145 Several other delivery techniques, such as solid lipid nanoparticles and nanostructured lipid carriers, have been studied for controlled delivery of resveratrol in the gastrointestinal tract. The same research group found that nanoresveratrol with 150–200 nm particles is biologically active with controlled delivery through the gastrointestinal tract in vitro.92 Similar to PD, Alzheimer’s disease can be controlled effectively by treatment with resveratrol-loaded lipid-core nanocapsules.33,152 Nano resveratrol developed using a suitable delivery technique produces a sustainable protective effect against PD and will lead to the development of more nanodelivery techniques for controlled delivery to the brain and enhanced neuroprotective activity.
Nanocatechins
Catechins are a group of polyphenols in many plant foods, including tea, fruits, and beverages and show multiple health beneficial aspects, such as anti-aging, anticancer, antimicrobial, antiviral, anti-PD, and antioxidative effects.177–180 The antioxidant activities of catechins are highly protective against oxidative stress-induced PD, as shown by various cell and animal models.181–183 Although catechins have various health benefits, their bioavailability is low following oral consumption, resulting in reduced circulating levels.184 Several nanotechnological approaches have been used to enhance their bioavailability with an enhanced protective effect against various disease models by reducing the size to the nanolevel or encapsulating the catechin in a suitable nanoencapsulating system.185,186 Nanoliposome encapsulation of (-)-epigallocatechin gallate produced at a mean particle size of 71.7 nm enhances antioxidant activity and controls bioavailability.187 Similarly, tea catechin-loaded nanoparticles with sizes of 134–354 nm prepared from chitosan show enhanced transport to the intestine with higher antioxidant activity.188,189 Some studies suggest that epigallocatehin-3-gallate reduced to approximately 50 nm by co-solubilization methods greatly enhances its bioavailability in a rat brain model of Alzheimer’s disease.190 These studies confirm that catechins can be efficiently encapsulated at a nanosize using a suitable nanotechnology involving nanoliposome, nanoemulsion, or nanoencapsulation techniques, thereby protecting the catechin from the gastrointestinal tract.
Nanoquercetin
Quercetin is found at high levels in plant foods, such as fruits, vegetables, and juices. This bioflavonoid has multiple neurobeneficial activities, such as free radical scavenging, antianxiety, neuroprotection, and cognitive enhancing effects.191–193 Quercetin is chemo labile and thermo labile, which leads to lower bioavailability at the target site.194 In addition, quercetin has poor solubility and distribution, resulting in less bioavailability to the brain.194 Nanosizing quercetin greatly increases bioavailability and increases the protective effect at the target site without much loss in the gastrointestinal tract during oral administration.98,135,195–197 Oral delivery of nanoencapsulated quercetin with a size of 270 nm protects rat brain and liver cells from toxicity induced by arsenic. These studies have confirmed that quercetin is highly protected in the gastrointestinal tract and can be safely delivered to the target site in the brain.198 The same research group also studied quercetin encapsulated using an emulsion-diffusion-evaporation method to produce nanoquercetin with a size range of 20–50 nm, which showed higher bioavailability in various parts of the brain, such as hypothalamus, cerebellum, and hippocampus, in young and aged rats.196 Similarly, nanoquercetin developed using a solid lipid nanoparticle delivery technique with a size of 200 nm showed enhanced permeability and a high brain protective effect from Alzheimer’s disease.197 Nanosized quercetin developed using a nanoliposome delivery technique with a size of 200 nm shows enhanced anti-inflammatory activity in MCF-10A cells and enhances cognitive function in a rat model.199,200 Furthermore, quercetin encapsulated with poly-D,L-lactide nanoparticles with a size of approximately 130 nm produced using a solvent evaporation method enhances retention time to 96 hours.55,201 These studies confirmed that nanosizing quercetin using various delivery techniques enhances its protective role against various neurological disorder animal models through its antioxidative effects.
Nanolycopene
Lycopene is a naturally occurring carotenoid compound widely found in tomato, watermelon, and pink guava.54,56,57,202–204 Lycopene has a protective effect against neurological disorders including Alzheimer’s and PD by reducing oxidative stress.205–211 Lycopene supplementation of a rotenone-induced rat model of PD enhances the protective effect against oxidative stress and reduces neurobehavioral abnormalities.208 However, bioavailability in the gastrointestinal tract was limited after oral administration.212 Nanosizing lycopene using a self-emulsifying nanodelivery system or nanoemulsion greatly enhances bioavailability of the lycopene.212–214 Nanosized lycopene prepared using a nanoemulsion delivery technique with a size of 100 nm enhances in vitro antioxidant activity213 with increased bioaccessibility. Nanolycopene developed using a nanostructured lipid carrier delivery technique with a size of 150–160 nm shows less degradation and enhanced in vitro antioxidant activity.215 These studies confirm that lycopene can be stabilized using various delivery techniques and is potentially bioavailable for an extended duration to protect against oxidative stress leading to PD. Nanolycopene developed using various delivery techniques will be used in future studies for its role in various diseases including PD.
Nanokaempferol
Kaempferol is a flavonoid found in many plant foods, including tea, broccoli, tomato, drumstick leaves, and beans. Kaempferol has a variety of beneficial effects, including antioxidant, anti-inflammatory, neuroprotective, and anticancer activities.162,216–222 Kaempferol enhances autophagy in a rotenone-induced acute toxicity model of PD by enhancing mitochondrial antioxidant activity.223 Kaempferol has a neuroprotective effect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in a mouse PD model.224 However, bioavailability is limited to approximately 2% after oral administration.225 Nanosized kaempferol enhances the antioxidant activity of kaempferol.226 Nanokaempferol developed using a layer-bi-layer technique with a size range of 149–161 nm enhances the bioavailability of kaempferol in bone marrow.227 Oral bioavailability of kaempferol is enhanced using self-nanoemulsifying drug delivery system and nanoniosome delivery techniques with a size range of 34–141 nm in dog and rat models.228
Nanosilibinin
Silibinin is a flavonoid found mostly in milk thistle that has a variety of bioactivities, including anticancer, antioxidant, neuroprotective, and antidiabetic effects.43,229–232 Silibinin protects against neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of PD by stabilizing mitochondria potential, antioxidative, and anti-neuroinflammatory reactions.230 Similarly, silibinin attenuates mitochondrial dysfunction, oxidative stress, and neuronal loss following injection of MPP+ in a rat model of PD.229 Higher doses of silibinin enhance the protective effect in a 1-methyl-4-phenylpyridinium ion-treated animal model of PD in vivo.43 However, the bioavailability of silibinin to various organs is limited but can be greatly enhanced by nanosizing the compound.233,234 The bioavailability of silibinin-loaded nanotubes with a size range of 20–30 nm is greatly enhanced in cancer cell lines, even at very low concentrations.234 PEG-loaded nanoliposomes with a size range of 164–194 nm have also been designed for controlled delivery of silibinin to the liver.235 These studies confirm that silibinin, which has low bioavailability after oral intake, can be enhanced using a nanotechnological delivery method. Further herbal-derived nanoparticles are a budding approach to treat PD their toxicity was very minimal. It will be a future promising approach to treat PD.
