Back to Journals » International Journal of Nanomedicine » Volume 19
Recent Advances of Emerging Metal-Containing Two-Dimensional Nanomaterials in Tumor Theranostics
Authors Li C, Fang X, Zhang H, Zhang B
Received 30 October 2023
Accepted for publication 15 January 2024
Published 24 January 2024 Volume 2024:19 Pages 805—824
DOI https://doi.org/10.2147/IJN.S444471
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Chenxi Li,1,2,* Xueyang Fang,1,* Han Zhang,1,3 Bin Zhang1
1Shenzhen Key Laboratory of Nanozymes and Translational Cancer Research, Institute of Translational Medicine Department of Otolaryngology Shenzhen Second People’s Hospital, the First Affiliated Hospital of Shenzhen University, Health Science Center, Shenzhen, 518035, People’s Republic of China; 2Graduate Collaborative Training Base of Shenzhen Second People’s Hospital, Heng Yang Medical School, University of South China, Hengyang, Hunan, 421001, People’s Republic of China; 3International Collaborative Laboratory of 2D, Materials for Optoelectronics Science and Technology of Ministry of Education, Institute of Microscale Optoelectronics, College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Zhang; Xueyang Fang, Email [email protected]; [email protected]
Abstract: In recent years, metal-containing two-dimensional (2D) nanomaterials, among various 2D nanomaterials have attracted widespread attention because of their unique physical and chemical properties, especially in the fields of biomedical applications. Firstly, the review provides a brief introduction to two types of metal-containing 2D nanomaterials, based on whether metal species take up the major skeleton of the 2D nanomaterials. After this, the synthetical approaches are summarized, focusing on two strategies similar to other 2D nanomaterials, top-down and bottom-up methods. Then, the performance and evaluation of these 2D nanomaterials when applied to cancer therapy are discussed in detail. The specificity of metal-containing 2D nanomaterials in physics and optics makes them capable of killing cancer cells in a variety of ways, such as photodynamic therapy, photothermal therapy, sonodynamic therapy, chemodynamic therapy and so on. Besides, the integrated platform of diagnosis and treatment and the clinical translatability through metal-containing 2D nanomaterials is also introduced in this review. In the summary and perspective section, advanced rational design, challenges and promising clinical contributions to cancer therapy of these emerging metal-containing 2D nanomaterials are discussed.
Keywords: metal-containing two-dimensional nanomaterials, nanotechnology-based therapy, cancer precision treatment, theranostic platform
Graphical Abstract:
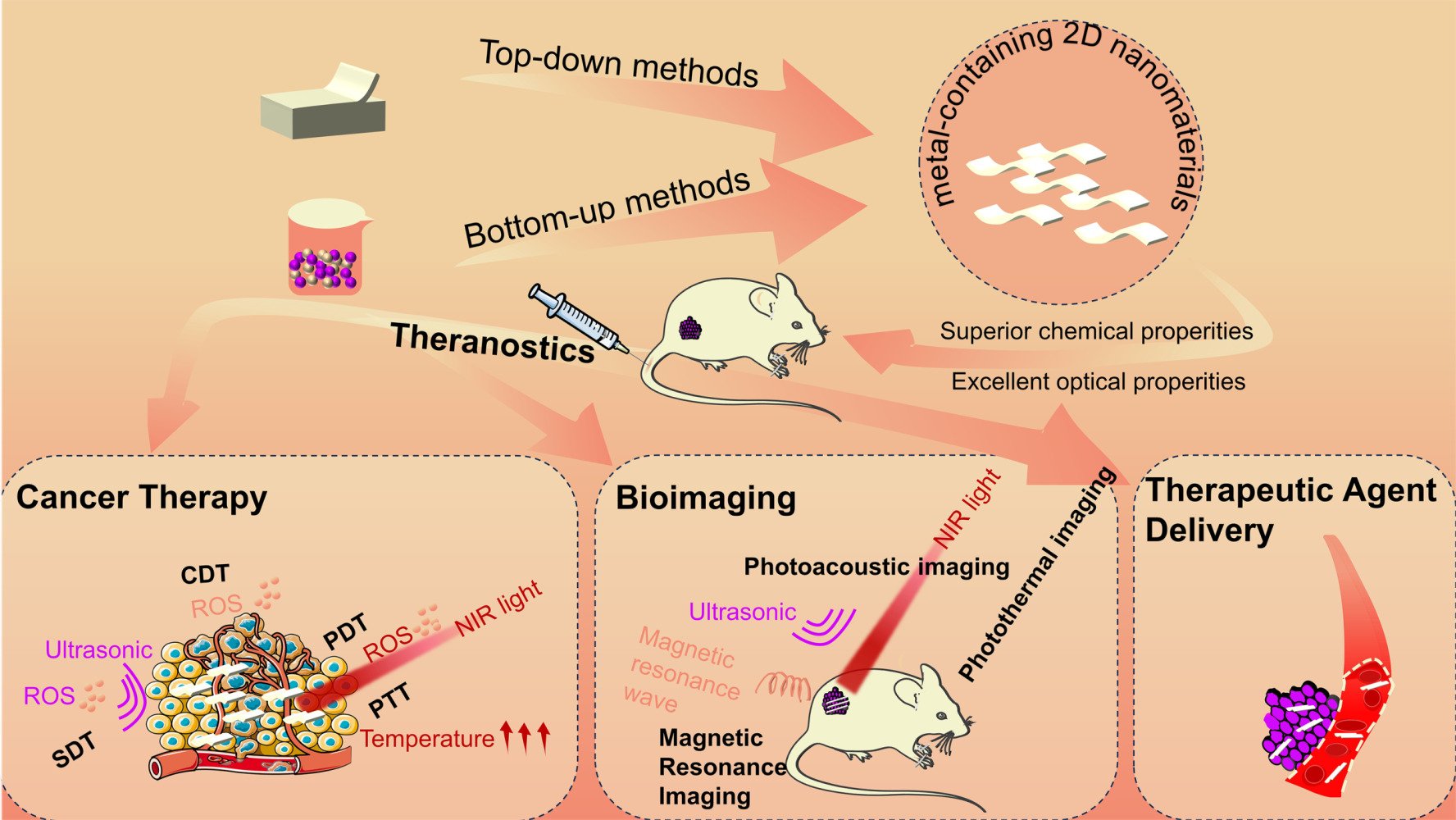
Introduction
Cancer has a significant impact on human health and societal advancement due to its high incidence and mortality rates. There are downsides to using conventional therapeutic approaches including surgery, radiation, chemotherapy, etc.1 Nanotechnology-based therapy, which includes photodynamic therapy (PDT), photothermal therapy (PTT), chemodynamic therapy (CDT), sonodynamic therapy (SDT) and integrated treatment approaches, etc., has received a lot of interest recently as a potentially promising strategy for cancer suppression.2
Two-dimensional (2D) materials are a category of materials with high lateral dimension-to-thickness ratios and a sheet-like structure.3–5 Numerous 2D nanomaterials have been employed in biomedicine, such as graphene, transition metal dichalcogenides (TMDs), transition metal carbides, nitrides and carbonitrides (MXenes), monoelemental nanosheets like black phosphorus (BP) and graphdiyne, layer like double hydroxides (LDHs), 2D metal-organic frameworks (MOFs), etc.6–16 Among them, metal-containing 2D materials have become a research frontier in nano-medicine, benefiting from their strong near-infrared (NIR) light absorption, ultrasonic responsiveness and the capacity to produce reactive oxygen species (ROS).17–20 Currently, there are mainly two types of metal-containing 2D materials, depending on whether metal species are decorated on the surface of 2D materials or take up the skeleton structures. Thanks to the performance modulation of metal species, which endows 2D materials with highly catalytic performance as well as light and sonic responsive ability, these materials are well suited for cancer PDT, PTT, CDT, SDT, and other types of therapy.21–25 Due to the large surface area of 2D nanomaterials, various functional molecules, such as chemotherapeutic drugs and fluorescent probes, can be loaded via covalent or non-covalent interactions, as well as various functional nanoparticles (NPs), including Au NPs, Pt NPs and some metal quantum dots.26–28 These extra molecules and NPs enhance the features of 2D nanomaterials, such as electrochemical performance, magnetic functionality, radioactivity and imaging capabilities. Thanks to their superior physicochemical characteristics, such as their high surface-to-mass ratio, distinctive surface chemical activity and inherent optical features, 2D nanomaterials have emerged as preferred nanoplatforms for biomedicine.29–31
Reviews of the categorization and applications of 2D nanomaterials in the literature are reported, but there are limited specific reviews of metal-containing 2D materials discussing the diverse mechanisms of action in the treatment of tumors and the most recent advancements.32–35 Here, we concentrate on outlining the many approaches that metal-containing 2D nanomaterials have been used to treat cancer in recent years, as well as their use in the integration of cancer diagnostics and therapy. This article conducts a thorough overview of current developments in metal-containing 2D nanomaterials applied to biomedicine, particularly in cancer treatment (Table 1). First, we review the methods used for constructing different kinds of these materials, such as mechanical exfoliation, liquid-phase exfoliation, hydrothermal/solvothermal techniques and Chemical Vapor Deposition (CVD). Then, we discuss the varied applications of these materials in cancer therapy (Figure 1). Afterwards, we analyze the clinical translatability and challenge of metal-containing 2D nanomaterials. Finally, the difficulties now facing them as an integrated nanoplatform for the diagnosis and treatment of cancer are discussed. We have high hopes that this review will stimulate broader interest in the research and development of advantageous uses of metal-containing 2D nanomaterials for cancer treatment.
