Back to Journals » International Journal of Nanomedicine » Volume 14
Recent insights on nanomedicine for augmented infection control
Authors Singh S , Hussain A, Shakeel F , Ahsan MJ , Alshehri S , Webster TJ , Lal UR
Received 5 April 2018
Accepted for publication 23 August 2018
Published 1 April 2019 Volume 2019:14 Pages 2301—2325
DOI https://doi.org/10.2147/IJN.S170280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Carlos Rinaldi
Sima Singh,1,* Afzal Hussain,1,* Faiyaz Shakeel,2,* Mohamed Jawed Ahsan,3 Sultan Alshehri,2 Thomas J Webster,4,* Uma Ranjan Lal5
1Department of Pharmaceutical Sciences and Technology, Birla Institute of Technology, Ranchi 835215, Jharkhand, India; 2Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 3Department of Pharmaceutical Chemistry, Maharishi Arvind College of Pharmacy, Jaipur, Rajasthan, 302023, India; 4Department of Chemical Engineering, Northeastern University, Boston, MA 02115, USA; 5School of Pharmaceutical Sciences, Shoolini University, Solan 173229, Himacahal Pradesh, India
*These authors contributed equally to this work
Abstract: Antimicrobial agents have been widely investigated for protecting against microbial infections in modern health. Drug-related limitations, poor bioavailability, toxicity to mammalian cells, and frequent bacteria drug resistance are major challenges faced when exploited in nanomedicine forms. Specific attention has been paid to control nanomaterial-based infection against numerous challenging pathogens in addition to improved drug delivery, targeting, and pharmacokinetic (PK) profiles, and thus, efficient antimicrobials have been fabricated using diverse components (metals, metal oxides, synthetic and semisynthetic polymers, natural or biodegradable polymers, etc). The present review covers several nanocarriers delivered through various routes of administration, highlighting major findings to control microbial infection as compared to using the free drug. Results over the past decade support the consistent development of various nanomedicines capable of improving biological significance and therapeutic benefits against an array of microbial strains. Depending on the intended application of nanomedicine, infection control will be challenged by various factors such as weighing the risk–benefits in healthcare settings, nanomaterial-induced (eco)toxicological hazards, frequent development of antibiotic resistance, scarcity of in vivo toxicity data, and a poor understanding of microbial interactions with nanomedicine at the molecular level. This review summarizes well-established informative data for nanomaterials used for infection control and safety concerns of nanomedicines to healthcare sectors followed by the significance of a unique “safe-by-design” approach.
Keywords: recent nanomedicines, infection control, antimicrobial agents, drug delivery, biosafety
Introduction
Traditional antimicrobial (AM) agents have several challenges, such as frequent drug resistance, pre-formulation-related limitations, poor drug targeting, and poor drug release which may lead to mammalian cell toxicity or ineffectiveness at the site of action. This was highlighted in a recent Centers for Disease Control prediction where more people are predicted to die from antibiotic-resistant bacteria than all cancers combined by 2050.1 This subsequent global antibiotic disaster has led to a decline in the number of new AM agents and marketed products to kill bacteria further resulting in serious implications.1 There are two strategies for a potential solution: 1) development of new antibiotics that are costly, time consuming, and may never receive regulatory approval, or 2) repurpose existing effective antibiotics (or new nanomedicines without drugs) into new formulations that are more effective. Of course, the latter (if done appropriately) can accelerate translation to the clinic economically with a faster life-saving capability.1
A new field termed “pharmaceutical nanotechnology” repurposes existing antibiotics to attract researchers working with approved AM agents. Several efforts have been implemented for numerous drugs using novel therapeutic strategies. Broadly speaking, nanomedicines are composed of either polymers or lipids as the basic component of the nanocarrier systems. A major concern in this area is the need for new and efficient approaches to enhance drug therapy using existing AM agents or a potent new compound as a substitute for existing drugs. In general, low and high molecular weight AM agents face common physiological barriers inside the body when administered using these delivery systems. Characteristic features of these nanocarrier delivery systems make for optimal carriers for the delivery of AM agents. For example, nanostructures (nanostructured lipid carriers [NLCs]) can be created to have water loving surface properties which are advantageous for carrying drug molecules to the target site by the reticuloendothelial system in the tissues and organs leading to prolonged mean resident times for maximizing AM activity. Conversely, nanostructures can also be developed to have hydrophobic properties which would be useful for loading hydrophobic antibiotics, prolonging their release and creating an anti-adhesive-type antimicrobial coating (AMC) in the healthcare setting. Furthermore, the modification of surface chemistry of these nanocarriers can enable conjugation of molecules as targeting signals, taking advantage of their high surface-area-to-volume ratios.2,3 Finally, nanocarriers can enter biofilms and subsequently bacteria (or even clog bacterial membrane nanospores) more easily than micron particles for maximum microorganism killing. Nanotechnology, thus, has been proven beneficial in infection diagnosis and control due to site-specific delivery and modified drug performance (via conjugation, ligand binding, and carrier-dependent cell internalization).
With all of the promises mentioned above, it is obvious that the application of nanomaterials for infection control has been investigated for improved cellular uptake using nanoparticles (NPs). These nanomaterials can lead to higher drug accumulation in microbial cells to toxic levels and subsequently produce tandem effects.4 Solid lipid nanoparticles (SLNs), NLCs, liposomes, nanoemulsions, self-emulsifying drug delivery systems (SEDDS), and polymeric NPs have all been established to enhance AM efficacy.5 This review emphasizes the current advances and challenges of such nanostructures, understanding the design principles for the development of novel nanocarriers, the cellular interplay between the nanocarriers and cells, prevention of drug degradation, stability enhancement of the integrated drug molecules, and improvement in PK profiles and challenges of nanomaterials used in the healthcare sector, involving ecosystems.
Despite the profound application of nanomedicine as drug delivery carriers to improve PK and pharmacodynamic profiles, safety aspects are of prime concerns for humans and other living systems in diverse ecosystems. The present review also highlights the challenges (surface coating) arising from employing nanomaterials in healthcare units and their current design criteria (ie, “safe by design”).
Nanomedicines as effective AM drug delivery systems
Approximately 33.3% of microbial infections are non-responsive to existing antibiotics generally prescribed to eradicate infections.6 Common challenges for formulation scientists is the frequent development of drug resistance against well-established antibiotics. Thus, the demand for newly developed antibiotics is increasing enormously. Presently, there is a serious demand to employ advanced approaches to improve their therapeutic effectiveness. Figure 1 illustrates this developmental progress involving several in vitro and in vivo assessments. Table 1 lists several nanocarrier formulations with basic components and their major advantages in medicine.
Possible mechanisms of nanocarriers (or nanomedicine) as AM systems
Infectious diseases are a result of microbial infections. Understanding the chemical composition of the microbial cell wall, cellular physiology, and behavior is a prerequisite for developing an effective therapy using nanomedicine to identify a specific surfactant, lipid, drug, and/or polymer. NPs encapsulating noble metals have been recognized to exhibit potential AM activity.7 The materials employed for fabricating and designing NPs have several important parameters that affect AM efficacy, such as surface-to-volume ratios of the NPs wherein smaller ones have been reported to have the strongest bactericidal effect.8 Moreover, the mechanism of bactericidal activity is due to the association of a positive charge of the metal encapsulated in the NPs with the negatively charged surface of the microbial cell by virtue of the electrostatic attraction. Over the last two decades, many researchers have addressed the application of the excipients possessing innate AM activity acting synergistically with the drug for the treatment of bacterial, fungal, viral, and parasitic infections. Recently, the concept of synergistic activity of excipients with drugs has been implemented to formulate nanomedicine with innate AM action.4,9 A schematic representation of the mechanism of AM action of nanomedicine is illustrated in Figure 2 wherein possible mechanisms have been portrayed. These mechanisms are: (A) adherence of nanocarriers around the microbial cell owing to increased surface area and smaller size which thereby causes a maximum detrimental effect, (B) electrostatic attraction of cationic charges by the negatively charged bacterial surface or lipid-lipid internalization of nanoglobules within the bacterial membrane, (C) detergent-like action of lipids and surfactants to extract lipids of cytoplasmic membranes as reported, (D and E) creation of tiny pores in the membrane which then causes a loss of cytoplasmic content outside of the cell, (F) accumulation of lipids and surfactants to a toxic level for an enhanced detrimental effect, and (G) inhibition of P-gp efflux to produce increased residence of the absorbed drug or such excipients to produce a prolonged AM effect.10
  | Figure 2 Schematic illustration of the possible mechanisms involved in microbial killing using nanomedicines (nanocarriers) composed of lipids and surfactants. Possible mechanisms are: (A) lipid–lipid interactions, (B) cationic nanomedicine interactions with negatively charged bacterial surfaces, (C) detergency-like action, (D) cytoplasmic content oozing out through developed tiny pores, (E) micelles loaded with extracted cellular lipids, (F) accumulated excipients to toxic levels to produce detrimental effects, and (G) P-gp efflux pump inhibition by specific excipients (reported labrasol, tween 80) and drugs (ketoconazole).4,19,31,67 |
Metal and metal oxide NPs
Silver NPs (Ag NPs) and Gold NPs (Au NPs)
Metal NPs have been investigated extensively due their elite catalytic, magnetic, and AM properties for effective delivery to bacterial and fungal strains.11 Ag possesses action against microbes and their spores, such as those in the size range of 20–40 nm which could possess better entry and residence in bacteria and fungi. They inhibit fungal proliferation from spores without causing toxicity as compared to other artificial fungicides.3 Au NPs are relatively nontoxic and are considered effective against bacteria due to highly optimized properties (such as an inability to generate reactive oxygen species [ROS] that can kill bacteria as well as ease functionalization for targeting and polyvalency). The AM activity of Au was reported due to: 1) adherence to the bacterial cell membrane, 2) modification in membrane potential and thereby resulting in ATP reduction, and 3) interruption between tRNA and ribosome assembly.12 The role of Au NPs in killing microorganisms have also been explored due to its nanosize. Au NPs of sizes <5 nm were effective against Escherichia coli and Salmonella typhi by reducing the bacterial load (90%–95%). The bactericidal effect of Au-mediated lethality was rationalized due to the roughness and dispersion of Au NPs.13
Metal oxide NPs
Leung et al14 determined that magnesium oxide (MgO) NPs damaged microbial cell membranes and caused leakage of intracellular content leading to bacterial killing. In addition, MgO NPs exhibited strong antibacterial activity which might be due to their role in mediating ROS production.14 The AM activity of calcium oxide released from NPs was investigated against Escherichia spp. and Salmonella spp.15 A CaCO3 (calcium carbonate) and MgO combination-based NP reported activity against Escherichia spp. and Salmonella spp. Vidic et al confirmed the microbicidal activity of mixed nanostructures of zinc oxide (ZnO) and MgO.16 The study evaluated the activity of the combined oxides (MgO and ZnO) encapsulated in NPs against microbes as compared to individual ZnO and MgO NPs. ZnO nanocrystals showed greater AM activity against both Escherichia spp. and Salmonella spp. ZnO has gained the highest attention in the pharmaceutical industry among all metal oxides due to its excellent antibacterial activities against a broad spectrum of bacteria with low toxicity and normal clearance. The stereochemical properties of novel green ZnO–aloe vera NPs were optimized with significant activity against several pathogenic bacteria (such as Escherichia spp. and Staphylococcus spp.). From the results, it was concluded that the ZnO–aloe vera NPs possessed increased surface energy, crystallinity, and AM activities against infectious pathogens.17 Thus, metal oxide NPs with minimal toxicity may be used to eradicate various infectious diseases. It is believed that simple and low-cost inorganic AM agents (such as metal and metal oxide NPs) serve as another nanomedicine substitute of conventional antibiotics, promising for the future of medicine.