Conclusion
The role of oxidative stress in PD is well understood but treatments using current phytotherapies are limited. Phytobioactive compounds are more vulnerable to various conditions during treatment, leading to lower bioavailability and lower anti-PD effects. Nanotechnology may solve these disadvantages and effectively deliver phytobioactive compounds with sustained activity. Development of nanodelivery techniques is more important for delivery to target organs and cross the blood–brain barrier. Delivery techniques can vary based on the bioactive compound. Several nanodelivery techniques and nanophytobioactive compounds discussed in this review increase the delivery efficiency of compounds to target sites. Further, research should focus on co-delivery of phytobioactive compounds to prevent oxidative stress that leads to various disorders including PD.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (number 2014R1A2A2A04007791).
Disclosure
The authors report no conflicts of interest in this work.
References
Blesa J, Przedborski S. Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat. 2014;8. | ||
Cai ZB, Zeng WJ, Tao K, Lu FF, Gao GD, Yang Q. Myricitrin alleviates MPP+-induced mitochondrial dysfunction in a DJ-1-dependent manner in SN4741 cells. Biochem Biophys Res Commun. 2015;458(2):227–233. | ||
Kim BW, Koppula S, Park SY, et al. Attenuation of neuroinflammatory responses and behavioral deficits by Ligusticum officinale (Makino) Kitag in stimulated microglia and MPTP-induced mouse model of Parkinson’s disease. J Ethnopharmacol. 2015;164:388–397. | ||
Chen YP, Zhang DQ, Liao Z, et al. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol Neurodegener. 2015;10(1):4. | ||
Hu WC, Wang GC, Li PX, et al. Neuroprotective effects of macranthoin G from Eucommia ulmoides against hydrogen peroxide-induced apoptosis in PC12 cells via inhibiting NF-kappa B activation. Chem Biol Interact. 2014;224:108–116. | ||
Phom L, Achumi B, Alone DP, Muralidhara, Yenisetti SC. Curcumin’s neuroprotective efficacy in Drosophila model of idiopathic Parkinson’s disease is phase specific: implication of its therapeutic effectiveness. Rejuvenation Res. 2014;17(6):481–489. | ||
Rowinska-Zyrek M, Salerno M, Kozlowski H. Neurodegenerative diseases – understanding their molecular bases and progress in the development of potential treatments. Coord Chem Rev. 2015;284:298–312. | ||
Solanki L, Parihar P, Mansuri ML, Parihar MS. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr. 2015;6(1):64–72. | ||
Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, Pinto MD. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. BrJ Clin Pharmacol. 2013;75(3):588–602. | ||
Scheepens A, Tan K, Paxton JW. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010;5(1):75–87. | ||
Shi Y, Johnson J, O’Shea M, Chu YF. The bioavailability and metabolism of phenolics, a class of antioxidants found in grains. Cereal Food World. 2014;59(2):52–58. | ||
Podsedek A, Redzynia M, Klewicka E, Koziolkiewicz M. Matrix effects on the stability and antioxidant activity of red cabbage anthocyanins under simulated gastrointestinal digestion. Biomed Res Int. 2014;2014:365738. | ||
Tsai YM, Jan WC, Chien CF, Lee WC, Lin LC, Tsai TH. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011;127(3):918–925. | ||
Augustin MA, Hemar Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem Soc Rev. 2009;38(4):902–912. | ||
Pereira DM, Valentao P, Andrade PB. Nano- and microdelivery systems for marine bioactive lipids. Mar Drugs. 2014;12(12):6014–6027. | ||
Yao MF, Xiao H, McClements DJ. Delivery of lipophilic bioactives: assembly, disassembly, and reassembly of lipid nanoparticles. Annu Rev Food Sci Technol. 2014;5:53–81. | ||
Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Tech. 2012;23(1):13–27. | ||
Mun S, Kim YR, McClements DJ. Control of beta-carotene bioaccessibility using starch-based filled hydrogels. Food Chem. 2015;173:454–461. | ||
Li Y, Hu M, Du YM, Xiao H, McClements DJ. Control of lipase digestibility of emulsified lipids by encapsulation within calcium alginate beads. Food Hydrocolloid. 2011;25(1):122–130. | ||
McClements DJ. Design of nano-laminated coatings to control bioavailability of lipophilic food components. J Food Sci. 2010;75(1):R30–R42. | ||
McClements DJ. Utilizing food effects to overcome challenges in delivery of lipophilic bioactives: structural design of medical and functional foods. Expert Opin Drug Deliv. 2013;10(12):1621–1632. | ||
McClements DJ, Decker EA, Park Y, Weiss J. Designing food structure to control stability, digestion, release and absorption of lipophilic food components. Food Biophys. 2008;3(2):219–228. | ||
Weiss J, Decker EA, McClements DJ, Kristbergsson K, Helgason T, Awad T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008;3(2):146–154. | ||
Wu W, Lee SY, Wu XB, et al. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials. 2014;35(7):2355–2364. | ||
Yin NY, Yao XL, Zhou QF, Faiola F, Jiang GB. Vitamin E attenuates silver nanoparticle-induced effects on body weight and neurotoxicity in rats. Biochem Biophys Res Commun. 2015;458(2):405–410. | ||
Zare K, Eidi A, Roghani M, Rohani AH. The neuroprotective potential of sinapic acid in the 6-hydroxydopamine-induced hemi-Parkinsonian rat. Metab Brain Dis. 2015;30(1):205–213. | ||
Ahmad S, Khan MB, Hoda MN, et al. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine induced neurotoxicity in mice model: cellular, biochemical and neurochemical evidence. Neurochem Res. 2012;37(3):516–526. | ||
Amri A, Le Clanche S, Therond P, et al. Resveratrol self-emulsifying system increases the uptake by endothelial cells and improves protection against oxidative stress-mediated death. Eur J Pharm Biopharm. 2014;86(3):418–426. | ||
Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AHL, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15(2):324–336. | ||
Hemalswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20(4):239–249. | ||