 |
Figure 1 The synthesis and theranostic platform of metal-containing 2D nanomaterials, including cancer therapy, bioimaging and therapeutic agent delivery. |
 |
Table 1 Cancer Treatment of Metal-Containing Two-Dimensional Nanomaterials |
Synthetic Methods of Metal-Containing Two-Dimensional Nanomaterials
The methods for synthesizing metal-containing 2D nanomaterials are mainly classified into two categories: top-down and bottom-up methods (Figure 2A).60 The former relies on exfoliating bulk materials through physical exfoliation, such as mechanical tearing and sonication exfoliation, and then a single or few layers of metal-containing 2D nanomaterials are obtained.61 While the latter means preparing metal-containing 2D nanomaterials by chemical reaction of certain precursors at specific experimental conditions. This article summarizes some common methods in detail for preparing metal-containing 2D nanomaterials in the following parts.
 |
Figure 2 (A) The synthetic methods of metal-containing 2D nanomaterials, including top-down and bottom-up methods. (B) Top-down synthetic method (Exfoliation) of 2D nanomaterials. Reproduced from Lin S, Yang M, Chen J, et al. Two-Dimensional FePS3 nanosheets as an integrative sonosensitizer/nanocatalyst for efficient nanodynamic tumor therapy. Small. 2023;19(8):2204992. 62 Copyright 2022 Wiley-VCH GmbH. (C) Bottom-up synthetic method (CVD) of 2D nanomaterials. Reproduced from Huang H, Feng W, Chen Y. Two-dimensional biomaterials: material science, biological effect and biomedical engineering applications. Chem Soc Rev. 2021;50(20):11381–11485.63 Copyright 2021 The Royal Society of Chemistry. |
Top-Down Method
The top-down method means stripping bulk materials into a monolayer or a few layers. These methods include micromechanical cleavage (Figure 2B), sonication-assisted liquid exfoliation, shear force-assisted liquid exfoliation, oxidation-assisted liquid exfoliation, selective etching-assisted liquid exfoliation and so on.64,65 These physical exfoliation methods are simple but not precise because of the uncontrolled synthesis process, thus resulting in low quality and yield.
Micromechanical Cleavage
Micromechanical cleavage is a method that could weaken the van der Waals between the materials by mechanical tearing and then stripping bulk crystals into a single or few layers (Figure 2B). This method has a wide range of applications and can be used to peel various kinds of large 2D crystals. In 2004, Novoselov’s group successfully peeled layered graphene for the first time in this way and subsequently applied it to other bulk crystal exfoliation processes.66 The material stripped by this method has a large lateral dimension, high purity and superior quality. However, it also has many problems including low yield, unprecise control and difficulty in industrial production which hinders its further application.
Sonication-Assisted Liquid Exfoliation
It is the simplest and most common mechanical stripping method. The layered bulk crystals were dissolved in a specific solvent before being treated with sonication. After sonication, the suspension would be purified via centrifugation to obtain the nanosheet suspension. The theory of this method is that ultrasound can create bubbles in the liquid, and when the bubbles burst, microjets and shocks can pass through chunks of crystals, peeling them off into layered nanosheets. Coleman’s team first used this method in 2008 which peeled large graphite crystals into layered graphite nanosheets in n-methyl-pyrrolidone (NMP) solution.67 Subsequently, many kinds of polymers and surfactants were used to adjust the surface energy of liquid solvents, increasing the production of 2D nanomaterials and extending their application range. This method exhibits many advantages such as high yield, low cost and the possibility for large-scale production. However, there are some apparent disadvantages including small lateral size and nanomaterials attaching lots of by-polymers attaching on 2D nanomaterials and low yield of specific monolayers.
High-Shear Force-Assisted Liquid Exfoliation
High-shear force-assisted liquid exfoliation is a method that stripping materials by gradient centrifugation in a specific solvent by a high-shear rotor. The shear rate of the rotor rotator is the most critical factor in determining output and without specific requirements for equipment.68 Researchers stirred the materials in kitchen Blender and successfully sheared graphene crystals into graphene nanosheets.69 In this strategy, 2D nanomaterials can be easily synthesized with high yield and low cost. While it has to be carried out at a specific power which induces its low synthetic efficiency.
Oxidation-Assisted Liquid Exfoliation
Oxidation-assisted liquid exfoliation method depends on a strong oxidant to nanomaterials. For instance, the oxidized graphene will generate a large number of oxygen-containing functional groups in the interlayer, which could expand the interlayer distance of graphene, weaken the van der Waals force and peel the big bulk graphene into layers.70 Although this method exhibits a high yield, it is limited in low safety and results the difficulty in large-scale applications.
Selective Etching-Assisted Liquid Exfoliation
The selective etching-assisted liquid exfoliation method means strip bulk MAX material into nanosheets by strong acid, and then obtaining monolayer 2D MXenes nanomaterials by ultrasonication.71 This method can be used in tightly bonded layered materials and is easy to perform. However, the strong acid used in the exfoliating process is dangerous, and this method is limited to specific types of materials. It does not apply to all MXenes from the MAX phase, nor can it be applied to graphene, TMD and other 2D nanomaterials.
Bottom-Up Method
The bottom-up synthesis method is a traditional strategy for 2D nanomaterials. It includes hydrothermal reaction, co-precipitation, self-assembly, template, mediated synthesis, surface synthesis CVD (Figure 2C) and so on.60 These methods are used for the synthesis of 2D nanomaterials through ions or small molecules, and they are widely used for the synthesis of various 2D nanomaterials.
Hydrothermal Reaction
Hydrothermal reaction is a typical method for the synthesis of metal-containing 2D nanomaterials.72 Hydrothermal reaction can be defined as a method of formation and growth of crystals by chemical reactions in a sealed heated aqueous solution.73 Li et al synthesized monolayer rhodium (Rh) nanosheets based on poly-vinylpyrrolidone (PVP) for the first time by hydrothermal method.74 Qiu et al also synthesized 2D Bi/BiOx nanomaterials by this method in 2021.36 The materials synthetic by this method have high quality, large two dimensions area, controllable thickness and high yield. However, it requires precise control of interior reaction system conditions such as concentration, pH value, time, pressure, organic additives and the exterior reaction environment conditions.75
Co-Precipitation Reaction
Co-precipitation reaction is another typical synthesis strategy. The basic idea is to put all kinds of components into one condition and make them react spontaneously.76 For example, Weicheng et al synthesized CoMo-LDH colloid by reacting (NH4) 6Mo7O24·4H2O, Co (NO3)2·6H2O and NaOH in the N2 environment.39 This reaction is simple and easy to operate, but 2D nanomaterials synthesized in this way are always inaccuracy with many by-products.
Self-Assembly Method
The self-assembly method is based on the spontaneous bonding of pre-synthesized nanocrystals through non-covalent interaction.77 As a typical example, Wu and his group synthesized Cu nanosheets and gold nanosheets through Cu clusters and gold clusters, respectively.78,79 The thickness of these nanosheets can be adjusted by the concentration of cluster, reaction temperature and reaction time. The operation of this reaction is simple, only need to put materials into the ultrasonic pot. However, the synthetic concentration and temperature need to be precisely controlled, and the quality of this kind of material is relatively low, which is not suitable for large-scale production.
Template Method
The template method is a kind of self-assembly method, which uses the specific shape as a template. For example, Wei et al synthesized half-unit-cell α‑Fe2O3 nanosheets with a CuO template.80 In this process, CuO template could be dissolved by acid after Fe2O3 nanosheets were synthesized. The method of dissolving the outer template by acid is called the hard template method, while the corresponding soft template method means removing the outer template by cauterization. This method can obtain high-quality nanomaterials, but the synthesis process requires stern conditions and special templates, which hinders its large-scale industrialized production.