Nanoemulsions as nanostructures
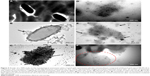
Nanoemulsions are thermodynamically stable nanocarrier systems widely used as a delivery system for drugs. In our research laboratory, we have fabricated nanoemulsions loaded with ethambutol and exposed them to Mycobacterium smegmatis to evaluate in vitro anti-Mycobacterium activities. Scanning electron microscopy revealed a detrimental effect of nanoemulsion-loaded ethambutol which could be due to the facilitated accumulation of nanoglobules loaded with drugs inside the membranes of bacilli.4 The control culture possessed a normal architecture with intact cell morphology, whereas the treated bacteria were found to be damaged, amalgamated, and fused after a loss of cellular integrity as shown in Figure 3A and B.4 Thus, bacterial membrane internalization of nanostructures has been reported by others as a major mechanism by which nanomaterials kill bacteria.4 This could be a promising therapeutic approach for efficient and effective drug delivery of antitubercular drugs.
In a report,18 an essential oil was formulated as a water-dispersible nanoemulsion by using high-energy ultrasound and its antibacterial activity was evaluated against E. coli. The mechanism to investigate antibacterial activity was completed by measuring potassium, protein, and nucleic acid leakage from cells using electron microscopy. The nanoemulsion possessed an enhanced effect against E. coli by increasing their ability to disrupt cell membrane integrity.18 Similar findings were reported recently by fabricating nanoemulsions using excipients (cationic and non-ionic nanoemulsions) with concerted antibacterial activity.19 The authors reported a detrimental effect of cationic placebo nanoemulsions comprising capmul oil against several Gram-positive and Gram-negative bacterial strains.19 They evaluated and corroborated such results based on the cytoplasmic content released after treatment followed by morphological assessment using transmission electron microscopy (TEM) and atomic force microscopy after exposure to bacteria.19
Amphotericin B (AmB)
AmB is a chemically macromolecular polyene (Figure 3C and D) which has been widely used for a long time in the treatment of systemic and local fungal infections. The chemical structure and three-dimensional structure of AmB are presented in Figures 3C and D, respectively. AmB remains a first-line drug for the treatment of severe visceral fungal infections and leishmaniasis despite the availability of several drugs without resistance.20 Moreover, AmB is often related with severe dose-dependent toxicities (such as nephrotoxicity, anemia, and phlebitis) and general malaise as side effects significantly limit its applications. An antifungal activity study was carried out against Candida albicans, which revealed that AmB/PEG5kDa-cholane possessed 15% more antifungal activity than AmB in buffer solution.21
Caldeira et al22 developed nanoemulsions to encapsulate AmB for the treatment of leishmaniasis. The main objective of the investigation was to evaluate the importance of stearylamine (STE) on drug encapsulation efficiency, toxicity, and in vitro effects. In vitro studies revealed that STE was capable of increasing the efficacy of AmB present in nanoemulsions, and Aforcin® showed almost the same results as nanoemulsions without STE.22 Thus, cationic nanoglobules present in the nanoemulsion might have been found to internalize more with negatively charged fungal cells due to electrostatic attraction. Similar reports were conducted recently using cationic nanoemulsions with an increased killing effect against bacteria showing that cationic nanoglobules were densely adherent around the bacterial membrane.19
Oral SLNs
SLNs are novel nanoparticulate structures fabricated from the mixing of triglyceride-containing oils in the solid core of the particles. Despite topical applications of SLNs, these carriers can be used for oral delivery in the form of tablets, capsules, pellets, and parenteral methods. Tobramycin (TB) was orally effective for the treatment of cystic fibrosis of a gastrointestinal tract (GIT) infection caused by Pseudomonas aeruginosa.23 Thus, SLNs are concentrated inside the cells after passing through the transmembrane increasing TB payloads inside the cells.24
Table 2 provides a short summary of the nanocarriers reported for the delivery of AM agents with informative data that should be of high value for research scientists and formulation designers working in the domain of nanomedicine.25–46
Oral polymeric NPs
Polymeric NPs have several unique features for AM drug delivery such as particle size, zeta potential, modified drug release data, and targeted AM ability using lectin as a ligand which attaches to simple or complex carbohydrates present in/on bacterial cell walls. During polymer NP preparation, AM drugs are either adsorbed to the nanocapsules incorporated during the polymerization process or covalently conjugated to the surface of the NPs after formation. The lipophilic molecules are loaded easily into the oil phase of the lipid-based NPs. Similarly, water soluble drugs are generally attached through covalent conjugation. It is noteworthy that their covalent linkage leads to inactivation of AM activity that needs to be verified before application. Polymeric NPs have been explored to deliver various AM agents with improved therapeutic efficacy.
Cationic bioadhesive chitosan NPs have also been established for a broad spectrum of antibacterial, antiviral, and antifungal activity. Later, chitosan conjugation was found to have high bactericidal action based on their biocompatibility, non-toxic nature, antibacterial properties, low immunogenicity, and their ability to act as an absorption enhancer.47 Chitosan NPs have demonstrated considerable antibacterial action depending on several factors such as pH and the solvent used. Moreover, chitosan was found to reduce the activity of metallic NPs despite its unknown reason for mode of action. Recently, comprehensive studies have focused on the effect of chitosan on Burkholderia cenocepacia and corroborated many membrane-related functions such as respiration, resistance modulation, cell division, the drug efflux system, and transport.47 This might be due to interactions of lipopolysaccharides with chitosan leading to the destabilization of membrane proteins and membrane lysis for cell death.47
AIDS is a deadly CD4+ cell disorder caused by the HIV that leads to the death of millions of people across the world. Protease inhibitors (PIs) are one of the main classes of anti-HIV drugs recently approved for the treatment of AIDS. However, eight PIs are approved, and saquinavir is the most potent among them. The treatment of AIDS with conventional saquinavir is not optimal due to its poor bioavailability.48 Multi-drug resistant 1 proteins led to the efflux of the drug because of its resemblance with the substrate analog of the P-gp-mediated system. The development of nanocarriers to encapsulate antiretroviral drugs may be a solution to the above-mentioned problems. The study concerning the antiviral activity of NPs was carried out by inducing viral infection of the target cells. The drug encapsulation efficiency and cell targeting efficiency were more than 70% and 92%, respectively. The developed formulation caused better control of viral proliferation when it was applied to the viral strain (NL4-3 and Indie-C1). Moreover, the same study was carried out at two different targets (T-cells) such as Jurkat and CEM-CCR5 cells to compare with a saturated solution of a drug (control). Chitosan NPs were optimized and showed better efficiency for cell targeting. This was to efficiently control viral proliferation in target T-cells.24
The minimum inhibitory concentration (MIC90) for plain (AmB), anionic, and Polyethylene glycol-lipid nanoparticles (PEG-LNPs) was 0.125 μg/mL and was found to be fivefold higher than AmBisome®.49 In vivo antifungal tests were conducted using an immunosuppressed mouse infection model. Efficiency of the PEG-LNPs was found to be higher than AmBisome®.49 The highly cleft architecture and high density gave higher surface area to size ratios and provided immense reactivity with microorganisms in vivo.50 Polyamidoamine (PAMAM) has been quite extensively studied as a dendrimer owing to its higher density of functional groups for AM drug delivery by causing a reaction to AM conjugation.51 Dendrimers having quaternary ammonium salts which act as biocides are responsible to break down bacterial membranes and show increased AM activity against target bacteria.50 The polycationic nature of this polymeric biocide facilitates electrostatic adsorption to the negatively charged bacterial surface. This leads to increased membrane permeability and enhanced accumulation of the polymer into the bacteria which further results into an escape of potassium ions followed by complete fragmentation of the bacteria.50 PAMAM dendrimers with silver salts have been investigated for considerable AM activity against Staphylococcus spp., Pseudomonas spp., and Escherichia spp.51 Sulfamethoxazole encapsulated into the PAMAM dendrimers in the core of ethylenediamine has profoundly increased water miscibility and efficacy as an antibacterial agent, the same as several other drugs.51
Poly(D,L-lactide-co-glycolic acid) (PLGA) has also been exploited to prepare AmB-loaded PLGA NPs and nanosuspensions as an alternative to Fungizone® and AmBisome®. An in vivo study using an Aspergillus fumigatus–infected mouse model showed that PLGA NPs and nanosuspensions were more effective than Fungizone® while both were two- and fourfold more efficient as compared to AmBisome®.52 An in vivo study was carried out to determine the amoebicidal activity of the formulation against Balamuthia mandrillaris and was compared to a drug solution and AmBisome®. Conclusively, the nanosuspension showed maximum efficacy followed by the pure drug solution and AmBisome®.53
Oral micellar-based drug delivery
AmB has also been loaded into polymeric micelles composed of poly(ethylene glycol)-block-poly(ε-caprolactone-co-trimethylene carbonate) [PEG-p(CL-co-TMC)] and in vitro antifungal activity was investigated against C. albicans. Moreover, it was noted that the MIC of PEG-p(CL-co-TMC) was in the range of 0.30–1.19 μg/mL (at different concentrations) and Fungizone® showed an MIC of 0.15 μg/mL.54 Furthermore, a new micellar carrier was applied to deliver AmB using PLGA/dextran graft copolymer micelles. Particle size and drug loading efficiency of the formulation was 200 nm and 80%, respectively. The AM effect of the formulation was investigated against C. albicans, and it was found that micelles and pure AmB showed almost the same MIC value (~0.625 μg/mL). It was concluded from that study that AmB-loaded polymeric micelles are a potent AM agent carriers requiring further investigation.55
Oral cubosomal (liquid crystalline nanostructured) drug delivery
Cubosome is a cubical crystal NP composed of basic glyceryl monoolein and a surfactant stable in the gastrointestinal (GI) fluid. Yang et al used a solEmuls technique for developing AmB-loaded cubosomes to enhance its oral bioavailability with an encapsulation efficiency of 87.8%.56 In vivo studies showed zero nephrotoxicity and improved PK parameters as compared to Fungizone® in a rat model.56 Angra et al57 developed AmB-loaded microspheres using cross-linked BSA with a particle size of <5 μm and a zeta potential of ~30 mV. In vitro studies suggested that the formulation did not show any signs of toxicity and had comparable antifungal activity to that in solution.57
Rizwan et al58 investigated the effect of the hydrotrope type (ethanol, polyethylene glycol-200, and propylene glycol), concentration, and the lipid-to-stabilizer (pluronic F127) ratio on cubosome formation. Optimized formulations were studied for their capability to include and maintain ovalbumin (Ova), a model hydrophilic protein regularly utilized to develop the vaccine.58 They further reported the development and physicochemical profiles of modified cubosomes to encapsulate the agonist of the toll-like receptor, monophosphoryl lipid A, and imiquimod as a new vaccine delivery system as well as the capacity of the delivery system to encourage immune responses toward the model antigen Ova. They studied the comparison of the immune stimulating capacity of cubosomes and liposomes. The results confirmed that cubosomes could deliver an antigen to the antigen-presenting cells and prime native T-cells.58 Yang et al used the solEmuls technique for developing AmB-loaded cubosomes with glyceryl monoolein to present a more stable and efficient cubosomal formulation as compared to the marketed Fungizone®.56 Moreover, a Caco-2 cell line study confirmed the active transport of AmB cubosomes with a 5.1-fold higher transport than Fungizone®. Finally, it has been concluded that cubosomal AmB enhanced the oral delivery of drugs without causing toxicity.56 Various components used in the preparation of nanomedicines for AM applications are listed in Table 3.