Mehta A, Kaur G, Chintamaneni M. Piperine and quercetin enhances antioxidant and hepatoprotective effect of curcumin in paracetamol induced oxidative stress. Int J Pharmacol. 2012;8(2):101–107. | ||
Nair P, Malhotra A, Dhawan DK. Curcumin and quercetin trigger apoptosis during benzo(a)pyrene-induced lung carcinogenesis. Mol Cell Biochem. 2015;400(1–2):51–56. | ||
Frozza RL, Bernardi A, Hoppe JB, et al. Lipid-core nanocapsules improve the effects of resveratrol against A beta-induced neuroinflammation. J Biomed Nanotechnol. 2013;9(12):2086–2104. | ||
Lu XW, Xu HE, Sun B, Zhu ZS, Zheng DH, Li XL. Enhanced neuroprotective effects of resveratrol delivered by nanoparticles on hydrogen peroxide-induced oxidative stress in rat cortical cell culture. Mol Pharm. 2013;10(5):2045–2053. | ||
Coradini K, Lima FO, Oliveira CM, et al. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur J Pharm Biopharm. 2014;88(1):178–185. | ||
Shin KS, Choi HS, Zhao TT, et al. Neurotoxic effects of berberine on long-term l-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch Pharm Res. 2013;36(6):759–767. | ||
Kumar H, Lim HW, More SV, et al. The role of free radicals in the aging brain and Parkinson’s disease: convergence and parallelism. Int J Mol Sci. 2012;13(8):10478–10504. | ||
Kumar H, Song SY, More SV, et al. Traditional Korean East Asian medicines and herbal formulations for cognitive impairment. Molecules. 2013;18(12):14670–14693. | ||
Lim HW, Park JI, More SV, et al. Anti-neuroinflammatory effects of DPTP, a novel synthetic clovamide derivative in in vitro and in vivo model of neuroinflammation. Brain Res Bull. 2015;112:25–34. | ||
Kim IS, Ko HM, Koppula S, Kim BW, Choi DK. Protective effect of Chrysanthemum indicum Linne against 1-methyl-4-phenylpridinium ion and lipopolysaccharide-induced cytotoxicity in cellular model of Parkinson’s disease. Food Chem Toxicol. 2011;49(4):963–973. | ||
Lee MY, Choi EJ, Lee MK, Lee JJ. Epigallocatechin gallate attenuates L-DOPA-induced apoptosis in rat PC12 cells. Nutr Res Pract. 2013;7(4):249–255. | ||
More SV, Kumar H, Kang S, Song SY, Lee K, Choi DK. Advances in neuroprotective ingredients of medicinal herbs by using cellular and animal models of Parkinson’s disease. Evid Based Complement Alternat Med. 2013;2013:957875. | ||
Jung UJ, Jeon MT, Choi MS, Kim SR. Silibinin attenuates MPP+-induced neurotoxicity in the substantia nigra in vivo. J Med Food. 2014;17(5):599–605. | ||
Choi HS, Kim HJ, Suh YH. Therapeutic potentials of human adipose-derived stem cells in mouse model of Parkinson’s disease. J Neurochem. 2014;130:68–68. | ||
Jeong KH, Jeon MT, Kim HD, et al. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treated rat model of Parkinson’s disease. J Med Food. 2015;18(4):409–414. | ||
Kim IS, Koppula S, Kim BW, et al. A novel synthetic compound PHID (8-Phenyl-6a, 7, 8, 9, 9a, 10-hexahydro-6H-isoindolo [5, 6-g] quinoxaline-7, 9-dione) protects SH-SY5Y cells against MPP+-induced cytotoxicity through inhibition of reactive oxygen species generation and JNK signaling. Eur J Pharmacol. 2011;650(1):48–57. | ||
Kumar H, Kim IS, More SV, Kim BW, Bahk YY, Choi DK. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson’s disease model. Evid Based Complement Alternat Med. 2013;2013:514095. | ||
Leem E, Nam JH, Jeon MT, et al. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson’s disease. J Nutr Biochem. 2014;25(7):801–806. | ||
Son HJ, Lee JA, Shin N, et al. A novel compound PTIQ protects the nigral dopaminergic neurones in an animal model of Parkinson’s disease induced by MPTP. Brit J Pharmacol. 2012;165(7):2213–2227. | ||
Pahwa R, Lyons KE. Levodopa-related wearing-off in Parkinson’s disease: identification and management. Curr Med Res Opin. 2009;25(4):841–849. | ||
Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL. Limitations of current Parkinson’s disease therapy. Ann Neurol. 2003;53(Suppl 3):S3–S12; discussion S12–S15. | ||
Yuan H, Zhang ZW, Liang LW, et al. Treatment strategies for Parkinson’s disease. Neurosci Bull. 2010;26(1):66–76. | ||
Ghosh S, More P, Derle A, et al. Diosgenin from Dioscorea bulbifera: novel hit for treatment of type II diabetes mellitus with inhibitory activity against alpha-amylase and alpha-glucosidase. PLoS One. 2014;9(9): e106039. | ||
Hong MY, Hartig N, Kaufman K, Hooshmand S, Figueroa A, Kern M. Watermelon consumption improves inflammation and antioxidant capacity in rats fed an atherogenic diet. Nutr Res. 2015;35(3):251–258. | ||
Kumari A, Kumar V, Yadav SK. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: a green approach. PLoS One. 2012;7(7):e41230. | ||
Shah K, Singh M, Rai AC. Bioactive compounds of tomato fruits from transgenic plants tolerant to drought. Lwt Food Sci Technol. 2015;61(2):609–614. | ||
Srivastava S, Srivastava AK. Lycopene, chemistry, biosynthesis, metabolism and degradation under various abiotic parameters. J Food Sci Technol. 2015;52(1):41–53. | ||
Barbosa M, Valentao P, Andrade PB. Bioactive compounds from macroalgae in the new millennium: implications for neurodegenerative diseases. Mar Drugs. 2014;12(9):4934–4972. | ||
Sun AJ, Xu XX, Lin JS, Cui XL, Xu RA. Neuroprotection by saponins. Phytother Res. 2015;29(2):187–200. | ||
Shukitt-Hale B, Galli RL, Meterko V, et al. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age. 2005;27(1):49–57. | ||
Del Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A. Dietary (Poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18(14):1818–1892. | ||
Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. | ||
Catarino MD, Alves-Silva JM, Pereira OR, Cardoso SM. Antioxidant capacities of flavones and benefits in oxidative-stress related diseases. Curr Top Med Chem. 2015;15(2):105–119. | ||
Seyoum A, Asres K, El-Fiky FK. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 2006;67(18):2058–2070. | ||
Gundimeda U, McNeill TH, Barseghian BA, et al. Polyphenols from green tea prevent antineuritogenic action of Nogo-A via 67-kDa laminin receptor and hydrogen peroxide. J Neurochem. 2015;132(1):70–84. | ||
Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–1382. | ||
Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–494. | ||
Singh G, Pai RS. In-vitro/in-vivo characterization of trans-resveratrol-loaded nanoparticulate drug delivery system for oral administration. J Pharm Pharmacol. 2014;66(8):1062–1076. | ||
Singh G, Pai RS. Optimized self-nanoemulsifying drug delivery system of atazanavir with enhanced oral bioavailability: in vitro/in vivo characterization. Expert Opin Drug Del. 2014;11(7):1023–1032. | ||
Gidwani M, Singh AV. Nanoparticle enabled drug delivery across the blood brain barrier: in vivo and in vitro models, opportunities and challenges. Curr Pharm Biotechnol. 2013;14(14):1201–1212. | ||
Ratnam DV, Chandraiah G, Sonaje K, et al. A potential therapeutic strategy for diabetes and its complications in the form of co-encapsulated antioxidant nanoparticles (NanoCAPs) of ellagic acid and coenzyme Q(10): preparation and evaluation in streptozotocin induced diabetic rats. J Biomed Nanotechnol. 2008;4(1):33–43. | ||
Sachdeva AK, Misra S, Kaur IP, Chopra K. Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: behavioral and biochemical evidence. Eur J Pharmacol. 2015;747:132–140. | ||
Kumar A, Chen F, Mozhi A, et al. Innovative pharmaceutical development based on unique properties of nanoscale delivery formulation. Nanoscale. 2013;5(18):8307–8325. | ||
Lockman PR, Oyewumi MO, Koziara JM, Roder KE, Mumper RJ, Allen DD. Brain uptake of thiamine-coated nanoparticles. J Control Release. 2003;93(3):271–282. | ||
Li L, Tuo J, Xie YQ, et al. Preparation, transportation mechanisms and brain-targeting evaluation in vivo of a chemical delivery system exploiting the blood-cerebrospinal fluid barrier. J Drug Target. 2014;22(8):724–731. | ||
Yin HT, Si J, Xu HE, et al. Resveratrol-loaded nanoparticles reduce oxidative stress induced by radiation or amyloid-beta in transgenic Caenorhabditis elegans. J Biomed Nanotechnol. 2014;10(8):1536–1544. | ||
Gonullu U, Uner M, Yener G, Karaman EF, Aydogmus Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015;65(1):1–13. | ||
Mustafa S, Pai RS, Singh G, Devi VK. Nanocarrier-based interventions for the management of MDR/XDR-TB. J Drug Target. 2015;23(4):287–304. | ||
Svetlichny G, Kulkamp-Guerreiro IC, Cunha SL, et al. Solid lipid nanoparticles containing copaiba oil and allantoin: development and role of nanoencapsulation on the antifungal activity. Pharmazie. 2015;70(3):155–164. | ||
Zhang YT, Han MQ, Shen LN, Zhao JH, Feng NP. Solid lipid nanoparticles formulated for transdermal aconitine administration and evaluated in vitro and in vivo. J Biomed Nanotechnol. 2015;11(2):351–361. | ||
Zhao S, Zhang YL, Han YZ, Wang J, Yang J. Preparation and Characterization of cisplatin magnetic solid lipid nanoparticles (MSLNs): effects of loading procedures of Fe3O4 nanoparticles. Pharm Res. 2015;32(2):482–491. | ||
Chan HK, Kwok PCL. Production methods for nanodrug particles using the bottom-up approach. Adv Drug Deliv Rev. 2011;63(6):406–416. | ||
Mehnert W, Mader K. Solid lipid nanoparticles – Production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–196. | ||
Mehnert W, Mader K. Solid lipid nanoparticles Production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101. | ||
Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349–358. | ||
Scalia S, Young PM, Traini D. Solid lipid microparticles as an approach to drug delivery. Expert Opin Drug Deliv. 2015;12(4):583–599. | ||
Nance E, Timbie K, Miller GW, et al. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood–brain barrier using MRI-guided focused ultrasound. J Control Release. 2014;189:123–132. | ||
Esposito E, Fantin M, Marti M, et al. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm Res. 2008;25(7):1521–1530. | ||
Bicker J, Alves G, Fortuna A, Falcao A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. Eur J Pharm Biopharm. 2014;87(3):409–432. | ||
Bose T, Latawiec D, Mondal PP, Mandal S. Overview of nano-drugs characteristics for clinical application: the journey from the entry to the exit point. J Nanopart Res. 2014;16(8). | ||
Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JEN, Omidfar K. Drug targeting using solid lipid nanoparticles. Chem Phys Lipids. 2014;181:56–61. | ||
Neves AR, Lucio M, Martins S, Lima JLC, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomed. 2013;8:177–187. | ||
Pandita D, Kumar S, Poonia N, Lather V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res Int. 2014;62:1165–1174. | ||
Vandita K, Shashi B, Santosh KG, Pal KI. Enhanced apoptotic effect of curcumin loaded solid lipid nanoparticles. Mol Pharm. 2012;9(12):3411–3421. | ||
Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;85(3):339–345. | ||
Aditya NP, Macedo AS, Doktorovov S, et al. Development and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). Lwt Food Sci Technol. 2014;59(1):115–121. | ||
Dora CL, Silva LFC, Tagliari MP, Silva MAS, Lemos-Senna E. Formulation study of quercetin-loaded lipid-based nanocarriers obtained by hot solvent diffusion method. Lat Am J Pharm. 2011;30(2):289–296. | ||
Li HL, Zhao XB, Ma YK, Zhai GX, Li LB, Lou HX. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–244. | ||
Lacatusu I, Badea N, Niculae G, Bordei N, Stan R, Meghea A. Lipid nanocarriers based on natural compounds: an evolving role in plant extract delivery. Eur J Lipid Sci Technol. 2014;116(12):1708–1717. | ||
Mussi SV, Sawant R, Perche F, et al. Novel Nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm Res. 2014;31(8):1882–1892. | ||
Mussi SV, Torchilin VP. Recent trends in the use of lipidic nanoparticles as pharmaceutical carriers for cancer therapy and diagnostics. J Mater Chem B. 2013;1(39):5201–5209. | ||
Weber S, Zimmer A, Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22. | ||
Zhang WJ, Li XD, Ye TT, et al. Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. Int J Nanomed. 2014;9:4305–4315. | ||
Zhang XY, Qiao H, Zhang TF, Shi YB, Ni JM. Enhancement of gastrointestinal absorption of isoliquiritigenin by nanostructured lipid carrier. Adv Powder Technol. 2014;25(3):1060–1068. | ||