Interface-Mediated Synthesis
Interface-mediated synthesis is a typical bottom-up synthesis method.81 It refers to the process of confining the ligand in the water/air interface and then reacting with salts dissolved in solution to form coordination polymers (CPs) nanosheet. In Bauer’s report, hexafunctionalterpyridine-based organic ligand is dissolved in chloroform, and then it is dropwise added into the metal salt solution.82 Due to the rapid evaporation of chloroform and the low solubility of organic ligands in water, this ligand will be confined to the water/air interface. The ligand at the interface can react with metal ions in solution and finally form a metal coordination polymer. The synthesized nanosheets are located in the interface between water and air, which are easy to transfer into other media. This method has been applied in the synthesis of a variety of metal-containing 2D nanomaterials and it is expected to be further extended.
Surface Synthesis
The surface synthesis method is often used for covalent-organometallic frame (COF) generation. It refers to casting the desired COF monomer onto an appropriate solid substrate and then sealing the solid substrate in a container that is filled with needed volatile monomers. When the container is heated at an appropriate temperature, the volatile monomers in it will gradually volatilize and react with the monomers on the solid substrate. Then, the COF nanosheets will be formed in the shape of the solid substrate. Lei et al first synthesized monolayer imine COF on the surface of highly oriented pyrolytic graphite (HOPG) by this method.83 With this method, we can synthesize high-quality COF nanosheets with regular shapes, but it requires a constant temperature, which is hard to control.
Chemical Vapor Deposition (CVD)
Due to its high efficiency and controllability, CVD is a popular method in the synthesis of 2D nanomaterials.84–86 The CVD method involves two main steps: firstly, the preselected substrate is placed into the Furnace Chamber, and then one or more gas/vapor precursors cycle in the chamber and react with the substrate. Under appropriate experimental conditions, ultra-thin metal-containing 2D nanomaterials can be obtained. This method can obtain the desired materials through interatomic reaction and the materials ratio is easy to control. However, there are many factors affecting the synthesis process, including precursors, substrates, catalysts, temperature, atmospheres, etc. Accounting for its complicated synthetic conditions, expensive cost and low yield, it still needs to be optimized.
Biomedical Applications
Cancer Therapy
PDT
PDT is a non-invasive treatment method that differs from traditional surgical treatment due to the fact it can generate cytotoxic ROS by irradiating photosensitizers gathered in tumor sites with laser irradiation, mainly including singlet oxygen (1O2), hydroxyl radicals (·OH) and superoxide ions (O2−). In tumor areas, a huge quantity of cytotoxic ROS builds up and thus may trigger intracellular mitochondrial apoptosis or activate immune responses to destroy tumor cells. As a result, the generation of ROS is the most important factor influencing PDT efficiency.21 Increased production of ROS during the PDT process may be accomplished by strengthening the photosensitizer’s properties, increasing the oxygen level in the tumor microenvironment and extending the duration of light exposure.87
The ability of photosensitizers to generate ROS is a crucial factor in determining the PDT effect. Ge et al synthesized H-germanene 2D nanosheets with a 1.65eV bandgap as a novel inorganic photosensitizer for PDT, which can generate more ROS than conventional 2D nanosheets with a narrower bandgap such as MXene when exposed to 660nm laser light (Figure 3A and B).47 Besides, the TME is a hypoxic microenvironment that influences the rate at which oxygen is converted into ROS. Chen et al loaded Pt on 2D Cu-TCPP MOF to alleviate the hypoxia at the tumor site, thereby increasing the production of ROS and enhancing the PDT effect (Figure 3C).44 In addition, Chang et al developed a sustainable luminescent 2D CaAl2O4:Eu, Nd persistent luminescence nanosheets (CAO PLNSs), which eliminated the need for external light source irradiation in PDT over the long term (Figure 3D).38 Their continuous luminescence is caused by Ca and O vacancies in the 2D nanosheets as well as the electronic transition of Eu2+ between the 4f and 5d states after illumination (Figure 3E). So, after 10 minutes of irradiation at 365nm, the 2D nanosheets continued to emit light, resulting in the continuous production of ROS that enhanced the tumor-killing effect (Figure 3F). Moreover, the inherent properties of metal-containing 2D nanomaterials, such as their large surface area and ease of modification, are extremely advantageous for PDT.
 |
Figure 3 (A) Illustrating PDT-induced cell apoptosis on H-germanene under laser irradiation. Reproduced from Ge M, Guo H, Zong M, et al Bandgap-engineered germanene nanosheets as an efficient photodynamic agent for cancer therapy. Angew Chem Int Ed Engl. 2023:62.47 Copyright 2023 Wiley-VCH GmbH. (B) UV-vis diffuse reflectance spectra and tauc plots of H-germanene nanosheets. Reproduced from Ge M, Guo H, Zong M, et al Bandgap-engineered germanene nanosheets as an efficient photodynamic agent for cancer therapy. Angew Chem Int Ed Engl. 2023:62.47 Copyright 2023 Wiley-VCH GmbH. (C) Schematic illustration of dual-enhanced PDT therapy. Reproduced from Chen Z, Wu Y, Yao Z, et al 2D copper (II) metalated metal-organic framework nanocomplexes for dual-enhanced photodynamic therapy and amplified antitumor immunity. ACS Appl Mater Interf. 2022;14(39):44199–44210.44 Copyright 2022 Journal of the American Chemical Society. (D) Schematic diagram of the mechanism of photodynamic tumor nanotherapy. Reproduced from Nano Today. Volume 42. Chang M, Dai X, Dong C, et al Two-dimensional persistent luminescence “optical battery” for autophagy inhibition-augmented photodynamic tumor nanotherapy.38 Copyright 2022, with permission from Elsevier. (E) Illustration of persistent luminescence mechanism of CAO PLNSs. Reproduced from Nano Today. Volume 42. Chang M, Dai X, Dong C, et al Two-dimensional persistent luminescence “optical battery” for autophagy inhibition-augmented photodynamic tumor nanotherapy.38 Copyright 2022, with permission Elsevier. (F) The persistent luminescence images at different time intervals (0–120 min) of CAO PLNSs after 10 min under irradiation of 365 nm UV lamp, then reactivated by white LED lamp for 5 min (130–180 min). Reproduced from Nano Today. Volume 42. Chang M, Dai X, Dong C, et al Two-dimensional persistent luminescence “optical battery” for autophagy inhibition-augmented photodynamic tumor nanotherapy.38 Copyright 2022, with permission from Elsevier. |
PTT
PTT means under the irradiating of NIR laser, photothermal agents (PTAs) can elicit protein ablation to eliminate tumor cells through high temperature. The efficacy of PTT is therefore dependent on the photothermal conversion efficiency (PTCE) of metal-containing 2D nanomaterials.88
Jiang et al synthesized 2D stanene nanosheets (SnNSs) via a combination of low-temperature exfoliation and liquid phase exfoliation, achieving a PTCE of 48.6% (Figure 4A).89 According to calculations based on density functional theory (DFT), free electrons in the upper and lower layers of the 2D SnNSs contribute to the conversion of light to heat. As for the therapeutic effect, SnNSs with an 808nm laser irradiation kill tumor cells in vitro; experiments in vivo also validated the local heating and potentially fatal effect of PTT on tumor tissue. As another example, Xu et al prepared a 2D nanocomposite Mn-HDCLwith good stability in aqueous, high photothermal stability, a large extinction coefficient and good PTCE (71%), under the conditions of 730 nm laser light irradiation (Figure 4B).50 Moreover, GeP nanosheets and 2D silicene nanocomposites have also been noted as outstanding PTAs in the literature, with PTCE reaching 68.6% and 37.7%, under NIR laser irradiation, and both materials also exhibit strong biodegradability and biocompatibility.46,90 Hence, 2D nanomaterials exhibit powerful PTCE and hold promise as materials for PTT against cancer.
 |
Figure 4 (A) Illustrating the synthetic procedure of 2D SnNSs and the Multifunction of SnNSs@PEG in vivo. Reproduced from Ouyang J, Zhang L, Li L, et al. Cryogenic exfoliation of 2D stanene nanosheets for cancer theranostics. Nanomicro Lett. 2021;13(1):90,89 Creative Commons. (B) Schematic diagram showing the preparation process of Mn-HDCL and its potential use in MRI and PTT. Reproduced from Xu Y, Li C, Wu X, et al. Sheet-like 2D Manganese (IV) Complex with High Photothermal Conversion Efficiency. J Am Chem Soc. 2022;144(41):18834–18843.50 Copyright 2022 American Chemical Society. |
CDT
CDT has received lots of attention in the field of tumor treatment.91 With the help of the characteristics of weak acid and excessive H2O2 in the tumor microenvironment, CDT uses Fenton or Fenton-like reactions to catalyze weakly oxidizing H2O2 into strongly oxidizing ·OH, resulting in increased intracellular oxidation levels, DNA necrosis and protein inactivation, lipid oxidation, and ultimately induce apoptosis in cancer cells. Increasing the quantity of ·OH generated by a Fenton or a Fenton-like reaction is therefore the key to enhancing the antitumor effect of CDT.