Oral SEDDS
SEDDS are capable of emulsification into nanocarriers or microcarriers (several structures) in gastric fluid media under mild agitation of physiological gastric movement. Wasan et al developed AmB-loaded SEDDS (Peceol/1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]-PEG lipid) formulations to increase stability, drug solubility, and antifungal activity.59 These formulations with drug content in the range of 100–500 μg/mL significantly decreased the number of colony-forming units (CFUs) in all organs by 80%. Kidneys showed a CFU reduction by 75% and 95% at a 5 and 10 mg/kg dose, respectively, as compared to the untreated group. No change in the level of creatinine in C. albicans-infected rats was found.59 Cytotoxicity studies confirmed a negligible toxicity to macrophages, and it was concluded to be a suitable carrier for AmB.43 Several authors reported the AM activity of lipids and surfactants wherein excipients revealed innate antibacterial, antifungal, antiviral, and antiparasitic potential.10,60–62 Table 4 shows a brief summary concerning lipids reported to have inherent anti-Mycobacterium activities.63–66
  | Table 4 Reports suggesting anti-Mycobacterium activity of certain lipids or fatty acids |
Recently, rifampicin (RIF) and isoniazid (INH) were delivered by fabricating self-nanoemulsifying drug delivery systems (SNEDDS) employing excipients with innate anti-Mycobacterium activities (M. smegmatis and M. tuberculosis).67 The authors reported improved PK parameters followed by a profound detrimental effect against these strains at the explored concentrations by the optimized SNEDDS formulation.67 Figure 4A–F shows a three-dimensional response surface graph, interaction curve, and residual curves of labrasol and capmul content present in the nanoemulsion formulation, suggesting a mathematical relationship of the zone of inhibition (ZOI) against both strains (MS-942 and MS-995), respectively. There was a linear relationship of ZOI with the labrasol content against M. smegmatis (MS-942) while the ZOI was found to be quardratic with M. smegmatis (MS-995) (Figure 4A–D). Moreover, there was no interaction between the components on the response, and the model was found to be the best fit for the selection of the optimized nanoemulsion to produce a more detrimental effect with an optimized blend of labrasol and capmul MCM C8 (labrasol, campmul, and cremophor-EL were 500, 250, and 250 mg in the final optimized formulation, respectively).67 Thus, it was corroborated that the explored excipients at their concentrations tested were capable of producing an extremely lethal effect against Mycobacterium acting synergistically with INH or RIF.
This finding was further confirmed by a morphological study using TEM wherein the treated tubercular strain (M. tuberculosis H37 Rv) was observed to be fragmented, lysed, amalgamated, and profoundly damaged (Figure 5). Untreated cells were found to be normal with a smooth margin of intact bacilli without an abnormal structure as shown in Figure 5A.67 Figure 5B–D revealed the sequential damage of the bacterial cell wall, and cell membrane followed by a loss of cytoplasmic content. Figure 5E showed internalization of nano globules with bacterial membranes responsible to produce severe structural damage (black arrows). Moreover, Figure 5F presents the possible mechanistic view of action of the nanoemulsions leading to the creation of tiny pores in the cell wall and thereby removal of the cytoplasmic material from the cell (red encircled area). Such findings have been supported by several authors wherein nanoemulsions comprising labrasol and glyceryl monocaprylate showed cytoplasmic content lost after treatment to Gram-negative and Gram-positive bacterial strains.16 Collectively, it can be inferred that the explored labrasol- and capmul-based nanoemulsions address the concern of the safe delivery of anti-tubercular drugs with reduced resistance development and dose-dependent side effects with maximum efficacy per economic cost compared to conventional dosage forms.
Topical and transdermal nanomedicines
Several nanostructured carriers have been investigated for the topical and transdermal delivery of AM agents. These routes are preferred for local and systemic delivery, respectively, using various lipid- and polymer-based NPs. In this section, polymeric NPs, lipid-based vesicular systems (liposomes and elastic liposomes), SLNs, and nanoemulsions are emphasized with in vitro and in vivo findings.
SLNs
Various unique features of SLNs show a promising option for AM drug delivery in cosmetic and skin products for local action and transdermal products for systemic action. It contains occlusive components which rapidly form a thin film after application and reduces water evaporation to retain skin moisture. Thus, their occlusive nature facilitates molecule penetration into a deeper layer of the skin, such as SLNs encapsulated with retinol and retinyl palmitate which have shown enhanced drug diffusion rates from the epidermis and lesser amounts of drug expulsion than the free drug.68 SLNs can deliver azole (an extremely poorly soluble drug) antifungal drugs to cutaneous fungal infections such as tioconazole, miconazole, econazole, oxiconazole, and clotrimazole.69 These drugs are complicated to apply and deliver to infected cells. Therefore, they can be loaded in an efficient manner into SLNs which causes an occlusive effect, and the nanosized particles increase drug residence time into the upper layer of the skin through enhanced penetration across the skin.