Luan JJ, Zheng F, Yang XY, Yu AH, Zhai GX. Nanostructured lipid carriers for oral delivery of baicalin: in vitro and in vivo evaluation. Colloid Surf A. 2015;466:154–159. | ||
Nanjwade BK, Kadam VT, Manvi FV. Formulation and characterization of nanostructured lipid carrier of ubiquinone (coenzyme Q10). J Biomed Nanotechnol. 2013;9(3):450–460. | ||
Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 2012;20(10):813–830. | ||
Aditya NP, Shim M, Lee I, Lee Y, Im MH, Ko S. Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agric Food Chem. 2013;61(8):1878–1883. | ||
Aditya NP, Shim M, Yang H, Lee Y, Ko S. Antiangiogenic effect of combined treatment with curcumin and genistein on human prostate cancer cell line. J Funct Foods. 2014;8:204–213. | ||
Mourtas S, Canovi M, Zona C, et al. Curcumin-decorated nanoliposomes with very high affinity for amyloid-beta 1-42 peptide. Biomaterials. 2011;32(6):1635–1645. | ||
Gao DW, Tang SN, Tong Q. Oleanolic acid liposomes with polyethylene glycol modification: promising antitumor drug delivery. Int J Nanomed. 2012;7:3517–3526. | ||
Hadian Z, Sahari MA, Moghimi HR, Barzegar M. Formulation, characterization and optimization of liposomes containing eicosapentaenoic and docosahexaenoic acids; a methodology approach. Iran J Pharm Res. 2014;13(2):393–404. | ||
Li T, Yang SB, Liu W, et al. Preparation and characterization of nanoscale complex liposomes containing medium-chain fatty acids and vitamin C. Int J Food Prop. 2015;18(1):113–124. | ||
Haeri A, Alinaghian B, Daeihamed M, Dadashzadeh S. Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iran J Pharm Res. 2014;13:3–14. | ||
Rasti B, Jinap S, Mozafari MR, Abd-Manap MY. Optimization on preparation condition of polyunsaturated fatty acids nanoliposome prepared by Mozafari method. J Liposome Res. 2014;24(2):99–105. | ||
Gharib A, Faezizadeh Z, Godarzee M. Preparation and characterization of nanoliposomal beta-cryptoxanthin and its effect on proliferation and apoptosis in human leukemia cell line K562. Trop J Pharm Res. 2015;14(2):187–194. | ||
Mohammadi R, Mahmoudzade M, Atefi M, Khosravi-Darani K, Mozafari MR. Applications of nanoliposomes in cheese technology. Int J Dairy Technol. 2015;68(1):11–23. | ||
Wu JL, Liu H, Ge SY, et al. The preparation, characterization, antimicrobial stability and in vitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocolloid. 2015;43:427–435. | ||
Pilakka-Kanthikeel S, Nair M. Targeted BDNF delivery across the blood-brain barrier for neuro-protection using liposome formulated magnetic nano carriers: an in-vitro study. J Neurovirol. 2012;18:85–86. | ||
Zhao M, Chang J, Fu XP, et al. Nano-sized cationic polymeric magnetic liposomes significantly improves drug delivery to the brain in rats. J Drug Target. 2012;20(5):416–421. | ||
Aisha AFA, Majid AMSA, Ismail Z. Preparation and characterization of nano liposomes of Orthosiphon stamineus ethanolic extract in soybean phospholipids. BMC Biotechnol. 2014;14:23. | ||
Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009;57(19):9141–9146. | ||
Mourtas S, Lazar AN, Markoutsa E, Duyckaerts C, Antimisiaris SG. Multifunctional nanoliposomes with curcumin-lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur J Med Chem. 2014;80:175–183. | ||
Bragagni M, Mennini N, Ghelardini C, Mura P. Development and characterization of niosomal formulations of doxorubicin aimed at brain targeting. J Pharm Pharm Sci. 2012;15(1):184–196. | ||
Manosroi A, Ruksiriwanich W, Abe M, Sakai H, Manosroi W, Manosroi J. Biological activities of the rice bran extract and physical characteristics of its entrapment in niosomes by supercritical carbon dioxide fluid. J Supercrit Fluid. 2010;54(2):137–144. | ||
Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Rel. 2014;185:22–36. | ||
Mukherjee B, Patra B, Layek B, Mukherjee A. Sustained release of acyclovir from nano-liposomes and nano-niosomes: an in vitro study. Int J Nanomed. 2007;2(2):213–225. | ||
Hamishehkar H, Rahimpour Y, Kouhsoltani M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin Drug Deliv. 2013;10(2):261–272. | ||
Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm. 2012;423(2):303–311. | ||
Gaafar PME, Abdallah OY, Farid RM, Abdelkader H. Preparation, characterization and evaluation of novel elastic nano-sized niosomes (ethoniosomes) for ocular delivery of prednisolone. J Liposome Res. 2014;24(3):204–215. | ||
Akhter S, Kushwaha S, Warsi MH, et al. Development and evaluation of nanosized niosomal dispersion for oral delivery of Ganciclovir. Drug Dev Ind Pharm. 2012;38(1):84–92. | ||
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. | ||
Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnology. 2011;9:55. | ||
Bennet D, Marimuthu M, Kim S, An J. Dual drug-loaded nanoparticles on self-integrated scaffold for controlled delivery. Int J Nanomed. 2012;7:3399–3419. | ||
Chakraborty S, Stalin S, Das N, Choudhury ST, Ghosh S, Swarnakar S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials. 2012;33(10):2991–3001. | ||
Tosi G, Vergoni AV, Ruozi B, et al. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: In vivo pharmacological evidence and biodistribution. J Control Release. 2010;145(1):49–57. | ||
Li JW, Feng L, Fan L, et al. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials. 2011;32(21):4943–4950. | ||
Cheng KK, Chan PS, Fan SJ, et al. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials. 2015;44:155–172. | ||
Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416(1):331–338. | ||
McClements DJ, Rao J. Food-Grade Nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci. 2011;51(4):285–330. | ||
Ahmed K, Li Y, McClements DJ, Xiao H. Nanoemulsion- and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem. 2012;132(2):799–807. | ||
Chang YH, McClements DJ. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: influence of the oil phase, surfactant, and temperature. J Agric Food Chem. 2014;62(10):2306–2312. | ||