Kang et al constructed a 2D interfacial heterojunction nanosheets (FeOCl/FeOOH NSs).45 The holes on the valence band of FeOCl have a superior ability to catalyze H2O to generate O2, and the resulting O2 is subsequently reduced to H2O2 by the electrons present on the conduction band of FeOOH (Figure 5A). The proficient generation of ·OH via the Fenton-like reaction, which is facilitated by FeOCl/FeOOH nanosheets, is guaranteed by the commendable self-sufficient capacity of H2O2. Another study conducted by Hu et al revealed that superlattice nanosheets polyaniline/MoO3−x (PANI/MoO3−x) facilitated electron transfer with the assistance of good conductivity of PANI through the Fenton reaction, thereby enhancing the efficiency of CDT and exhibiting potent antitumor properties (Figure 5B–D).51 Besides, due to the high levels of hydrogen sulfide (H2S) found in colon cancer tumor tissue, Wang’s research team created a novel 2D Cu-bipyridine MOF nanosheet called Cu(bpy)2(OTf)2, which can consume H2S in tumor tissue to produce ultra-small CuS, thus encouraging the Fenton-like reaction for CDT against colon cancer.43 Taken these together, the utilization of 2D nanomaterials in catalyzing the production of ·OH from H2O2 through Fenton or Fenton-like reactions has garnered significant interest in the scientific community, and this method does not necessitate external energy input or O2 as substrates, thereby presenting a promising avenue for future development.
 |
Figure 5 (A) Schematic diagram of bioimaging and anti-tumor performance of FeOCl NSs and FeOCl/FeOOH NSs. Reproduced from Kang Y, Mao Z, Wang Y, et al. Design of a two-dimensional interplanar heterojunction for catalytic cancer therapy. Nat Commun. 2022;13(1):2425.45 Copyright 2022 Nature Publishing Group (B) The illustration of the preparation of 2D PVP@ PANI/MoO3−x superlattice nanosheets for efficient chemodynamic cancer therapy. Reproduced from Hu T, Xue B, Meng F, et al. Preparation of 2D Polyaniline/MoO3-x superlattice nanosheets via intercalation-induced morphological transformation for efficient chemodynamic therapy. Adv Healthc Mater. 2023:12.51 Copyright 2023 Wiley-VCH GmbH. (C) Cytotoxicity of the PVP@ PANI/MoO3−x nanosheets to 4T1 cells at different PH levels in the presence of H2O2. Reproduced from Hu T, Xue B, Meng F, et al. Preparation of 2D Polyaniline/MoO3-x superlattice nanosheets via intercalation-induced morphological transformation for efficient chemodynamic therapy. Adv Healthc Mater. 2023:12.51 Copyright 2023 Wiley-VCH GmbH. (D) Images of mice after treatment with PBS (control) and the PVP@PANI/ MoO3−x nanosheets at different time points. Reproduced from Hu T, Xue B, Meng F, et al. Preparation of 2D Polyaniline/MoO3-x superlattice nanosheets via intercalation-induced morphological transformation for efficient chemodynamic therapy. Adv Healthc Mater. 2023:12.51 Copyright 2023 Wiley-VCH GmbH. |
SDT
Ultrasound is a periodic vibrating mechanical wave with a frequency over 20 kHz. It is extensively used in clinical diagnosis due to its controllability, non-invasiveness and high tissue penetrability.92 SDT refers to a treatment modality that generates cytotoxic ROS by activating sonosensitizers via ultrasound, similar to the underlying principle of PDT.93 SDT has been extensively researched as an emergent non-invasive method for eliminating cancer due to its high specificity, low damage to normal cells and deep tissue penetration. The sonosensitizers are the main factor impacting the effect of SDT, specifically, the quantity of ROS generated by the sonosensitizers when activated by ultrasound determines the effect of SDT.
Due to their excellent biocompatibility, acid-responsive biodegradability and varied chemical compositions and structures, 2D LDHs have been demonstrated to be potential nanomedicines across multiple biomedical disciplines. Hu et al designed an ultra-thin 2D CoW-LDH as an efficient SDT sonosensitizer using a simple acid etching process (Figure 6A–C).40 And the CoW-LDH sonosensitizer demonstrated a higher ROS production, which was approximately 17 times greater than that of the commercially applied titanium dioxide sonosensitizer. The enhanced property may be a result of the crystalline-to-amorphous phase transformation-induced bandgap narrowing, electronic structure changing and defect generation, all of which promote the separation of electron–hole pairs and thus enhance ROS generation. Dong et al designed ultrathin 2D Bi2MoO6 (BMO NRs) nanocomposites to realize efficient SDT.94 The ultrathin BMO NRs are piezoelectric, where ultrasonic waves introduce mechanical strain to the nanoribbons leading to piezoelectric polarization and band tilting, which can accelerate the production of ROS (Figure 6D). This process not only provides new options for improving SDT but also broadens the application of 2D nanomaterials as sonosensitizers in SDT. Lin et al reported a kind of high-efficiency sonosensitizers based on 2D tetra(p-benzoate) porphyrin metal-organic layers (TBP@MOL) that created more ROS than TBP ligand and TBP-based MOF, respectively, and showed excellent effectiveness for the treatment of colorectal cancer and breast cancer.54 In contrast to PDT and PTT which rely on light activation, SDT utilizes ultrasound that does not require precise tumor localization. This characteristic endows SDT with the capability to serve as a feasible and non-intrusive alternative for the treatment of tumors that are located at a significant depth or are of considerable size, and it thus has considerable promise as a therapeutic approach.
 |
Figure 6 (A) Schematic diagram of the preparation of a-CoW-LDH-PEG nanosheets and their application in SDT. Reproduced from Hu T, Shen W, Meng F, et al Boosting the sonodynamic cancer therapy performance of 2D layered double hydroxide nanosheet-based sonosensitizers via crystalline-to-amorphous phase transformation. Adv Mater. 2023;35(17):e2209692.40 Copyright 2023 Wiley-VCH GmbH. (B) Calcein-AM/PI co-stained 4T1 cells under different conditions. Reproduced from Hu T, Shen W, Meng F, et al Boosting the sonodynamic cancer therapy performance of 2D layered double hydroxide nanosheet-based sonosensitizers via crystalline-to-amorphous phase transformation. Adv Mater. 2023;35(17):e2209692.40 Copyright 2023 Wiley-VCH GmbH. (C) H&E, Ki-67 and TUNEL staining assays of anti-tumor performance of 4T1 tumor-bearing mice in different groups after 16-day treatment. Reproduced from Hu T, Shen W, Meng F, et al Boosting the sonodynamic cancer therapy performance of 2D layered double hydroxide nanosheet-based sonosensitizers via crystalline-to-amorphous phase transformation. Adv Mater. 2023;35(17):e2209692.40 Copyright 2023 Wiley-VCH GmbH. (D) Schematic illustration of the 2D piezoelectric Bi2MoO6 sonosensitizer for GSH-enhanced sonodynamic therapy. Reproduced fromDong Y, Dong S, Liu B, et al 2D piezoelectric Bi2 MoO6 nanoribbons for GSH-enhanced sonodynamic therapy. Adv Mater. 2021;33(51).94 Copyright 2021 Wiley-VCH GmbH. |
Therapeutic Agent Delivery
After years of research, many nanomaterials have been discovered for drug delivery. With a large surface area and easily surface-modified character, 2D nanomaterials can load specific genes or target nanomedicine for smart therapeutic agent delivery.