Lv et al70 prepared penciclovir-loaded NPs for the topical delivery of drugs for the treatment of hepatitis virus, herpes simplex virus type-1 (HSV-1) and HSV-2, varicella-zoster virus (VZV), Epstein-barr virus (EBV), and cytomegalo virus. Penciclovir is a potent synthetic nucleoside analog (prodrug) extensively used for the treatment of HSV with very low oral bioavailability (6%–10%). However, its clinical use is significantly limited. Results showed that the percent of drug penetrating through excised rat skin from the developed NPs was more than twice as that compared to the marketed cream after 12 hours of administration.70
Corware et al71 used soluble AmB with polymethacrylic acid for the treatment of cutaneous leishmaniasis in non-healing BALB/c mice. In vitro studies revealed less toxicity and high effectiveness against leishmaniasis-infected macrophages as compared to deoxycholate AmB. From PK studies in BALB/c mice, it was found that a dose amount of 18 mg/kg body weight showed effective healing of lesions. It was concluded that AmB polymethacrylic acid can be used pharmacologically for lesion healing.71 Chen et al developed SLN as a podophyllotoxin (POD)-loaded topical nanocarrier for targeting the superficial layer of skin for the treatment of the human papilloma virus (HPV).72 The growth of the upper layer of skin (epithelial cells) infected with HPV can be prevented by POD in the epidermis. Hence, this drug has been used as the first-line drug in genital warts. This formulation showed a fivefold more effectiveness with minimum side effects.72
Liposomes
Vesicular lipid bilayer carriers such as liposomes have been explicitly employed to deliver numerous drug candidates by researchers in various domains of drug delivery. They have been investigated for higher transdermal permeation and prolonged stability with positively charged liposome formulations.73 Devi et al formulated different vesicular systems to deliver AmB to treat topical fungal infections and the prepared formulations were characterized for loading efficiency, particle shape, size, stability, in vitro release profile, and anti-fungal activity.74 Moreover, transfersome dispersion showed the highest transdermal flux (1.66 μg/cm2/h) and drug retention into the skin (77 μg) among them, suggesting a threefold higher flux value as compared to ethosome dispersions and relatively higher than liposomal dispersions (0.51 μg/cm2/h). In vitro anti-fungal studies revealed that transfersome dispersions maximized ZOI as compared to other formulations. In vivo skin irritation assessment was performed using albino rats that confirmed insignificant irritation scores as compared to conventional AmB therapy.74
Dithranol is a first-line drug for the treatment of topical psoriasis and has been delivered using liposomes. The use of conventional dithranol in disease conditions leads to a necrotizing effect, irritation, burning sensation, and discoloration effects on healthy and diseased skin. The entrapped drug in vesicles was visualized for the localized delivery and improved availability at the site. This could reduce the dose and dose-dependent side effects, such as irritation and staining.75
Polymyxin B-loaded liposomes have been used as an alternative successful delivery vehicle. This molecule has been well established to treat P. aeruginosa infections, such as lung disease and cystic fibrosis in chronic bronchopneumonia.76 However, in vivo clinical use has been restricted owing to some kind of toxic effects on vital organs (such as kidney, neuromuscular, etc).77 Interestingly, an investigation has been completed concerning the encapsulation of the drug molecule in the liposomes which significantly diminished severe side effects and increased efficacy against microbes, such as strains of P. aeruginosa.77 The mechanistic view of membrane fusion has been reported for the development of polymyxin B encapsulated in liposomes which was used against P. aeruginosa. Studies using TEM, flow cytometry, and fluorescent resonance energy transfer confirmed that there were reorganizations in the lipid structure when it was incubated with P. aeruginosa membranes.76 Membrane fusion of drug-loaded liposomes with bacterial strains is a quite swift and spontaneous process driven by non-covalent forces (Van der Waals forces and hydrophobic interactions) that minimize the free energy of the system. A more commonly known factor for antibiotic resistance is the P-gp efflux pump and has been extensively reported by several researchers as a strong mechanism for microbial drug resistance. The pump is made of proteins and acts as a transporter embedded and assembled in bacterial membranes leading to the efflux of AM drugs outside the cells even after absorption.78 After fusion with the bacterial membrane of liposomes, an increased amount of drug was instantly transported to the cytoplasmic portion through a membrane, which can profoundly suppress drug resistance by inhibiting the efflux pumps system and eventually improves AM activity of a drug.78
Several liposome-based novel formulations for AM drugs have been fabricated for many therapeutic applications such as ampicillin and benzyl penicillin for higher AM efficacy as compared to the drug solution.79,80 Kadimi et al81 developed AmB-loaded liposomal formulations using the fluid state of a carbon dioxide (SFC-CO2) process and a probe sonication method. Liposomes were characterized by phase contrast microscopy. Particle sizes of the formulations prepared by the SFC-CO2 method were 0.15–3 μm and when formed by a sonication method were from 0.15 to 6 μm. In vitro antifungal activity revealed that both liposomes and standard AmB solutions had the same ZOI.81
Elastic liposomes
Zidovudine (AZT) has been loaded into elastic liposomes for the management of HIV and AIDS. However, limitations with its use have been due to dose-dependent hematological toxicity, low plasma half-life, extensive hepatic first-pass metabolism, and low bioavailability. The targeted and sustained drug delivery of AZT using transfersomal formulations has been accomplished. Furthermore, researchers have developed elastic liposomes using PEG AZT formulations and studied their transdermal flux on rat skin which confirmed results of biodistribution, indicating a 28-fold higher accumulation of AZT in lymphoid tissues when it was applied as compared to the free drug.82 Furthermore, these authors prepared elastic liposomal formulations of acyclovir sodium for transdermal delivery in the treatment of various viral diseases and reported enhanced transdermal flux on rat skin compared to conventional liposomes bearing drug and plain drug solutions. Hussain et al reviewed several elastic liposomes with their chemical compositions, advantages, and limitations.83
Ethosome
Topical delivery is patient friendly and non-invasive as compared to parenteral routes of administration. Kaur et al reported the enhanced topical delivery of AmB in nanoethosomal formulations with vesicle sizes in the range of 186.0±2.0 to 298.0±4.0 nm in the treatment of cutaneous fungal infections.84 The prepared nanocarriers were included into the gel base to obtain a nanogel which was elastic in nature and restraint to deformation at 37°C. The value of yield stress of the nanoethogel was calculated (109.05±2.4 Pas) and compared against marketed formulations (52.15±0.9 Pas). Enhanced drug–skin permeation and deposition studies were also carried out followed by drug penetration using confocal laser scanning microscopy (CLSM) and an in vivo Draize test study confirmed zero skin irritation.84 Jain et al developed transdermal ethosomal formulations of lamivudine (an anti-HIV drug) for the management of AIDS and hepatitis. Lamivudine has a very small biological half-life (4–6 h), and there is a need for frequent administration for over a prolonged period of time to achieve a desired antiviral effect.85 Moreover, they studied ethosomes loaded with hepatitis B surface antigens for in vitro qualitative and quantitative uptake by human dendritic cells and its ability to stimulate T lymphocytes for reducing the viral load.85
Nanoemulsions as AM agents
Drugs used in the management of microbial diseases of the skin belong to antibiotics which hinder important microbial biochemical activities. Indiscriminate utility of these drugs precipitates consequences of multiple drug resistance against pathogens and, thus, leads to the need of new AM development. The non-toxic agents are of great importance to kill a variety of microbial organisms. The toxic nature of certain drugs has limited their topical application. Quaternary ammonium compounds have low potential efficacy against various pathogens (fungi and bacteria), whereas chlorhexidine is a broad-spectrum antibiotic agent due to its potential activity and low toxic effect on the skin.86 The activity was pH dependent and found to be reduced significantly in the presence of organic matter.86 Thus, there is a constant need for the development of safe AM formulations with a broad range of rapid biocidal activities. For this reason, non-ionic surfactant-based nanoemulsions are constantly being prepared and tend to be stable with the addition of a minimum content of surfactants. Nanoemulsions are the first choice with antimicrobicidal activity against various infection-causing pathogens in the skin.87 Hamouda et al investigated nanoemulsions possessing a strong efficiency to kill spores of Bacillus spp.87 In some studies, 1% emulsions were found to be effective against influenza virus, Neisseria spp., Streptococcus spp., Bacillus spp., and Cholerae spp.87 They further evaluated activities against HSV-1, influenza, and other viruses and fungistatic activity against C. albicans.87
In our preceding publications, a nanoemulsion was developed as a nanocarrier for the enhanced topical delivery of AmB.88 An oil-in-water nanoemulsion was prepared using different ratios of surfactants and co-surfactants following a spontaneous titration method as shown in Figure 3E and F. Of the different formulations, F1 was considered optimal with a 96.9%±1.0% transmittance value. The formulation was characterized for particle size (67.37±0.8 nm) and zeta potential (−3.7±1.2 mV). An optimized formulation showed a higher drug release rate as compared to commercial Fungisome®. In vivo skin deposition studies reported a 2.11-fold higher drug deposition from formulations as compared to Fungisome®.88 Furthermore, the same nanocarrier was exploited employing a sefsol-218 lipid for enhanced stability and permeation potential for topical delivery.9 Different formulations were developed at varying pH and temperatures of a continuous phase. Formulations showed the highest flux rate (17.85±0.5 μg/cm2/h) as compared to Fungisome® (7.97±0.01 μg/cm2/h) and drug solution (5.37±0.01 μg/cm2/h). The CLSM results demonstrated enhanced skin penetration of the drug at pH 6.8 and 7.4. Conclusively, the formulation was stable at pH 7.4 with a higher shelf life (12 months) at refrigerated conditions. The prepared nanoemulsion gel (0.1% w/w AmB in carbopol base) showed the highest flux rate (18.08±0.6 μg/cm2/h) as compared to the nanoemulsion (4.59±0.01 μg/cm2/h) without skin irritation (as conformed in a Draize test). Finally, the nanoemulsion gel served as an economic and effective topical delivery system of AmB.9 A breakthrough of recent developments for the topical delivery of AmB was reported employing the synergistic activity of excipients (lipid and surfactant) in nanoemulsion form using peceol and labrasol which revealed potential in vitro antifungal activities.89
Ophthalmic drug delivery
Ocular drug delivery is well established by several researchers and has been employed for various drug molecules available commercially. The potential issue associated with ophthalmic formulations is its residence time after instillation and frequent washing by the eyelids while blinking. However, bioadhesive biocompatible polymers (chitosan and carbopol) have been explored to enhance drug residence time followed by release improvement by various researchers for the treatment of eye infections. Das and Suresh reported an improved delivery of AmB to the ocular surface employing nanosuspensions prepared by a solvent displacement technique with a mean size range and zeta potential of 150–290 nm and +19–28 mV, respectively.90 From in vitro studies, it was found that there was 60% of drug release in 24 h with the same efficacy as that of the free AmB solution.90 Several authors have reported to deliver AmB using other nanostructures such as NPs, NLCs, SLNs, liposomes, elastic liposomes, ethosomes, transfersomes, and gel formulations.4,88 The basic fundamental purpose of these carriers is to deliver drugs with enhanced ocular permeation across the corneum layer of the eye for an extended time period. All nanostructures have been fabricated using several lipids, surfactants, co-surfactants, stabilizers, and other particular components. Aminoglycosides were loaded in liposomes and served as a reservoir to prolong drug release directly to the site of action after topical application. This study witnessed that the therapeutic efficacy would be improved in eye infections of AIDS patients.91 These excipients have been tabulated in Table 3.
Inhalation nanostructures
Shah and Misra developed AmB-loaded liposomal dry powder inhalation formulations.92 The formulations were prepared using hydrogenated soya phosphatidylcholine (PC), cholesterol, saturated soya phosphatidylglycerol (7:3:0.5) with ethyl acetate and ethanol (1:1) as an organic solvent by reverse phase evaporation methods and were characterized for mean particle size as 1.8±0.2 and 2.0±0.3 μm, respectively.92 In one report, the effect of temperature (at 35°C and 4°C) on the transfer of drugs from AmBisome® to fungal cell membranes was investigated followed by antifungal activity of AmBisome® against S. cerevisiae. At 35°C, the formulation showed effective antifungal activity as compared to 4°C. The binding ability of AmBisome® to the yeast cells was further studied and it was observed that the formulation binding was achieved after 3 h of exposure at 35°C.93 Imam et al prepared and characterized distigmasteryl hemisuccinoyl-glycero-phosphatidylcholine liposomes.94 The liposome particle size was found to be ~100 nm with a negative zeta potential (~0.2 mV). The formulation was found to be less toxic than Fungizone®. In vitro antifungal studies revealed lower values of MIC against Candida as compared to AmBisome®. Moreover, the anti-leishmanial potential of the formulation was found to be similar to AmBisome® and Fungizone®. The liposome maximum tolerated dose was estimated to be 60 mg/kg using BALB/c mice.94
Optical properties of metal NPs
Noble metals have been used for years for radiation therapy of cancer, diagnosis of diseases (X-ray), drug delivery, and for sensors and detectors. Moreover, these have been explored for fine tuning their surface plasmon absorption band by changing particle size into the nanometer regime which further results into increased interactions between the electric field of light and oscillation of electrons.95 It was observed that ultraviolet emission intensity is enhanced (60-fold) when the core of the Ag NP was coated with ZnO as compared to Au (17-fold).95 The investigators suggested that metal NPs are suitable tools for the enhanced detection of ionizing radiation.95
Current challenges of nanomedicines/nanomaterials
Recently published reviews have summarized and compiled informative data from the literature concerning the safety profile of nanomaterials followed by addressing challenges for the delivery of AM agents.96 Authors have comprehensively viewed various aspects of (eco)toxicological risks and a potency to induce antimicrobial resistance (AMR) owing to AMCs when applied to healthcare sectors. They have discussed issues in light of recently published scientific reports and expert opinions (eg, members of the Cooperation in Science and Technology Programme, COST and Action AMiCI, AntiMicrobial Coating Innovations).96 It is noteworthy that weighing the risk and benefits of AMCs in healthcare settings requires a thorough assessment of 1) ecotoxicological hazards in healthcare settings, 2) experiences obtained from AMR evaluations, 3) addressing the quality, efficacy, and safety evaluation of AMCs at the level of a “Safety by Design Approach,” and 4) involving stakeholders for the risk–benefit analysis of AMCs created AMR.96 A potentially promising tool against microbial growth is AMCs employed in healthcare sectors which are based on chemical strategies and technologies such as 1) AMCs containing active eluting agents (ions, NPs of metals, antibiotics, chloride, iodine), 2) immobilized molecules on contact surfaces, and 3) light-activated molecules (such as TiO2 or photosensitizers).97–99 These authors have compiled a comprehensive review on the risk of AMR to biocides used as AMCs wherein several issues need to be resolved through regulatory bodies, such as the European Commission.95 It is noteworthy that NPs of metals (silver, copper, zinc, etc) are extensively used in AMCs due to innate superior biocidal efficacy introduced as additives in hospital fabric bandages, wound dressings, and intravenous catheters.100–102 A comprehensive approach for the risk–benefit analysis in the healthcare sector is still required and limited data have been reported on high-quality studies for these concerned issues.