Komaiko J, McClements DJ. Low-energy formation of edible nanoemulsions by spontaneous emulsification: factors influencing particle size. J Food Eng. 2015;146:122–128. | ||
Young NA, Bruss MS, Gardner M, et al. Oral administration of nano-emulsion curcumin in mice suppresses inflammatory-induced NF kappa B signaling and macrophage migration. PLoS One. 2014;9(11):e111559. | ||
Pangeni R, Sharma S, Mustafa G, Ali J, Baboota S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology. 2014;25(48):485102. | ||
Mizrahi M, Friedman-Levi Y, Larush L, et al. Pomegranate seed oil nanoemulsions for the prevention and treatment of neurodegenerative diseases: the case of genetic CJD. Nanomed Nanotechnol. 2014;10(6):1353–1363. | ||
Cavazos-Garduno A, Flores AAO, Serrano-Nino JC, Martinez-Sanchez CE, Beristain CI, Garcia HS. Preparation of betulinic acid nanoemulsions stabilized by omega-3 enriched phosphatidylcholine. Ultrason Sonochem. 2015;24:204–213. | ||
Sessa M, Balestrieri ML, Ferrari G, et al. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014;147:42–50. | ||
Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81(1):317s–325s. | ||
Correa-Betanzo J, Allen-Vercoe E, McDonald J, Schroeter K, Corredig M, Paliyath G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014;165:522–531. | ||
Tenore GC, Campiglia P, Ritieni A, Novellino E. In vitro bioaccessibility, bioavailability and plasma protein interaction of polyphenols from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013;141(4):3519–3524. | ||
Frozza RL, Bernardi A, Hoppe JB, et al. Neuroprotective effects of resveratrol against A beta administration in rats are improved by lipid-core nanocapsules. Mol Neurobiol. 2013;47(3):1066–1080. | ||
Cai YZ, Sun M, Xing J, Luo Q, Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78(25):2872–2888. | ||
Lee KS, Lee BS, Semnani S, et al. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010;13(5):561–570. | ||
Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32(5):1119–1123. | ||
Siddique YH, Naz F, Jyoti S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. Biomed Res Int. 2014:606928. | ||
Zhao JW, Dyson SC, Kriegel C, et al. Modelling of a targeted nanotherapeutic ‘stroma’ to deliver the cytokine LIF, or XAV939, a potent inhibitor of Wnt-beta-catenin signalling, for use in human fetal dopaminergic grafts in Parkinson’s disease. Dis Model Mech. 2014;7(10):1193–1203. | ||
Anand P, Nair HB, Sung BK, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79(3):330–338. | ||
Ramalingam P, Ko YT. A validated LC-MS/MS method for quantitative analysis of curcumin in mouse plasma and brain tissue and its application in pharmacokinetic and brain distribution studies. J Chromatogr B. 2014;969:101–108. | ||
Ramalingam P, Ko YT. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm Res. 2015;32(2):389–402. | ||
Nazari QA, Takada-Takatori Y, Hashimoto T, et al. Potential protective effect of highly bioavailable curcumin on an oxidative stress model induced by microinjection of sodium nitroprusside in mice brain. Food Funct. 2014;5(5):984–989. | ||
Dey A, De JN. Neuroprotective therapeutics from botanicals and phytochemicals against Huntington’s disease and related neurodegenerative disorders. J Herb Med. 2015;5(1):1–19. | ||
Jo SK, Kim IS, Yoon KS, Yoon HH, Yoo HH. Preparation of ginsenosides Rg3, Rk1, and Rg5-selectively enriched ginsengs by a simple steaming process. Eur Food Res Technol. 2015;240(1):251–256. | ||
Kim SN, Kim S, Hong YD, et al. The ginsenosides of Panax ginseng promote hair growth via similar mechanism of minoxidil. J Dermatol Sci. 2015;77(2):132–134. | ||
Li T, Shu YJ, Cheng JY, et al. Pharmacokinetics and efficiency of brain targeting of ginsenosides Rg1 and Rb1 given as Nao-Qing microemulsion. Drug Dev Ind Pharm. 2015;41(2):224–231. | ||
Quan K, Liu Q, Wan JY, et al. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci Rep. 2015;5. | ||
Liu QA, Kou JP, Yu BY. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-kappa B activation. Neurochem Int. 2011;58(1):119–125. | ||
Ge KL, Chen WF, Xie JX, Wong MS. Ginsenoside Rg1 protects against 6-OHDA-induced toxicity in MES23.5 cells via Akt and ERK signaling pathways. J Ethnopharmacol. 2010;127(1):118–123. | ||
Xu HM, Jiang H, Wang J, Xie JX. Rg1 protects iron-induced neurotoxicity through antioxidant and iron regulatory proteins in 6-OHDA-treated MES23.5 cells. J Cell Biochem. 2010;111(6):1537–1545. | ||
Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb-1 and Rg(1) from Panax notoginseng in rats. J Ethnopharmacol. 2003;84(2–3):187–192. | ||
Ryu JS, Lee HJ, Bae SH, et al. The bioavailability of red ginseng extract fermented by Phellinus linteus. J Ginseng Res. 2013;37(1):108–116. | ||
Tsai WC, Li WC, Yin HY, Yu MC, Wen HW. Constructing liposomal nanovesicles of ginseng extract against hydrogen peroxide-induced oxidative damage to L929 cells. Food Chem. 2012;132(2):744–751. | ||
Wen HW, Li WC, Chung RJ, et al. Evaluation of nanofabricated ginseng extract powders. J Nanosci Nanotechnol. 2009;9(7):4108–4115. | ||
Zhang J, Han XZ, Li X, et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomed. 2012;7:4299–4310. | ||
Singh G, Pai RS. Trans-resveratrol self-nano-emulsifying drug delivery system (SNEDDS) with enhanced bioavailability potential: optimization, pharmacokinetics and in situ single pass intestinal perfusion (SPIP) studies. Drug Deliv. 2015;22(4):522–530. | ||
Singh G, Pai RS. Optimized PLGA nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opin Drug Deliv. 2014;11(5):647–659. | ||
Goh R, Gao J, Ananingsih VK, Ranawana V, Henry CJ, Zhou WB. Green tea catechins reduced the glycaemic potential of bread: an in vitro digestibility study. Food Chem. 2015;180:203–210. | ||
Jaworska G, Pogon K, Bernas E, Duda-Chodak A. Nutraceuticals and antioxidant activity of prepared for consumption commercial mushrooms Agaricus Bisporus and Pleurotus Ostreatus. J Food Quality. 2015;38(2):111–122. | ||
Rashidinejad A, Birch EJ, Sun-Waterhouse D, Everett DW. Total phenolic content and antioxidant properties of hard low-fat cheese fortified with catechin as affected by in vitro gastrointestinal digestion. Lwt Food Sci Technol. 2015;61(1):393–399. | ||