For the first time, Liu et al developed the 2D PEGylated nanographene oxide (NGO) for the delivery of water-insoluble cancer drugs-SN38 in 2008.95 The water-soluble NGO-PEG-SN38 complex could afford strong noncovalent banding and exhibit obvious cell toxicity. While no toxicity was measured of plain NGO-PEG without drug loading. This 2D structure and ultrasmall size (down to 5 nm) of NGO-PEG offered unexpected anticancer ability and provided a new idea for a drug delivery application. This promising work paved a way for the subsequent explosion of 2D nanomaterials-related research in cancer therapy. In 2022, a newly 2D Copper (II) based MOF nano complexes (Cu-TCPP(Al)), decorated with folate (FA) and platinum (Pts) NPs, were prepared for specific targeting drug-delivery and tumor therapy (Figure 7A).44 FA in these nanocomplexes was used for targeting tumor tissue and Pt NPs could produce ROS under laser irradiation, which endows 2D Cu-TCPP(Al) excellent therapeutic efficiency. At the same time, scientists came up with the idea of sandwich-like 2D nanosheets (HA-CQ/HT@sSiO2 NSs), which were modified by CD44− targeted hyaluronic acid (HA) and ultimately developed targeted 2D nanosheets (Figure 7B).53 This material has CD44− targeting specificity which allows it to achieve effective enrichment in tumor cells, and the in vitro and in vivo experiments in this research both collectively demonstrated the superb effect of cancer cell apoptosis and tumor growth inhibition.
 |
Figure 7 (A) The preparation process of Cu-TCPP(Al)-Pt-FA. Reproduced from Chen Z, Wu Y, Yao Z, et al 2D copper (II) metalated metal-organic framework nanocomplexes for dual-enhanced photodynamic therapy and amplified antitumor immunity. ACS Appl Mater Interf. 2022;14(39):44199–44210.44 Copyright 2022 American Chemical Society. (B) Fabrication procedure of targeted sandwich-like 2D NSs and schematic illustration of 2D NSs for activatable autophagy inhibition-primed CDT. Reproduced from Chem Eng J. Volume 431. Wu H, Wu F, Zhou T, et al Activatable autophagy inhibition-primed chemodynamic therapy via targeted sandwich-like two-dimensional nanosheets.53 Copyright 2022, with permission from Elsevier B.V. |
Bioimaging
Cancer imaging is essential not only for early detection of cancer but also for determining the specific tumor location and stage, as well as for directing therapy and monitoring for cancer recurrence following treatment. 2D nanomaterials may be employed for a variety of imaging applications due to their superior physical, electronic, chemical and optical properties.
The 2D nanocomplex (Mn-HDCL), as prepared by Xu et al, exhibits a favorable photothermal effect and possesses paramagnetic properties.50 These characteristics contribute to an enhanced T1-weighted contrast of magnetic resonance images (MRI) in vitro and in vivo, as reported in their study. The study revealed a positive correlation between the concentration of Mn-HDCL in an aqueous solution and the increase in magnetic resonance signal intensity, and Mn-HDCL could passively target tumors and differentiate tumor margins in vivo (Figure 8A–C). Zhang et al introduced an H2S-responsive nanoplatform known as ZNNPs, which produces a photoacoustic (PA) signal and exhibits an intelligent reaction to H2S, enabling the quantitative and real-time imaging of endogenous H2S.96 This platform therefore offers a method for accurate H2S -related diagnosis and efficient treatment of diseases such as acute liver toxicity, cerebral hemorrhage and colorectal cancer therapy.
 |
Figure 8 (A) T1-weighted and color-mapped phantom MRI for aqueous solutions at different concentrations of Mn-HDCL. Reproduced from Xu Y, Li C, Wu X, et al. Sheet-like 2D Manganese (IV) Complex with High Photothermal Conversion Efficiency. J Am Chem Soc. 2022;144(41):18834–18843.50 Copyright 2022 Journal of the American Chemical Society (B) Coronal T1-weighted MR images of a BALB/c mouse at different time points after intravenous injection of Mn-HDCL. Reproduced from Xu Y, Li C, Wu X, et al. Sheet-like 2D Manganese (IV) Complex with High Photothermal Conversion Efficiency. J Am Chem Soc. 2022;144(41):18834–18843.50 Copyright 2022 Journal of the American Chemical Society (C) Axial T1-weighted MR images of a BALB/c mouse bearing 4T1 tumor xenografts at different time points after intravenous injection of Mn-HDCL. Reproduced from Xu Y, Li C, Wu X, et al. Sheet-like 2D Manganese (IV) Complex with High Photothermal Conversion Efficiency. J Am Chem Soc. 2022;144(41):18834–18843.50 Copyright 2022 Journal of the American Chemical Society. |
Combination Therapy
Synergistic Therapy
As previously stated, the exceptional physical, electronic, chemical and optical characteristics of 2D nanomaterials render them highly promising for potential applications in the field of cancer therapy. Numerous 2D nanomaterials exhibit diverse catalytic functionalities and effectively synergize multiple mechanisms to accomplish cytotoxic effects on tumors.22
For combined PDT and PTT, Lu et al incorporated the organic compound chlorophyll (Chl) with metal-supported vanadium carbide (V2C) nanosheets (Figure 9C).97 They studied the killing ability of 2D Chl/V2C NSs on tumor cells under different conditions (Figure 9A), among which 2D Chl/V2C NSs have PDT effect under 670 nm laser and PTT effect can be produced under 808 nm laser. The killing effect of the two methods on cells is 64% and 80%, respectively. When irradiated with both 670 and 808nm lasers, the killing effect of 2D Chl/V2C NSs on cells can reach 99%, which verifies that the combined application of PDT and PTT can achieve better tumor-killing effect. The tumor volume of mice also shows the same trend (Figure 9B). In this work, the appropriate heating by PTT enables increased blood flow and improves oxygen supply for enhanced PDT, and PDT can disturb TME conditions and result in increased heat sensitivity of cancer cells. Zhang et al employed the liquid exfoliation method to synthesize FePS3 nanosheets.98 Their research findings indicate that these nanosheets exhibit a notable PTCE of 43.3% when exposed to 1064nm laser irradiation. This characteristic positions them as a promising type of efficient NIR-II PTA. This iron-based 2D nanosheet exhibits outstanding Fenton catalytic activity that triggers the CDT effect, which is augmented by its photothermal impact, thereby providing a synergistic PTT/CDT therapeutic effect. Liu et al also successfully synthesized 2D FePS3 by liquid phase exfoliation and improved its biocompatibility by modifying it with lipoic acid-polyethylene glycol (LA-PEG).62 These research findings indicate that FePS3-LA-PEG has the capability to generate ROS when subjected to ultrasound, thereby facilitating the SDT effect (Figure 9D). The intrinsic Fenton catalytic activity of FePS3 can synergistically collaborate with SDT to augment the therapeutic efficacy and efficiently eradicate tumors. 2D nanomaterials, which possess the ability to induce therapeutic effects through various mechanisms, exhibit enhanced efficacy in eliminating tumors and hold significant potential for diverse applications.