Despite several benefits of AMCs, these may cause serious adverse effects on human, livestock, and microbiota after reaching the ecosystem. Particularly, a slow and steady diffusion of nanomaterials leached from AMCs may induce different types of AMR as compared to current antibiotics.103 Thus, scientific committees recommend the limited use of AM in a balanced way in all areas and highlight the urgent need for assessing major AMR contributors.104 The issues that need to be resolved are 1) information on currently used biocides in terms of volumes and production sources, 2) epidemiological data of the public health on the relevance to AMR, 3) data on environmental stability, 4) triggering factors for the emergence of AMR, and 5) generation of international standards for testing and surveillance on AMR.
In brief, major current issues for nanomedicines/nanomaterials include:
- Major challenges of AMC applications in healthcare settings (solid–air interface and solid–liquid interface), particularly for nanomaterials.
- Ecotoxicological hazards of nanomaterials for mammalian cells at environmental levels.
- Risk–benefit weighing of AMCs before development and application.
- Challenges in the drug delivery of nanomedicines.
- Importance of “safe by design” strategies.
Some of these issues are described shortly in the present review.
Possible (eco)toxicological risks related to AM agents used
Broadly, AMCs can be grouped into three categories such as 1) antibacterial agent release-based coatings, 2) antibacterial agent contact killing-based surfaces, and 3) anti-adhesion surfaces (eg, topographically modified surface). A majority of AMCs are based on category (1) wherein biocides (silver ions) are released either from Ag NP or polymeric NPs, such as nanosilver-based coatings. A slow release of silver results into AMR against bacteria and therefore a long shelf life is desired to tackle the AMR.105 In contact killing, there is an interaction between the bacterial cell surface and subsequent inhibition by virtue of surface topography (a smooth surface with more bacterial as compared to a rough surface), releasing agents in the vicinity, and bacterial repulsion (hydrophilic/hydrophobic interactions).106,107
AMs incorporated in AMC are inherently toxic to proliferating pathogens. However, these intrinsically show harmful effects on animals, humans, and microorganisms such as algae. Most commonly noted notorious metal NPs are those containing silver, copper, and zinc. These metal ions are water soluble salts released into the aquatic ecosystem that then produce toxic effects on aquatic bioorganisms/animals.108,109 Moreover, the authors expanded on the use of AgNPs with sizes ranging from 10 to 40 nm for widespread use in AMCs.95 AgNPs could be a promising approach for AM action due to the release of Ag ions and the subsequent release of ROS for producing detrimental effects to bacteria.95 However, there are now new concerns over bacterial resistance to Ag as well as its cytotoxicity to mammalian cells; such studies highlight the complex biosafety issues by using Ag (and all) NPs for antibacterial applications which require more investigation especially at the in vivo level. A dossier of the project “NanoTrust” addressed the adverse effect of AgNP on human and aquatic animals due to the 1) development of silver-resistant bacteria owing to released subtoxic levels of Ag ions, 2) compromised human skin microflora to regularly exposed cosmetic containing silver nanomaterials, and 3) accumulation of silver at elevated concentrations into aquatic animals/microflora.110 Recently, nanosilver nanomedicines have been reported to have toxic effects on the same ecosystems and human beings.59 Moreover, meta-analysis of toxicity due to NPs of ZnO, CuO, and ZnO used in AMCs suggested remarkable lethality on ecotoxicological test organisms (aquatic crustaceans and phytoplanktons) as compared to bacteria which could be due to waste released to the aquatic environment.111
Possible risks of AMCs related to the potential development of AMR
It has been pointed out that there is horizontal gene transfer of AMR to pathogens or environmental bacteria. The effect of concentration, exposure time, cleaning procedure and hospital wastewater management are aspects to consider as prime precautions to avoid AMR and gene transfer. Thus, AMR is a global problem arising from potential AMC inducers which contribute to a developed resistance.112
Current challenges in drug delivery of nanomedicines
The use of nanomaterials is gaining interest with the greatest attention in diverse fields such as theranostics, personalized medicine, tissue engineering, highly sensitive diagnostics (biosensors), and nanostructured lab-on-a-chip systems interacting with a biological interface.113,114 Despite the tremendous benefits of nanomedicine in patients and society, nanomedicine is moving in new directions possessing certain challenges to the field at a fundamental level in future decades.115 This might be due to a limited understanding of the complex interaction among developed artificial nanomaterials, biological surfaces (at the protein, cellular, and whole organism level), and immediate environment (or ecosystem). Furthermore, there is a need to improve our understanding of surface chemistry, morphology (nanoscale to macroscale), material softness, and biosafety aspects greatly affecting biological behavior.113–115 We would like to emphasize the intravenous administration of nanomaterials that has certain challenges, but researchers have moved very quickly to establish technologies (and companies). Today, these assumptions still remain, such as a highly warranted investigation of the so-called enhanced permeation and retention effect in humans instead of xenograft models (where usually 4%–8% of the injected doses reach the solid tumor).113,116 Another challenge is a very poor understanding of the barrier constituted by the extracellular matrix, in a drug delivery context and a lack of data from larger animals and humans with spontaneous tumors. In addition, a lack of understanding of targeting ligands for overexpressed receptors on the tumor cells conjugated with NPs, the interplay between the target and nanomaterials, quantitative assessment of in vivo cellular uptake, and unavailable techniques for the quantitative assessment of drug targeting are also continuous problems that need to be solved before the field can grow.
The principle of effective doxorubicin delivery to the active/target site of the current commercial product DOXIL is based on using physico-chemical characteristics for successful delivery which is only applicable for a small dose range and unsuitable for large dose biologicals. Recent advanced approaches in drug release have been explored, such as the application of heat, light, ultrasound-sensitive systems, and pH- and enzyme-based strategies (such as the lower pH of tumor cells and endosomes inducing drug release).113 Employing pH/enzyme-based targeted delivery could be an interesting approach wherein drug binding is not based on tumor identification; however, transferring in vitro findings to in vivo performance has been a challenging issue. Investigations using drug delivery systems are not available to evaluate the pharmacokinetics of drugs from nanocarriers directly inside the cancer tissues. Nanomedicines as drug delivery systems are still facing challenges due to a lack of a thorough understanding of the interplay between protein adsorption on nanomaterials resulting in a significant variation in their biological/therapeutic behavior.117
Despite programmable nanomaterials offering great promises in the healthcare system, the dichotomy of the nanotechnology and nanotoxicology paradigms must be of the utmost consideration. Therefore, before nanomaterials can be routinely integrated into mainstream therapeutics, several key gaps must be considered. The US Food and Drug Administration and the Alliance for NanoHealth in 2008 organized a joint workshop in this area where six areas were identified as critical priorities that remain as priorities to date.118 These include 1) the development of imaging technologies, 2) determination of the distribution of nano-vehicles in the body upon their systemic administration, 3) the biological affinity of nanodrugs, the pathways by which they are internalized, retention time, and their ability to translocate across barriers, 4) the development of new computational models for predicting the human health risks of nanomaterial exposures, 5) the establishment of consensus toxicity testing protocols, and 6) understanding the unexpected secondary effects of NPs.119
Exposure of nanomaterials (at specific concentrations) leads to undesirable outcomes. Thus, there is need to understand the mechanistic view of nanomaterial uptake, interaction with the physiological molecules, environment, and toxicity to inform a “safe by design” leading to safer nanomaterial applications. Several nanomaterials may be a significant challenge with a “systems biology” approach and can be feasible to build efficient molecular and computational screening tools to overcome those challenges.118
Recently, Jennifer et al reported a critical review for challenges in the delivery of nanomedicines for cancer treatment.120 However, queries still need to be answered such as: 1) a complete understanding of the tumor heterogeneity and the biological factors responsible for the behavior of nanomedicines in cancers, 2) translation from formulation-driven research to disease-driven development, 3) to implement the most relevant animal model and diagnostic protocol, and 4) the prior selection of the patients most likely to respond to specific nanomedicine therapies.120 Conclusively, the present review also highlighted the major concerns about the deficiency of studies for a clinical and mechanistic understanding of nanomaterials at the molecular level to produce sufficient evidence to exploit nanomaterials safely for human applications and keeping the ecosystem healthy.