Sardana A, Kalra S, Khanna D, Balakumar P. Nephroprotective effect of catechin on gentamicin-induced experimental nephrotoxicity. Clin Exp Nephrol. 2015;19(2):178–184. | ||
Kang KS, Yamabe N, Wen YJ, Fukui M, Zhu BT. Beneficial effects of natural phenolics on levodopa methylation and oxidative neurodegeneration. Brain Res. 2013;1497:1–14. | ||
Mehra P, Garg M, Koul A, Bansal DD. Effect of (+)-catechin hydrate on oxidative stress induced by high sucrose and high fat diet in male Wistar rats. Indian J Exp Biol. 2013;51(10):823–827. | ||
Teixeira MDA, Souza CM, Menezes APF, et al. Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol Biochem B. 2013;110:1–7. | ||
Chow HHS, Hakim IA, Vining DR, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11(12):4627–4633. | ||
Krishnaswamy K, Orsat V, Thangavel K. Synthesis and characterization of nano-encapsulated catechin by molecular inclusion with beta-cyclodextrin. J Food Eng. 2012;111(2):255–264. | ||
Luo XB, Guan RF, Chen XQ, Tao M, Ma JQ, Zhao J. Optimization on condition of epigallocatechin-3-gallate (EGCG) nanoliposomes by response surface methodology and cellular uptake studies in Caco-2 cells. Nanoscale Res Lett. 2014;9(1):291. | ||
Zou LQ, Peng SF, Liu W, et al. Improved in vitro digestion stability of (-)-epigallocatechin gallate through nanoliposome encapsulation. Food Res Int. 2014;64:492–499. | ||
Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur J Pharm Sci. 2010;41(2):219–225. | ||
Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the plasma exposure of (-)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur J Pharm Sci. 2011;44(3):422–426. | ||
Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int J Pharm. 2010;389(1–2):207–212. | ||
Kumar B, Gupta SK, Nag TC, et al. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res. 2014;125:193–202. | ||
Lopes CRP, Ferreira PEB, Zanoni JN, Alves AMP, Alves EPB, Buttow NC. Neuroprotective effect of quercetin on the duodenum enteric nervous system of streptozotocin-induced diabetic rats. Digest Dis Sci. 2012;57(12):3106–3115. | ||
Lu CW, Lin TY, Wang SJ. Quercetin inhibits depolarization-evoked glutamate release in nerve terminals from rat cerebral cortex. Neurotoxicology. 2013;39:1–9. | ||
Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20(20):2572–2582. | ||
Kumar VD, Verma PRP, Singh SK. Development and evaluation of biodegradable polymeric nanoparticles for the effective delivery of quercetin using a quality by design approach. Lwt Food Sci Technol. 2015;61(2):330–338. | ||
Ghosh A, Sarkar S, Mandal AK, Das N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One. 2013;8(4):e57735. | ||
Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63(3):342–351. | ||
Ghosh A, Mandal AK, Sarkar S, Panda S, Das N. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life Sci. 2009;84(3–4):75–80. | ||
Chulasiri M, Sutthiparinyanont S, Priprem A, Lee HH, Na HK, Surh YJ. Anti-inflammatory in induced MCF-10A cells of quercetin delivered by liposomes. Drug Metab Rev. 2009;41:110–111. | ||
Priprem A, Watanatorn J, Sutthiparinyanont S, Phachonpai W, Muchimapura S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomed Nanotechnol. 2008;4(1):70–78. | ||
Kumari A, Yadav SK, Pakade YB, et al. Nanoencapsulation and characterization of Albiziachinensis isolated antioxidant quercitrin on PLA nanoparticles. Colloid Surf B. 2011;82(1):224–232. | ||
Biddle MJ, Lennie TA, Bricker GV, Kopec RE, Schwartz SJ, Moser DK.Lycopene dietary intervention: a pilot study in patients with heart failure. J Cardiovasc Nurs. 2015;30(3):205–212. | ||
Chen F, Sun ZW, Ye LF, Fu GS, Mou Y, Hu SJ. Lycopene protects against apoptosis in hypoxia/reoxygenation-induced H9C2 myocardioblast cells through increased autophagy. Mol Med Rep. 2015;11(2):1358–1365. | ||
Gerszberg A, Hnatuszko-Konka K, Kowalczyk T, Kononowicz AK. Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell Tiss Org. 2015;120(3):881–902. | ||
Altas S, Kizil G, Kizil M, Ketani A, Haris PI. Protective effect of Diyarbakir watermelon juice on carbon tetrachloride-induced toxicity in rats. Food Chem Toxicol. 2011;49(9):2433–2438. | ||
Barros L, Oliveira S, Carvalho AM, Ferreira ICFR. In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind Crop Prod. 2010;32(3):572–579. | ||
Ishaq S, Rathore HA, Sabir SM, Maroof MS. Antioxidant properties of Elaeagnus umbellata berry solvent extracts against lipid peroxidation in mice brain and liver tissues. Food Sci Biotechnol. 2015;24(2):673–679. | ||
Kaur H, Chauhan S, Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem Res. 2011;36(8):1435–1443. | ||
Kuhad A, Sethi R, Chopra K. Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sci. 2008;83(3–4):128–134. | ||
Suganuma H, Hirano T, Arimoto Y, Inakuma T. Effect of tomato intake on striatal monoamine level in a mouse model of experimental Parkinson’s disease. J Nutr Sci Vitaminol. 2002;48(3):251–254. | ||
Suganuma H, Kaburagi S, Inakuma T, Ishiguro Y. Amelioratory effect of dietary ingestion of lycopene and tomato rich in lycopene on learning impairment in senescence-accelerated mice (SAMP8). Food Sci Technol Res. 2002;8(2):183–187. | ||
Ross AB, Vuong LT, Ruckle J, et al. Lycopene bioavailability and metabolism in humans: an accelerator mass spectrometry study. Am J Clin Nutr. 2011;93(6):1263–1273. | ||
Ha TVA, Kim S, Choi Y, et al. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015;178:115–121. | ||
Kim SO, Ha TVA, Choi YJ, Ko S. Optimization of homogenization-evaporation process for lycopene nanoemulsion production and its beverage applications. J Food Sci. 2014;79(8):N1604–N1610. | ||
Okonogi S, Riangjanapatee P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int J Pharm. 2015;478(2):726–735. | ||
Chandramohan G, Al-Numair KS, Alsaif MA, Veeramani C. Antidiabetic effect of kaempferol a flavonoid compound, on streptozotocin-induced diabetic rats with special reference to glycoprotein components. Prog Nutr. 2015;17(1):50–57. | ||