 |
Figure 9 (A) MTT analysis of MCF-7 cells under different conditions. Reproduced from Lu H, Zada S, Tang S, et al. Artificial photoactive chlorophyll conjugated vanadium carbide nanostructure for synergistic photothermal/photodynamic therapy of cancer. J Nanobiotechnology. 2022;20(1):121.97 Copyright 2022 BMC (B) The images of MCF-7 tumor-bearing mice with different treatments. Reproduced from Lu H, Zada S, Tang S, et al. Artificial photoactive chlorophyll conjugated vanadium carbide nanostructure for synergistic photothermal/photodynamic therapy of cancer. J Nanobiotechnology. 2022;20(1):121.97 Copyright 2022 BMC (C) Schematic diagram of Chl/V2C nanostructure for synergistic PTT/PDT. Reproduced from Lu H, Zada S, Tang S, et al. Artificial photoactive chlorophyll conjugated vanadium carbide nanostructure for synergistic photothermal/photodynamic therapy of cancer. J Nanobiotechnology. 2022;20(1):121.97 Copyright 2022 BMC (D) Schematic diagram of the preparation of 2D FePS3-PEG NSs and the proposed mechanism for combinatorial SDT/ CDT. Reproduced from Lin S, Yang M, Chen J, et al. Two-Dimensional FePS3 nanosheets as an integrative sonosensitizer/nanocatalyst for efficient nanodynamic tumor therapy. Small. 2023;19(8):2204992.62 Copyright 2022 Wiley-VCH GmbH. |
Theranostics
The development of an integrated approach of tumor diagnosis and therapy is the primary research objective of scientists due to the increased need for tumor therapy.99 To address the issue, 2D therapeutic nanosystems were developed, which combine anticancer ability and imaging capability into a unified nanoplatform.100
The 2D GeP nanosheets prepared by Ren et al are high-performance PTAs with a PTCE of 68.6% in the NIR region, which can realize multi-modal imaging including PA imaging and photothermal imaging.46 In addition, GeP nanosheets have a high loading capacity for the anticancer drug doxorubicin (DOX), thereby making the 2D nanocomposite a synergistic platform for chemotherapy and PTT (Figure 10A). Fang et al reported a novel 2D nanomaterials (FePSe3@APP@CCM) for multimodal imaging and synergistic cancer therapy. It exhibited good PTCE of PTT and a significant effect of immunotherapy.101 Meanwhile, the bioinspired 2D nanomaterials are also used as a new PAI and MRI contrast agent for precise and real-time monitoring. Ji et al synthesized 2D functional core layers (FCLs) nanosheets which were composed of MgO and Fe2O3 for cancer theranostics.49 It has high PTCE, great O2– -generation ability under 658nm laser irradiation and enhanced generation capacity of ·OH in TME via the Fenton reaction (Figure 10B). What’s more, FCL-PEG NSs also showed great performance in PA, photothermal and fluorescent imaging, which provides a foundation for its potential application in cancer theranostics. The 2D silicene material demonstrates remarkable light absorption capabilities.102 Research findings indicate that the 2D silicene nano photosensitizer exhibits outstanding photostability, superior ability to generate 1O2, and effective PA imaging capabilities, making it suitable for applications in tumor theranostics. The 2D PtBi-PEG nanomaterials developed by Liu et al are capable of PTT and radiotherapy (RT) for the treatment of cancer, as well as exhibiting favorable performance in infrared (IR) imaging, PA imaging and X-ray imaging; thus, these capabilities can be applied to image-guided cancer therapy (Figure 10C).103 As novel multifunctional therapeutic nanoplatforms, 2D nanomaterials with both imaging and therapeutic functions can inspire the design and application of future anticancer nanoplatforms in biology and medicine and have tremendous potential in cancer therapy.
 |
Figure 10 (A) The illustration of the 2D GeP nanosheets for cancer theranostics application. Reproduced from Chem Eng J. Volume 431. Ren X, Liu W, Zhou H, et al. Biodegradable 2D GeP nanosheets with high photothermal conversion efficiency for multimodal cancer theranostics.46 Copyright 2022, with permission from Elsevier B.V (B) The photonic therapy-enhanced CDT based on FCL-PEG NSs in tumor-bearing mice. Reproduced from Ji X, Ge L, Liu C, et al. Capturing functional two-dimensional nanosheets from sandwich-structure vermiculite for cancer theranostics. Nat Commun. 2021;12(1):1124.49 Copyright 2021 Nature Publishing Group (C) Schematic diagram of the tumor therapy mechanism of PtBi-PEG nanoplates. Reproduced from Liu Y, Li X, Shi Y, et al. Two-dimensional intermetallic PtBi/Pt core/shell nanoplates overcome tumor hypoxia for enhanced cancer therapy. Nanoscale. 2021;13(33):14245–14253.103 Copyright 2021 The Royal Society of Chemistry. |
Clinical Translatability and Challenges
With superior physicochemical properties, metal-containing 2D nanomaterials can achieve obvious antitumor effects through therapy modalities such as PDT, PTT, CDT, and SDT. To date, metal-containing 2D nanomaterials have achieved substantial advancements in the field of biomedical science, particularly in cancer therapy. However, to successfully complete the clinical translation, a number of obstacles still need to be overcome.
The poor stability and biocompatibility of metal-containing 2D nanomaterials in physiological environments is an important problem hindering their clinical application.104 Their large surface areas and easily modified surface properties allow for the enhancement of dispersion, stability and biocompatibility with the addition of surface modifiers such as CS, BSA, PEG, PVP, and others.
The key influencing factor of the biodegradation capacity of metal-containing 2D nanomaterials in non-targeted tissues and eliminating organs is the size of nanomedicine.105 Liu et al pointed out that 2D Pd NSs with different sizes exhibited good biocompatibility in mice, rats and rabbits.106 In their work, 5nm PdNSs exhibit a long blood half-life and can be cleared up through the kidney, while larger 2D PdNSs will accumulate in the liver and spleen for a long time. The long-term residence of metal-containing 2D nanomaterials in non-targeted tissues and their toxicity are major concerns that should be taken into consideration for biomedical applications in the clinic. The long-term retention of metal-containing 2D nanomaterials in non-targeted tissues may lead to systemic damage, such as thrombus and endothelial leakiness.107,108 The cytotoxicity of these metal-containing 2D nanomaterials is highly dependent on their own size, structure and surface chemistry. Therefore, we can work on suitable low-cytotoxicity 2D nanomaterials by controlling the size and surface modification, to reduce their damage to non-target tissues and promote the process of clinical transformation.
At present, the animal models used for the antitumor effect detection of metal-containing 2D nanomaterials still mainly feature subcutaneous xenografts. The preclinical animal tumor models may contribute to the difference between research and predicted clinical performance as they do not reflect clinical tumors and their environment.105 Genetically engineered mice models or patient-derived xenografts may be more effective in predicting the performance of metal-containing 2D nanomaterials in future clinical applications.
There are two challenges in addressing regulatory standards: the standard definition of metal-containing 2D nanomaterials and their related terms, and the systematic approach for the physical characterization and biological testing of nanomedicine, which have not yet been perfected in many countries and there is still a long way from basic research to clinical application.
Conclusions and Perspectives
In this review, we provide an overview of metal-containing 2D material manufacturing techniques and biomedical applications. These 2D nanomaterials have been successfully synthesized using both top-down and bottom-up techniques. The synthesis of simply layered metal-containing 2D nanomaterials may be accomplished using the top-down method since it is straightforward and practical. The bottom-up approach also produces materials with great accuracy and is appropriate for creating metal-containing 2D nanomaterials with intricate structures. In the field of biomedicine, current research works demonstrate that metal-containing 2D nanomaterials which are highly catalytic active and light/sonic responsive have tremendous potential, particularly for cancer therapy. Precision medicine based on metal-containing 2D nanomaterials in principle is more effective and safer than surgery combined with RT and chemotherapy for cancer patients. Metal-containing 2D nanomaterial-mediated cancer treatment methods, such as PDT, PTT, CDT and SDT, can destroy tumor cells through in situ generation of ROS or local high temperature while causing minimal harm to surrounding normal tissues. Combination therapy can also improve the therapeutic effect without increasing drug toxicity and is more conducive to the healthy recovery of patients. Additionally, most of the atoms in 2D nanomaterials are exposed on the surface, which facilitates material modification and efficient utilization. Moreover, owing to their large specific surface area, 2D nanomaterials may effectively load more chemotherapeutic drugs and tumor-imaging fluorescence probes, and be modified with tumor-targeted molecules more easily, suggesting that they can be utilized for targeted drug delivery, cancer imaging, etc. Therefore, the unique physical and chemical structure of metal-containing 2D nanomaterials, as well as their advantages of high loading capacity, make them the potential platform for the integration of tumor diagnosis and treatment.
Even though metal-containing 2D nanomaterials have a broad variety of applications in biomedicine, there are still certain obstacles and challenges in clinical translatability. First, the synthesis methods of these nanomaterials need to be improved. The top-down synthesis approach is straightforward and practical, but it does not provide the resulting materials with excellent quality and adequate accuracy. In addition, the bottom-up technique, which is the most prevalent approach, is capable of producing optimum nanomaterials; however, it is difficult to generate on a large industrial scale due to the laborious procedure involved in the process of synthesis as well as the high cost. Secondly, in the research on cancer therapy with these nanomaterials, most studies tend to explore the simple mechanism in vitro and in vivo. However, it is necessary to further explain their internal mechanisms, such as the interaction between metal species, the skeleton structures, interfaces and structural proteins, and how these materials affect or regulate various signaling pathways, etc. The in-depth and comprehensive research will help us to gain insight into the influence of the size, shape, chemical composition and surface properties of these nanomaterials on antitumor efficiency. Another existing problem is that some 2D nanomaterials are difficult to degrade in the physiological environment, so they may stay in the body for a long time and may cause damage. However, current research typically evaluates the efficacy of biological safety in just a short period. Therefore, it is essential to study the gene mutations, immune responses and changes in metabolic functions of organs such as the liver and kidney caused by the long-term retention of metal-containing 2D nanomaterials in the body to assess their biosecurity. In short, strict biomedical standards including long-term toxicity, cumulative effects, metabolism, biocompatibility and immunogenicity in animal models should be established systematically and comprehensively.