Carbon nanotube (CNT) and graphene oxide (GO) in AMCs: a safe alternative
Recently, some authors have focused on the solid–air interface (as a main concern considering tables, door handles, computer keyboards, textiles, etc) as well as the solid–liquid interface (taps, shower, and drains wherein biofilms appear frequently) in healthcare units.121 Thus, new alternative methods (state-of-the-art topographical modification, chemical modifications using polyethylene glycol or hydrogel) are needed to reduce microbial activity and infection and develop antibiotic resistance. Biocide release based strategies are “toxic by design” approaches where the slow release of biocides results into the development of antibiotic resistance which becomes toxic to humans as well as other ecosystems.96 However, CNTs, graphene (diamond like-carbons), and GO have promised prudent AM activity with relatively low cytotoxicity to human tissues and other ecological biosystems.121 It is noteworthy that whether these novel systems achieve AM activity on the surface or whether it occurs when released in an aqueous solution is still unknown. A synergistic effect has been reported when CNTs were combined with chitosan in hydrogen, dendrimers, and Ag2S quantum dots.122–124 Shorter CNTs embedded in a poly(lactic-co-glycolic acid) polymeric matrix exhibit greater AM activity as compared to longer CNTs.124 Several investigations have been published on plant extracts revealing significant AM action to protect food. However, such research is still limited for AM action on the surfaces in healthcare units, on medical devices, and diagnostic centers. Tea tree oil coatings have been investigated to possess AM action against methicillin-resistant Staphylococcus aureus when incubated for 2 days.125
Importance of “safe by design” in AMCs
Safe by design is a well-accepted approach for the timely assessment of risks associated with industrial innovation processes and the value chain of nanomaterials including nanoproducts. This was designed to ensure three inter-related communities such as workplace, consumers, and environment.96 Strategies based on the functional principle have been described in the previous section which may further be considered under safe by design such as 1) contact killing and 2) anti-adhesive, whereas biocide release can therefore be considered as toxic by design. Sometimes two functional strategies are combined to achieve a synergistic effect. Moreover, state-of-the-art is a new strategy identified at AMiCI meetings.121–124 Recently, upcoming “anti-adhesive” strategy approaches focus on 1) zwitterions, 2) morphology, and 3) hydrophobic materials. Similarly, polymeric brushes, bacteriophages, and enzymes are becoming strategies of “contact active.” Triggered release, quorum sensing, photo oxidation, plant extract, CNTs, and GOs are recently updated strategies used in AMCs.121 There are no standard test protocols, sufficient toxicity data, and fate of nanomaterials at the environmental and mammalian cell level. Therefore, EU programs (NanoFase, SafeNano, ProSafe, NaNoReg, and Euro-Nano-Tax) developed a procedure to establish and implement standardized toxicological measurements for the early assessment and fate of nanomedicine. They reported that four challenges in the safe design of AMCs are 1) the toxicity of the material, 2) potential impact of the material on the emergence of resistance, 3) the duration of the antimicrobial activity in the long term, and 4) the lack of a standardized method for the assessment of AM activity under representative environmental conditions.121 The rationale of adding an AM agent to a particular surface should be balanced between the potential impact of the AM agent on the development of antibiotic resistance and the impact of controlling the spread of the pathogenic strain within the healthcare environment.121
Summary and conclusion
The present review has compiled data from recently published research that addresses the challenges for the delivery of AM agents. Major findings concerning preformulation studies, drug degradation, cytotoxicity to healthy cells, and oral bioavailability are presented. In view of the challenges and limitations, numerous diverse nanocarriers have been successfully discovered for diverse routes of administration to improve local and systemic therapeutic efficacy for killing bacteria. The potential interplay of NPs with microbial cell membranes demonstrate facilitated AM delivery to the site of action working in tandem due to nanoscale carriers. Thus, with ongoing efforts in this domain, nanomedicine carriers will continue to improve the treatment of life-threatening diseases such as cancer, bacterial infection, tuberculosis, and HIV. Safety concerns of nanomaterials or nanomedicines must be of prime consideration including development, waste management, and regulatory aspects. Finally, this review provided a brief description of drug development over the last decade using nanocarriers which is hopefully valuable for their continued use in infection control followed by safety concerns of nanomedicines at environmental and mammalian cell levels.
Future prospects
Despite several advantages and the constant advancement in nanomedicine for the delivery of AM agents, we call special attention to a lack of strategies for the improved delivery of these candidates. There is still a united interest among nanotechnologists and microbiologists to implement theoretical and practical concepts against infectious diseases to overcome today’s challenges. One possible strategy is to employ safe excipients with innate AM agents which may be more detrimental against the microbes and, thus, develop an approach that may reduce unnecessary introduction of excipients and drugs into the patient’s body. This may mitigate drug-associated side effects and chances of drug resistance. Acquired drug resistance is a major challenge for most of the antibiotics used today which could be resolved by the concurrent delivery of more than one drug in the carrier against the same pathogen. Combination therapy has been expected to have high potency owing to synergistic effects and different mechanistic approaches to disturb the microbial defense system. Premature drug delivery is another major challenge for nanocarrier systems particularly in systemic delivery and targeting microbes. This loss of a drug may be reduced by drug targeting to intracellular microbes (such as M. tuberculosis) and modulating their release to the microenvironment of the infection site rather than the normal tissue (namely blood). The triggered release may be manipulated by pH, ligand binding, enzyme-mediation, and other properties, such as using pyrazinamide which is more active in an acidic medium at an infection site (pus area) than the blood pH. Lastly, it could be beneficial to use nanostructured carriers functionalized with antigen-specific ligands (site-specific drug targeting like aptamers, antigens and antibodies, antibody fragments, proteins, and peptides) for the treatment of infectious, cancerous, and cardiovascular diseases.
Acknowledgments
The authors would like to thank Northeastern University for constant support in manuscript writing and scientific suggestions.
Disclosure
The authors report no conflict of interest in this work.
References
Kalhapure RS, Suleman N, Mocktar C, Seedat N, Govender T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J Pharm Sci. 2015;104:872–905. doi:10.1002/jps.24298 | ||
Fukushima K, Pratt RC, Nederberg F, et al. Organocatalytic approach to amphiphilic comb-block copolymers capable of stereocomplexation and self-assembly. Biomacromolecules. 2009;9:3051–3056. doi:10.1021/bm800526k | ||
Jo YK, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009;93:1037–1043. doi:10.1094/PDIS-93-10-1037 | ||
Hussain A, Singh SK. Evidences for anti-mycobacterium activities of lipids and surfactants. World J Microbiol Biotechnol. 2016;32:E7. doi:10.1007/s11274-015-1965-4 | ||
Gould I, Bal A. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence. 2013;4:185–191. doi:10.4161/viru.24530 | ||
Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M. Antimicrobial effects of TiO2 and Ag2O NPs against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011;6:933–940. doi:10.2217/fmb.11.78 | ||
Malarkodi C, Kumar RS, Paulkumar VM, Gnanajobitha G, Annadurai G. Biosynthesis and antimicrobial activity of semiconductor NPs against oral pathogens. Bioinorg Chem Appl. 2014. http://dx.doi.org/10.1155/2014/347167. | ||
Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:17–71. doi:10.1116/1.2815690 | ||
Hussain A, Samad A, Singh SK, Ahsan MN, Faruk A, Ahmad FJ. Enhanced stability and permeation potential of nanoemulsion containing sefsol-218 oil for topical delivery of amphotericin B. Drug Develop Ind Pharm. 2015;41:780–790. doi:10.3109/03639045.2014.902957 | ||
Kondo E, Kanai K. Further studies on the lethal effect of long chain fatty acids on Mycobacteria. Japan J Med Sci Biol. 1976;29:25–37. doi:10.7883/yoken1952.29.25 | ||
Zarei M, Jamnejad A, Khajehali E. Antibacterial effect of silver nanoparticless against four foodborne pathogens. Jundishapur J Microbiol. 2014;7:E8720. doi:10.5812/jjm.8720 | ||
Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X. The molecular mechanism of action of bactericidal gold NPs on Escherichia coli. Biomaterials. 2012;33:2327–2333. doi:10.1016/j.biomaterials.2011.11.057 | ||
Lima E, Guerra R, Lara V, Guzmán A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem Cent J. 2013;7:E11. doi:10.1186/1752-153X-7-11 | ||
Leung YH, Ng A, Xu X, et al. Mechanisms of antibacterial activity of MgO: non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small. 2014;10:1171–1183. doi:10.1002/smll.201302188 | ||
Jeong MS, Park JS, Song SH, Jang SB. Characterization of antibacterial NPs fromthe scallop, Ptinopecten yessoensis. Biosci Biotechnol Biochem. 2007;71:2242–2247. doi:10.1271/bbb.70228 | ||