Das AK, Singh V. Antioxidative free and bound phenolic constituents in pericarp, germ and endosperm of Indian dent (Zea mays var. indentata) and flint (Zea mays var. indurata) maize. J Funct Foods. 2015;13:363–374. | ||
Fu R, Zhang YT, Peng T, Guo YR, Chen F. Phenolic composition and effects on allergic contact dermatitis of phenolic extracts Sapium sebiferum (L.) Roxb. leaves. J Ethnopharmacol. 2015;162:176–180. | ||
Kim SH, Park JG, Lee J, et al. The dietary flavonoid kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediators Inflamm. 2015;2015:904142. | ||
Wang M, Sun JG, Jiang ZH, Xie WY, Zhang XY. Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am J Chin Med. 2015;43(2):241–254. | ||
Zhang Q, Wu D, Wu J, et al. Improved blood-brain barrier distribution: effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J Ethnopharmacol. 2015;162:270–277. | ||
Zhao XY, Zhang WN, Yin XR, et al. Phenolic composition and antioxidant properties of different peach [Prunus persica (L.) Batsch] cultivars in China. Int J Mol Sci. 2015;16(3):5762–5778. | ||
Filomeni G, Graziani I, De Zio D, et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson’s disease. Neurobiol Aging. 2012;33(4):767–785. | ||
Li S, Pu XP. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol Pharm Bull. 2011;34(8):1291–1296. | ||
Barve A, Chen C, Hebbar V, Desiderio J, Saw CLL, Kong AN. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm Drug Dispos. 2009;30(7):356–365. | ||
Tzeng CW, Yen FL, Wu TH, et al. Enhancement of dissolution and antioxidant activity of kaempferol using a nanoparticle engineering process. J Agric Food Chem. 2011;59(9):5073–5080. | ||
Kumar A, Gupta GK, Khedgikar V, et al. In vivo efficacy studies of layer-by-layer nano-matrix bearing kaempferol for the conditions of osteoporosis: a study in ovariectomized rat model. Eur J Pharm Biopharm. 2012;82(3):508–517. | ||
Jin Y, Wen JY, Garg S, et al. Development of a novel niosomal system for oral delivery of Ginkgo biloba extract. Int J Nanomed. 2013;8:421–430. | ||
Geed M, Garabadu D, Ahmad A, Krishnamurthy S. Silibinin pretreatment attenuates biochemical and behavioral changes induced by intrastriatal MPP+ injection in rats. Pharmacol Biochem Behav. 2014;117:92–103. | ||
Lee Y, Park HR, Chun HJ, Lee J. Silibinin prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease via mitochondrial stabilization. J Neurosci Res. 2015;93(5):755–765. | ||
Baluchnejadmojarad T, Roghani M, Mafakheri M. Neuroprotective effect of silymarin in 6-hydroxydopamine hemi-Parkinsonian rat: involvement of estrogen receptors and oxidative stress. Neurosci Lett. 2010;480(3):206–210. | ||
Milic N, Milosevic N, Suvajdzic L, Zarkov M, Abenavoli L. New therapeutic potentials of milk thistle (Silybum marianum). Nat Prod Commun. 2013;8(12):1801–1810. | ||
Ruan XQ, Shen C, Meng Q. Establishment of a methodology for investigating protectants against ethanol-induced hepatotoxicity. Food Chem Toxicol. 2010;48(5):1145–1151. | ||
Tan JM, Karthivashan G, Arulselvan P, Fakurazi S, Hussein MZ. Characterization and in vitro sustained release of silibinin from pH responsive carbon nanotube-based drug delivery system. J Nanomater. 2014;2014:439873. | ||
Elmowafy M, Viitala T, Ibrahim HM, et al. Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. Eur J Pharm Sci. 2013;50(2):161–171. | ||
Siddique YH, Khan W, Singh BR, Naqvi AH. Synthesis of alginate-curcumin nanocomposite and its protective role in transgenic Drosophila model of Parkinson’s disease. ISRN Pharmacol. 2013:doi:10.1155/2013/794582. | ||
Wang W, Zhu R, Xie Q, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine. 2012;7:3667–3677. | ||
Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425(1–2):44–52. | ||
Doggui S, Sahni JK, Arseneault M, Dao L, Ramassamy C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J Alzheimers Dis. 2012;30(2):377–392. | ||
Chang CZ, Wu SC, Lin CL, Kwan AL. Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear factor κB (p65) and subarachnoid hemorrhage induced early brain injury in a rat model. Brain Res. 2015;1608:215–224. | ||
Mathiyalagan R, Subramaniyam S, Kim YJ, Kim YC, Yang DC. Ginsenoside compound K-bearing glycol chitosan conjugates: synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr Polym. 2014;112:359–366. | ||
Frozza RL, Bernardi A, Paese K, et al. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J Biomed Nanotechnol. 2010;6(6):694–703. | ||
Lu X, Ji C, Xu H, et al. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int J Pharm. 2009;375(1–2):89–96. | ||
Lee CW, Yen FL, Huang HW, et al. Resveratrol nanoparticle system improves dissolution properties and enhances the hepatoprotective effect of resveratrol through antioxidant and anti-inflammatory pathways. J Agric Food Chem. 2012;60(18):4662–4671. | ||
Guo W, Li W, Jia Z, Yuan Y, Dai H, Li H. Transferrin modified PEG–PLA-resveratrol conjugates: in vitro and in vivo studies for glioma. Eur J Pharmacol. 2013;718(1–3):41–47. | ||
Jung KH, Lee JH, Park JW, et al. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int J Pharm. 2015;478(1):251–257. | ||
Liang J, Li F, Fang Y, et al. Cytotoxicity and apoptotic effects of tea polyphenol-loaded chitosan nanoparticles on human hepatoma HepG2 cells. Mater Sci Eng C Mater Biol Appl. 2014;36:7–13. | ||
Chen CC, Hsieh DS, Huang KJ, et al. Improving anticancer efficacy of (−)-epigallocatechin-3-gallate gold nanoparticles in murine B16F10 melanoma cells. Drug Des Devel Ther. 2014;8:459–474. | ||
Hong Z, Xu Y, Yin JF, Jin J, Du Q. Improving the Effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J Agric Food Chem. 2014;62(52):12603–12609. | ||
Karadag A, Ozcelik B, Huang Q. Quercetin nanosuspensions produced by high-pressure homogenization. J Agric Food Chem. 2014;62(8):1852–1859. | ||
Wang G, Wang JJ, Yang GY, et al. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int J Nanomedicine. 2012;7:271–280. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.