In summary, as a specified category of 2D nanomaterials, metal-containing 2D materials with large surface areas, unique chemical functions and intrinsic optical/sonic properties are promising nanoplatforms for biomedical applications. Metal-containing 2D nanomaterials present unprecedented opportunities and challenges for technological development in the fields of cancer therapy, drug delivery and diagnostics. We believe that this review will contribute to a better comprehension of metal-containing 2D nanomaterials-mediated cancer therapy and facilitate the future clinical application of nanomedicine.
Acknowledgments
This work was supported by National Key Research and Development Project (2022YFA0912500), National Natural Science Foundation of China (52203335, 82192865, 81970875 and 32301128), Shenzhen Science and Technology Innovation Committee (RCBS20221008093329063, ZDSYS201707281114196, KCXFZ20201221173413038, LCYSSQ20220823091403007), Sanming Project of Medicine in Shenzhen (SZSM201612031), Shenzhen High-level Hospital Construction Fund, Development and Reform Commission of Shenzhen Municipality, Postdoctoral Science Foundation of China (2022M722208), Basic and Applied Basic Research Foundation of Guangdong Province (2022A1515110803). We thank Prof. Guohui Nie from the First Affiliated Hospital of Shenzhen University for his contributions during the revision.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future. Chem Rec. 2017;17(8):775–802. doi:10.1002/tcr.201600121
2. Zhang YQ, Liu WL, Luo XJ, et al. Novel self-assembled multifunctional nanoprobes for second-near-infrared-fluorescence-image-guided breast cancer surgery and enhanced radiotherapy efficacy. Adv Sci. 2023;10(10):2205294. doi:10.1002/advs.202205294
3. Wang L, Yin S, Yang J, et al. Moiré superlattice structure in two-dimensional catalysts: synthesis, property and activity. Small. 2023;19:2300165. doi:10.1002/smll.202300165
4. Kovalska E, Wu B, Liao L, et al. Electrochemical decalcification-exfoliation of two-dimensional siligene, sixgey: material characterization and perspectives for lithium-ion storage. ACS Nano. 2023;17(12):11374–11383. doi:10.1021/acsnano.3c00658
5. Lv Q, Tan J, Wang Z, et al. Ultrafast charge transfer in mixed-dimensional WO3-x nanowire/WSe2 heterostructure heterostructures for attomolar-level molecular sensing. Nat Commun. 2023;14(1):2717. doi:10.1038/s41467-023-38198-x
6. Sahoo BB, Pandey VS, Dogonchi AS, et al. A state-of-art review on 2D material-boosted metal oxide nanoparticle electrodes: supercapacitor applications. J Energy Storage. 2023;65:107335. doi:10.1016/j.est.2023.107335
7. Yang G, Liu F, Zhao J, et al. MXenes-based nanomaterials for biosensing and biomedicine. Coord Chem Rev. 2023;479:215002. doi:10.1016/j.ccr.2022.215002
8. Lu C, Li R, Miao Z, Wang F, Zha Z. Emerging metallenes: synthesis strategies, biological effects and biomedical applications. Chem Soc Rev. 2023;52(8):2833–2865. doi:10.1039/d2cs00586g
9. Xiong Z, Zhang X, White JC, et al. Transcriptome analysis reveals the growth promotion mechanism of enteropathogenic Escherichia coli induced by black phosphorus nanosheets. ACS Nano. 2023;17(4):3574–3586. doi:10.1021/acsnano.2c09964
10. Ning C, Bai S, Wang J, et al. Review of photo- and electro-catalytic multi-metallic layered double hydroxides. Coord Chem Rev. 2023;480:215008. doi:10.1016/j.ccr.2022.215008
11. Xiao Y, Xiong C, Chen MM, Wang S, Fu L, Zhang X. Structure modulation of two-dimensional transition metal chalcogenides: recent advances in methodology, mechanism and applications. Chem Soc Rev. 2023;52(4):1215–1272. doi:10.1039/d1cs01016f
12. Tang J, Huang C, Liu Y, et al. Metal-organic framework nanoshell structures: preparation and biomedical applications. Coord Chem Rev. 2023;490:215211. doi:10.1016/j.ccr.2023.215211
13. He Y, Li D, Wu L, et al. Metal-organic frameworks for gene therapy and detection. Adv Funct Mater. 2023;33(12):2212277. doi:10.1002/adfm.202212277
14. Peng G, Fadeel B. Understanding the bidirectional interactions between two-dimensional materials, microorganisms, and the immune system. Adv Drug Deliv Rev. 2022;188:114422. doi:10.1016/j.addr.2022.114422
15. Moharramnejad M, Malekshah RE, Ehsani A, et al. A review of recent developments of metal–organic frameworks as combined biomedical platforms over the past decade. Adv Colloid Interf Sci. 2023;316:102908. doi:10.1016/j.cis.2023.102908
16. Duan X, Liu Z, Xie Z, et al. Emerging monoelemental 2D materials (Xenes) for biosensor applications. Nano Res. 2023;16(5):7030–7052. doi:10.1007/s12274-023-5418-3
17. Mahadev Patil P, Poddar N, Parihar N, et al. Optoresponsive Pheophorbide-Silver based organometallic nanomaterials for high efficacy multimodal theranostics in Melanoma. Chem Eng J. 2023;470:144110. doi:10.1016/j.cej.2023.144110
18. Jiang J, Feng W, Wen Y, et al. Tuning 2D magnetism in cobalt monoxide nanosheets via in situ nickel-doping. Adv Mater. 2023;35(22):2301668. doi:10.1002/adma.202301668
19. Yang L, Tian B, Xie Y, et al. Oxygen-vacancy-rich piezoelectric BiO2-x nanosheets for augmented piezocatalytic, sonothermal, and enzymatic therapies. Adv Mater. 2023;35(29):e2300648. doi:10.1002/adma.202300648
20. Xie Z, Zhang B, Ge Y, et al. Chemistry, functionalization, and applications of recent monoelemental two-dimensional materials and their heterostructures. Chem Rev. 2022;122(1):1127–1207. doi:10.1021/acs.chemrev.1c00165
21. Yang Y, Hu T, Bian Y, et al. Coupling probiotics with 2D CoCuMo‐LDH nanosheets as a tumor‐microenvironment‐responsive platform for precise NIR‐II photodynamic therapy. Adv Mater. 2023;35(23):2211205. doi:10.1002/adma.202211205
22. Truong Hoang Q, Huynh KA, Nguyen Cao TG, et al. Piezocatalytic 2D WS2 nanosheets for ultrasound-triggered and mitochondria-targeted piezodynamic cancer therapy synergized with energy metabolism-targeted chemotherapy. Adv Mater. 2023;35(18):2300437. doi:10.1002/adma.202300437
23. Fan H, Guo Z. Tumor microenvironment-responsive manganese-based nanomaterials for cancer treatment. Coord Chem Rev. 2023;480:215027. doi:10.1016/j.ccr.2023.215027
24. Zhao Y, Wang S-B, Chen A-Z, Kankala RK. Nanoarchitectured assembly and surface of two-dimensional (2D) transition metal dichalcogenides (TMDCs) for cancer therapy. Coord Chem Rev. 2022;472:214765. doi:10.1016/j.ccr.2022.214765
25. Xie Z, Fan T, An J, et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem Soc Rev. 2020;49(22):8065–8087. doi:10.1039/d0cs00215a
26. Li B, Luo Y, Zheng Y, Liu X, Tan L, Wu S. Two-dimensional antibacterial materials. Prog Mater Sci. 2022;130:100976. doi:10.1016/j.pmatsci.2022.100976
27. Du R, Wang Y, Cheng M, et al. Two-dimensional multiferroic material of metallic p-doped SnSe. Nat Commun. 2022;13(1):6130. doi:10.1038/s41467-022-33917-2
28. Zhang B, Fan T, Xie N, et al. Versatile applications of metal single-atom @ 2D material nanoplatforms. Adv Sci. 2019;6(21):1901787. doi:10.1002/advs.201901787
29. Wu Q, Liao J, Yang H. Recent advances in kaolinite nanoclay as drug carrier for bioapplications: a review. Adv Sci. 2023;10(25):2300672. doi:10.1002/advs.202300672
30. Zhu S, Liu Y, Gu Z, et al. Research trends in biomedical applications of two-dimensional nanomaterials over the last decade – a bibliometric analysis. Adv Drug Deliv Rev. 2022;188:114420. doi:10.1016/j.addr.2022.114420