Vidic J, Stankic S, Haque F, et al. Selective antibacterial effects of mixed ZnMgO nanoparticles. J Nanoparticle Res. 2013;15:1–10. doi:10.1007/s11051-013-1595-4 | ||
Qian Y, Yao J, Russel M, Chen K, Wang X. Characterization of green synthesized nano-formulation (ZnO–Avera) and their antibacterial activity against pathogens. Env Toxicol Pharmacol. 2015;39:736–746. doi:10.1016/j.etap.2015.01.015 | ||
Moghimi R, Ghaderi L, Rafati H, Aliahmadi A, McClements DJ. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016;194:410–415. doi:10.1016/j.foodchem.2015.07.139 | ||
Singh N, Verma SM, Singh SK, Verma PRP. Evidence for bactericidal activities of lipidic nanoemulsions against Pseudomonas aeroginosa. Anton Van Leeuwen. 2015;107:1555–1568. doi:10.1007/s10482-015-0449-8 | ||
Gray KC, Palacios DS, Dailey I, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A. 2012;109:2234–2239. doi:10.1073/pnas.1117280109 | ||
Luengo-Alonso C, Torrado JJ, Ballesteros MP, et al. A novel performing PEG-cholane nanoformulation for amphotericin B delivery. Int J Pharm. 2015;495:41–51. doi:10.1016/j.ijpharm.2015.08.070 | ||
Caldeira LR, Fernandes FR, Costa DF, Frezard F, Afonso CC, Ferreira LM. Nanoemulsions loaded with amphotericin B: a new approach for the treatment of leishmaniasis. Eur J Pharm Sci. 2015;70:125–131. doi:10.1016/j.ejps.2015.01.015 | ||
Gilligan PH. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. | ||
Bargoni A, Cavalli R, Zara GP, Fundaro A, Caputo O, Gasco MR. Transmucosal transport of tobramycin incorporated in solid lipid nanoparticles (SLN) after duodenal administration to rats. Part II – tissue distribution. Pharm Res. 2001;43:497–502. | ||
Mosqueira VC, Loiseau PM, Bories C, Legrand P, Devissaguet JP, Barratt G. Efficacy and pharmacokinetics of intravenous nanocapsule formulations of halofantrine in Plasmodium berghei-infected mice. Antimicrob Agents Chemother. 2004;48:1222–1228. | ||
Ahmad Z, Pandey R, Sharma S, Khuller GK. Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Ind J Chest Dis Allied Sci. 2006;48:171–176. | ||
Pandey R, Khuller GK. Oral nanoparticle-based antituberculosis drug delivery to the brain in an experimental model. J Antimicrob Chemother. 2006;57:1146–1152. doi:10.1093/jac/dkl128 | ||
Espuelas MS, Legrand P, Campanero MA, et al. Polymeric carriers for amphotericin B: in vitro activity, toxicity and therapeutic efficacy against systemic candidiasis in neutropenic mice. J Antimicrob Chemother. 2003;52:419–427. doi:10.1093/jac/dkg351 | ||
Tyagi R, Lala S, Verma AK, et al. Targeted delivery of arjunglucoside I using surface hydrophilic and hydrophobic nanocarriers to combat experimental leishmaniasis. J Drug Target. 2005;13:161–171. doi:10.1080/10611860500046732 | ||
Pandey R, Khuller GK. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis. 2005;85:227–234. doi:10.1016/j.tube.2004.11.003 | ||
Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238:241–245. | ||
Souto EB, Wissing SA, Barbosa CM, Muller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278:71–77. doi:10.1016/j.ijpharm.2004.02.032 | ||
Gupta M, Vyas SP. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipids. 2012;165:454–461. doi:10.1016/j.chemphyslip.2012.01.006 | ||
Ahmed M, Ramadan W, Rambhu D, Shakeel F. Potential of nanoemulsions for intravenous delivery of rifampicin. Pharmazie. 2008;63:806–811. | ||
Gangadharam PR, Ashtekar DA, Ghori N, Goldstein JA, Debs RJ, Duzgunes N. Chemotherapeutic potential of free and liposome encapsulated streptomycin against experimental Mycobacterium avium complex infections in beige mice. J Antimicrob Chemother. 1991;28:425–435. | ||
Fielding RM, Lewis RO, Moon-McDermott L. Altered tissue distribution and elimination of amikacin encapsulated in unilamellar, low-clearance liposomes (MiKasome). Pharm Res. 1998;15:1775–1781. | ||
Onyeji CO, Nightingale CH, Marangos MN. Enhanced killing of methicillin-resistant Staphylococcus aureus in human macrophages by liposome-entrapped vancomycin and teicoplanin. Infection. 1994;22:338–342. doi:10.1007/BF01715542 | ||
Schiffelers R, Storm G, Bakker-Woudenberg I. Liposome encapsulated aminoglycosides in pre-clinical and clinical studies. J Antimicrob Chemother. 2001;48:333–344. | ||
Omri A, Suntres ZE, Shek PN. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem Pharmacol. 2002;64:1407–1413. | ||
Takemoto K, Yamamoto Y, Ueda Y, Sumita Y, Yoshida K, Niki Y. Comparative studies on the efficacy of AmBisome and Fungizone in a mouse model of disseminated aspergillosis. J Antimicrob Chemother. 2004;53:311–317. doi:10.1093/jac/dkh055 | ||
Kim JS, Kim HK, Chung H, Sohn YT, Kwon IC, Jeong SY. Drug formulations that form a dispersed cubic phase when mixed with water. Proc Int Symp Control Rel Bioact Mater. 2000;27:1118–1119. | ||
Jain S, Yadav P, Swami R, Swarnakar NK, Kushwah V, Katiyar SS. Lyotropic liquid crystalline nanoparticles of amphoteriicn B: implication of pytantriol glyceryl monooleate on bioavailability enhancement. AAPS PharmSciTech. 2018;19(4):1699–1711. | ||
Silva M, Ricelli NL, Seoud OE, et al. Potential tuberculostatic agent: micelle-forming pyrazinamide prodrug. Arch Pharm. 2006;339:283–290. doi:10.1002/ardp.200500039 | ||
Abeylath SC, Turos E, Dickey S, Lim DV. Glyconanobiotics: novel carbohydrated nanoparticle antibiotics for MRSA and Bacillus anthracis. Bioorg Med Chem. 2008;16:2412–2418. doi:10.1016/j.bmc.2007.11.052 | ||
Cheng Y, Qu H, Ma M, et al. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: an in vitro study. Eur J Med Chem. 2007;42:1032–1038. doi:10.1016/j.ejmech.2006.12.035 | ||
Bhadra D, Bhadra S, Jain NK. Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J Pharm Pharm Sci. 2005;8:467–482. | ||
Ibrahim M, Tao Z, Hussain A, et al. Deciphering the role of Burkholderia cenocepacia membrane proteins in antimicrobial properties of chitosan. Arch Microbiol. 2014;196:9–16. doi:10.1007/s00203-013-0936-0 | ||
Li X, Chan WK. Transport, metabolism and elimination mechanism of anti-HIV agents. Adv Drug Deliv Rev. 1999;39:81–103. doi:10.1016/S0169-409X(99)00021-6 | ||
Jung SH, Lim DH, Jung SH, et al. Amphotericin B entrapping lipid nanoparticles and their in vitro and in vivo characteristics. Eur J Pharm Sci. 2009;37:313–320. doi:10.1016/j.ejps.2009.02.021 | ||
Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi:10.1016/S0142-9612(02)00036-4 | ||
Balogh L, Swanson DR, Tomalia DA, Hagnauer GL, McManus AT. Dendrimer-silver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001;1:18–21. doi:10.1021/nl005502p | ||
Van de Ven H, Paulussen C, Feijens PB, et al. PLGA nanoparticles and nanosuspensions with amphotericin B: potent in vitro and in vivo alternatives to Fungizone and AmBisome. J Control Rel. 2012;161:795–803. doi:10.1016/j.jconrel.2012.05.037 | ||
Lemke A, Kiderlen AF, Petri B, Kayser O. Delivery of amphotericin B nanosuspensions to the brain and determination of activity against Balamuthia mandrillaris amebas. Nanomedicine. 2010;6:597–603. doi:10.1016/j.nano.2009.12.004 | ||
Vandermeulen G, Rouxhet L, Arien A, Brewster ME, Preat V. Encapsulation of amphotericin B in poly (ethylene glycol)-block-poly (ε-caprolactone-co-trimrthylene carbonate) polymeric micelles. Int J Pharm. 2006;309:234–240. doi:10.1016/j.ijpharm.2005.11.037 | ||
Choi KC, Bang GN, Kim P, Kim C, Song CE. Amphotericin B incorporated polymeric micelles composed by poly(D,L-lactide-co-glycolide)/dextran graft copolymer. Int J Pharm. 2008;355:224–230. doi:10.1016/j.ijpharm.2007.12.011 | ||
Yang Z, Chen M, Yang M, Chen J, Fan W, Xu P. Evaluating the potential of cubosomal nanoparticles for oral delivery of amphotericin B in treating fungal infection. Int J Nanomed. 2014;9:327–336. | ||
Angra PK, Oettinger C, Balakrishna PS, D’Souza MJ. Amphotericin B microspheres: a therapeutic approach to minimize toxicity while maintaining antifungal activity. J Microencap. 2009;26:580–587. doi:10.3109/02652040902797516 | ||
Rizwan SB, Assmus D, Boehnke A, et al. Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. Eur J Pharm Biopharm. 2011;79:15–22. doi:10.1016/j.ejpb.2010.12.034 | ||
Wasan EK, Bartlett K, Gershkovich P, et al. Development and characterization of oral lipid based amphotericin B formulation in enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans. Int J Pharm. 2009;372:76–84. doi:10.1016/j.ijpharm.2009.01.003 | ||
Cottle W. Chaulmoogra oil in leprosy. Br Med J. 1879;1:968–969. | ||
Bergstrom S, Theorell H, Davide H. Effect of some fatty acids on the oxygen uptake of Mycobact. Tubercul. Hum. in relation to their bactericidal action. Nature. 1946;157:306. doi:10.1038/157306a0 | ||
Walker EL, Sweeney MA. The chemotherapeutics of the chaulmoogric acid series and other fatty acids in leprosy and tuberculosis. J Infect Dis. 1920;26:238–264. doi:10.1093/infdis/26.3.238 | ||
Saito H, Tomioka H, Yoneyama T. Growth of group IV mycobacteria on medium containing various saturated and unsaturated fatty acids. Antimicrob Agents Chemother. 1984;26:164–169. | ||
Saito H, Tomioka H. Susceptibilities of transparent, opaque, and rough colonial variants of Mycobacterium avium complex to various fatty acids. Antimicrob Agents Chemother. 1988;32:400–402. | ||
Williams AA, Sugandhi EW, Macri RV, Falkinham III JO, Gandour RD. Antimicrobial activity of long-chain, water-soluble, dendritic tricarboxylato amphiphiles. J Antimicrob Chemother. 2007;59:451–458. doi:10.1093/jac/dkl515 | ||
Falkinham III JO, Macri RV, Maisuria BB, et al. Antibacterial activities of dendritic amphiphiles against non-tuberculous Mycobacteria. Tuberculosis. 2012;92:173–181. doi:10.1016/j.tube.2011.12.002 | ||
Hussain A, Singh SK, Singh N, Verma PRP. In vitro-in vivo in-silico simulation studies of anti-tubercular drugs doped with self-nanoemulsifying drug delivery system. RSC Adv. 2016;6:93147–93161. doi:10.1039/C6RA14122F | ||
Muller RH, Radtke M, Wissing SA. Solid lipid NPs (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S13–1S155. doi:10.1016/S0169-409X(02)00118-7 | ||
Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008;166:353–367. doi:10.1007/s11046-008-9109-0 | ||
Lv Q, Yu A, Xi Y, et al. Guangxi Zhai: development and evaluation of penciclovir-loaded nanoparticles for topical delivery. Int J Pharm. 2009;372:191–198. doi:10.1016/j.ijpharm.2009.01.014 | ||
Corware K, Harris D, Teo I, Rogers M, Naresh K. Accelerated healing of cutaneous leishmaniasis in non-healing BALB/c mice using water soluble amphotericin B-polymethacrylic acid. Biomaterials. 2011;32:8029–8039. doi:10.1016/j.biomaterials.2011.07.021 | ||
Chen H, Chang X, Du D, et al. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Control Rel. 2006;110:296–306. doi:10.1016/j.jconrel.2005.09.052 | ||
Manosroi A, Kongkaneramit A, Manosroi J. Stability and transdermal absorption of topical amphotericin B liposome formulations. Int J Pharm. 2004;270:279–286. doi:10.1016/j.ijpharm.2003.10.031 | ||
Devi M, Kumar MS, Manadevan N. Amphotericin-B loaded vesicular systems for the treatment of topical fungal infection. Int J Recent Adv Pharm Res. 2011;4:37–46. | ||
Vyas SP, Agarwal R, Katare OP. Preparation and in vitro evaluation of liposomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 2001;228:43–52. | ||
Mugabe C, Halwani M, Azghani AO, Lafrenie RM, Omri A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:2016–2022. doi:10.1128/AAC.01547-05 | ||
Alipour M, Halwani M, Omri A, Suntres ZE. Antimicrobial effectiveness of liposomal polymyxin B against resistant gram negative bacterial strains. Int J Pharm. 2008;355:293–298. doi:10.1016/j.ijpharm.2007.11.035 | ||
Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325:1089–1093. doi:10.1126/science.1176667 | ||
Kim HJ, Jones MN. The delivery of benzyl penicillin to Staphylococcus aureus biofilms by use of liposomes. J Liposome Res. 2004;14:123–139. doi:10.1081/LPR-200029887 | ||
Schumacher I, Margalit R. Liposome-encapsulated ampicillin: physicochemical and antibacterial properties. J Pharm Sci. 1997;86:635–641. doi:10.1021/js9503690 | ||
Kadimi US, Balasubramaniam DR, Ganni UR, Balaraman M, Govindaraju V. In vitro studies on liposomal amphotericin B obtained by supercritical carbon dioxide mediated process. Nanomedicine. 2007;3:273–280. doi:10.1016/j.nano.2007.08.003 | ||
Jain S, Tiwary AK, Jain NK. PEGylated elastic liposomal formulation for lymphatic targeting of zidovudine. Curr Drug Deliv. 2008;5:275–281. | ||
Hussain A, Singh S, Sharma D, Webster TJ, Shafaat K, Faruk A. Elastic liposomes as novel carrier: recent advances in drug delivery. Int J Nanomed. 2017;12:5087–5108. doi:10.2147/IJN.S138267 | ||
Kaur L, Jain SK, Manhas RK, Sharma D. Nanoethosomal formulation for skin targeting of amphotericin B: an in vitro and in vivo assessment. J Liposome Res. 2014;25:294–307. doi:10.3109/08982104.2014.995670 | ||
Jain S, Tiwary AK, Sapra B, Jain NK. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech. 2007;8(4):E111. doi:10.1208/pt0804111 | ||
Russell AD, Day MJ. Antibacterial activity of chlorhexidine. J Hosp Infect. 1993;25:229–238. doi:10.1016/0195-6701(93)90109-D | ||
Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker JR Jr. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol Res. 2001;156:1–7. doi:10.1078/0944-5013-00069 | ||
Hussain A, Samad A, Nazish I, Ahmed FJ. Nanocarrier-based topical drug delivery for an antifungal drug. Drug Develop Ind Pharm. 2014;40:527–541. doi:10.3109/03639045.2013.771647 | ||
Hussain A, Singh S, Webster TJ, Ahmad FJ. New perspectives in the topical delivery of optimized amphotericin B loaded nanoemulsions using excipients with innate anti-fungal activities: a mechanistic and histopathological investigation. Nanomedicine. 2017;13:1117–1126. doi:10.1016/j.nano.2016.12.002 | ||
Das S, Suresh PK. Nanosuspension: a new vehicle for the improvement of the delivery of drug to ocular surface. Application to amphotericin B. Nanomedicine. 2011;7:242–247. | ||
Peyman GA, Charles HC, Liu KR, Khoobehi B, Niesman M. Intravitreal liposome-encapsulated drugs: a preliminary human report. Int Ophthalmol. 1988;12:175–182. | ||
Shah SP, Misra A. Development of liposomal amphotericin B dry powder inhaler formulation. Drug Deliv. 2004;11:247–253. doi:10.1080/10717540490467375 | ||
Shimizu K, Osada M, Takemoto K, Yamamoto Y, Asai T, Oku N. Temperature dependent transfer of amphotericin B from liposomal formulation of Ambisome to fungal cell membrane. J Control Rel. 2010;141:208–215. doi:10.1016/j.jconrel.2009.09.019 | ||
Imam M, Huang Z, Szoka F, Jaafari M. Characterizatin of colloidal properties, in vitro antifungal, anti leishmanial and toxicity studies in mice of di-stigma-steryl-hemi-succinoyl-glycero-phosphatidylcholine liposomes-intercalated amphotericin B. Int J Pharm. 2011;408:163–172. doi:10.1016/j.ijpharm.2011.01.044 | ||
Guidelli EJ, Baffa O, Clarke DR. Enhanced UV emission from Silver/ZnO and Gold/ZnO core-shell nanoparticles: photoluminescence, radioluminescence, and optically stimulated luminescence. Sci Rep. 2015;5:14004. | ||
Ahonen M, Kahru A, Ivask A, et al. Proactive approach for safe use of antimicrobial coatings in healthcare settings: opinion of the COST action network AMiCI. Int J Environ Res Public Health. 2017;14:366. doi:10.3390/ijerph14040366 | ||
Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011;77:1541–1547. doi:10.1128/AEM.02766-10 | ||
Chen X, Hirt H, Li Y, Gorr SU, Aparicio C. Antimicrobial GL13K peptide coatings killed and ruptured the wall of streptococcus gordonii and prevented formation and growth of biofilms. PLoS One. 2014;9:e111579. doi:10.1371/journal.pone.0111579 | ||
Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–284. doi:10.1016/j.msec.2014.08.031 | ||
Antimicrobial nanocoatings market – global industry analysis, size, share, growth, trends and forecast 2015–2023. Transparency Market Research. Available from: http://www.transparencymarketresearch.com/antimicrobial-nanocoatingsmarket.html. Accessed July 19, 2018. | ||
Furno F, Morley KS, Wong B, et al. Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J Antimicrob Chemother. 2004;54:1019–1024. doi:10.1093/jac/dkh478 | ||
Elliott C. The effects of silver dressings on chronic and burns wound healing. Br J Nurs. 2010;19:S32–S36. doi:10.12968/bjon.2010.19.Sup5.77707 | ||
González-Zorn B, Escudero JA. Ecology of antimicrobial resistance: humans, animals, food and environment. Int Microbiol. 2012;15:101–109. doi:10.2436/20.1501.01.174 | ||
European Commission. Scientific Committee on Emerging and Newly Identified Health Risks. Brussels (Belgium): Assessment of the Antibiotic Resistance Effects of Biocides; European Commission; 2009. | ||
Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903–6913. doi:10.1021/nn102272n | ||
Pavithra D, Doble M. Biofilm formation, bacterial adhesion and host response on polymeric implants – issues and prevention. Biomed Mater. 2008;3:034003. doi:10.1088/1748-6041/3/3/034003 | ||
Sousa C, Teixeira P, Oliveira R. Influence of surface properties on the adhesion of staphylococcus epidermidis to acrylic and silicone. Int J Biomater. 2009;2009:718017. doi:10.1155/2009/718017 | ||
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro. A Critical Review Arch Toxicol. 2013;87:1181–1200. doi:10.1007/s00204-013-1079-4 | ||
Ivask A, Juganson K, Bondarenko O, et al. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology. 2014;8:57–71. doi:10.3109/17435390.2013.855831 | ||
Fries R, Gressler S, Simkó M, Gazsó A, Fiedeler U, Michael N. Nanosilver (Nanotrust Dossier No. 010en). Wien (Austria): Institut für Technikfolgen-Abschätzung (ITA); 2010. | ||
Käkinen A, Bondarenko O, Ivask A, Kahru A. The effect of composition of different ecotoxicological test media on free and bioavailable copper from CuSO4 and CuO nanoparticles: comparative evidence from a Cu-selective electrode and a Cu-biosensor. Sensors. 2011;11:10502–10521. doi:10.3390/s111110502 | ||
D’Costa VM, King CE, Kalan L, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi:10.1038/nature10388 | ||
Andresen TL, Berg RH, Challenges C. Future directions in nanomedicine. JSM Nanotechnol Nanomed. 2013;1(2):1013. | ||
Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48:5406–5415. doi:10.1002/anie.200805179 | ||
Petersen AL, Hansen AE, Gabizon A, Andresen TL. Liposome imaging agents in personalized medicine. Adv Drug Deliv Rev. 2012;64:1417–1435. doi:10.1016/j.addr.2012.09.003 | ||
Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi:10.1158/0008-5472.CAN-12-4561 | ||
Moghimi SM, Andersen AJ, Ahmadvand D, et al. Material properties in complement activation. Adv Drug Deliv Rev. 2011;63:1000–1007. doi:10.1016/j.addr.2011.06.002 | ||
Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3:242–244. doi:10.1038/nnano.2008.114 | ||
Halappanavar S, Vogel U, Wallin H, Yauk CL. Promise and peril in nanomedicine: the challenges and needs for integrated systems biology approaches to define health risk. WIREs Nanomed Nanobi. 2018;10:1–7. doi:10.1002/wnan.1465 | ||
Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2016. doi:10.1016/j.addr.2016.04.025 | ||
Adlhart C, Verran J, Azevedo NF, et al. Surface modifications for antimicrobial effects in the healthcare setting: a critical overview. J Hospl Infect. 2018;99:239–249. doi:10.1016/j.jhin.2018.01.018 | ||
Venkatesan J, Jayakumar R, Mohandas A, Bhatnagar I, Kim S-K. Antimicrobial activity of chitosan-carbon nanotube hydrogels. Materials. 2014;7:3946–3955. doi:10.3390/ma7053946 | ||
Neelgund GM, Oki A, Luo Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf B Biointerfaces. 2012;100:215–221. doi:10.1016/j.colsurfb.2012.05.012 | ||
Aslan S, Loebick CZ, Kang S, Elimelech M, Pfefferle LD, Van Tassel PR. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid). Nanoscale. 2010;2:1789–1794. doi:10.1039/c0nr00329h | ||
Park H, Jang CH, Cho YB, Choi CH. Antibacterial effect of tea-tree oil on methicillinresistant Staphylococcus aureus biofilm formation of the tympanostomy tube: an in vitro study. In Vivo. 2007;21:1027–1030. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.