31. Wang W, Chen C, Ying Y, et al. Smart PdH@MnO2 Yolk–Shell nanostructures for spatiotemporally synchronous targeted hydrogen delivery and oxygen-elevated phototherapy of melanoma. ACS Nano. 2022;16(4):5597–5614. doi:10.1021/acsnano.1c10450
32. Carrasco JA, Congost-Escoin P, Assebban M, et al. Antimonene: a tuneable post-graphene material for advanced applications in optoelectronics, catalysis, energy and biomedicine. Chem Soc Rev. 2023;52(4):1288–1330. doi:10.1039/d2cs00570k
33. Lin Y-C, Torsi R, Younas R, et al. Recent advances in 2D material theory, synthesis, properties, and applications. ACS Nano. 2023;17(11):9694–9747. doi:10.1021/acsnano.2c12759
34. Sakthivel R, Keerthi M, Chung R-J, He J-H. Heterostructures of 2D materials and their applications in biosensing. Prog Mater Sci. 2023;132:101024. doi:10.1016/j.pmatsci.2022.101024
35. Fan T, Yan L, He S, et al. Biodistribution, degradability and clearance of 2D materials for their biomedical applications. Chem Soc Rev. 2022;51(18):7732–7751. doi:10.1039/d1cs01070k
36. Qiu M, Wang D, Huang H, et al. A regioselectively Oxidized 2D Bi/BiOx lateral nano-heterostructure for hypoxic photodynamic therapy. Adv Mater. 2021;33(49):2102562. doi:10.1002/adma.202102562
37. Yang D, Yang G, Yang P, et al. Assembly of au plasmonic photothermal agent and iron oxide nanoparticles on ultrathin black phosphorus for targeted photothermal and photodynamic cancer therapy. Adv Funct Mater. 2017;27(18). doi:10.1002/adfm.201700371
38. Chang M, Dai X, Dong C, et al. Two-dimensional persistent luminescence “optical battery” for autophagy inhibition-augmented photodynamic tumor nanotherapy. Nano Today. 2022:42. doi:10.1016/j.nantod.2021.101362
39. Shen W, Hu T, Liu X, et al. Defect engineering of layered double hydroxide nanosheets as inorganic photosensitizers for NIR-III photodynamic cancer therapy. Nat Commun. 2022;13(1):3384. doi:10.1038/s41467-022-31106-9
40. Hu T, Shen W, Meng F, et al. Boosting the sonodynamic cancer therapy performance of 2D layered double hydroxide nanosheet-based sonosensitizers via crystalline-to-amorphous phase transformation. Adv Mater. 2023;35(17):e2209692. doi:10.1002/adma.202209692
41. Liu SY, Xu Y, Yang H, et al. Ultrathin 2D Copper(I) 1,2,4-triazolate coordination polymer nanosheets for efficient and selective gene silencing and photodynamic therapy. Adv Mater. 2021;33(18):2100849. doi:10.1002/adma.202100849
42. Ma W, Zhang H, Li S, et al. A multifunctional nanoplatform based on Fenton-like and Russell reactions of cu, mn bimetallic ions synergistically enhanced ros stress for improved chemodynamic therapy. ACS Biomater Sci Eng. 2022;8(3):1354–1366. doi:10.1021/acsbiomaterials.1c01605
43. Wang C, Xue F, Wang M, An L, Wu D, Tian Q. 2D Cu-Bipyridine MOF nanosheet as an agent for colon cancer therapy: a three-in-one approach for enhancing chemodynamic therapy. ACS Appl Mater Interf. 2022;14(34):38604–38616. doi:10.1021/acsami.2c11999
44. Chen Z, Wu Y, Yao Z, et al. 2D copper (II) metalated metal-organic framework nanocomplexes for dual-enhanced photodynamic therapy and amplified antitumor immunity. ACS Appl Mater Interf. 2022;14(39):44199–44210. doi:10.1021/acsami.2c12990
45. Kang Y, Mao Z, Wang Y, et al. Design of a two-dimensional interplanar heterojunction for catalytic cancer therapy. Nat Commun. 2022;13(1):2425. doi:10.1038/s41467-022-30166-1
46. Ren X, Liu W, Zhou H, et al. Biodegradable 2D GeP nanosheets with high photothermal conversion efficiency for multimodal cancer theranostics. Chem Eng J. 2022:431. doi:10.1016/j.cej.2021.134176
47. Ge M, Guo H, Zong M, et al. Bandgap-engineered germanene nanosheets as an efficient photodynamic agent for cancer therapy. Angew Chem Int Ed Engl. 2023:62. doi:10.1002/anie.202215795
48. Zhao J, Wu H, Zhao J, et al. 2D LDH-MoS2 clay nanosheets: synthesis, catalase-mimic capacity, and imaging-guided tumor photo-therapy. J Nanobiotechnology. 2021;19(1):36. doi:10.1186/s12951-020-00763-7
49. Ji X, Ge L, Liu C, et al. Capturing functional two-dimensional nanosheets from sandwich-structure vermiculite for cancer theranostics. Nat Commun. 2021;12(1):1124. doi:10.1038/s41467-021-21436-5
50. Xu Y, Li C, Wu X, et al. Sheet-like 2D Manganese (IV) Complex with High Photothermal Conversion Efficiency. J Am Chem Soc. 2022;144(41):18834–18843. doi:10.1021/jacs.2c04734
51. Hu T, Xue B, Meng F, et al. Preparation of 2D Polyaniline/MoO3-x superlattice nanosheets via intercalation-induced morphological transformation for efficient chemodynamic therapy. Adv Healthc Mater. 2023:12. doi:10.1002/adhm.202202911
52. Qiu M, Duo Y, Liang W, et al. Nanopoxia: targeting cancer hypoxia by antimonene‐based nanoplatform for precision cancer therapy. Adv Funct Mater. 2021;31(42). doi:10.1002/adfm.202104607
53. Wu H, Wu F, Zhou T, et al. Activatable autophagy inhibition-primed chemodynamic therapy via targeted sandwich-like two-dimensional nanosheets. Chem Eng J. 2022:431. doi:10.1016/j.cej.2021.133470
54. Lin G, Nash GT, Luo T, et al. Two-dimensional nanosonosensitizers facilitate energy transfer to enhance sonodynamic therapy. Adv Mater. 2023;35:2212069. doi:10.1002/adma.202212069
55. Liu G, Zou J, Tang Q, et al. Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl Mater Interf. 2017;9(46):40077–40086. doi:10.1021/acsami.7b13421
56. Ma L, Huang H, Feng W, et al. 2D Catalytic, Chemodynamic, and Ferroptotic Vermiculite Nanomedicine. Adv Funct Mater. 2022;32(51). doi:10.1002/adfm.202208220
57. Nie Y, Chen W, Kang Y, et al. Two-dimensional porous vermiculite-based nanocatalysts for synergetic catalytic therapy. Biomaterials. 2023;295:122031. doi:10.1016/j.biomaterials.2023.122031
58. Hang L, Zhang T, Wen H, et al. Controllable photodynamic performance via an acidic microenvironment based on two-dimensional metal-organic frameworks for photodynamic therapy. Nano Res. 2020;14(3):660–666. doi:10.1007/s12274-020-3093-1
59. Gao R, Mei X, Yan D, et al. Nano-photosensitizer based on layered double hydroxide and isophthalic acid for singlet oxygenation and photodynamic therapy. Nat Commun. 2018;9(1):2798. doi:10.1038/s41467-018-05223-3
60. Chen Y, Fan Z, Zhang Z, et al. Two-dimensional metal nanomaterials: synthesis, properties, and applications. Chem Rev. 2018;118(13):6409–6455. doi:10.1021/acs.chemrev.7b00727
61. Xing L, Jin Y, Weng Y, et al. Top-down synthetic strategies toward single atoms on the rise. Matter. 2022;5(3):788–807. doi:10.1016/j.matt.2021.12.015
62. Lin S, Yang M, Chen J, et al. Two-Dimensional FePS3 nanosheets as an integrative sonosensitizer/nanocatalyst for efficient nanodynamic tumor therapy. Small. 2023;19(8):2204992. doi:10.1002/smll.202204992
63. Huang H, Feng W, Chen Y. Two-dimensional biomaterials: material science, biological effect and biomedical engineering applications. Chem Soc Rev. 2021;50(20):11381–11485. doi:10.1039/d0cs01138j
64. Islam MA, Serles P, Kumral B, et al. Exfoliation mechanisms of 2D materials and their applications. Appl Phys Rev. 2022;9(4). doi:10.1063/5.0090717
65. Zheng W, Lee LYS. Beyond sonication: advanced exfoliation methods for scalable production of 2D materials. Matter. 2022;5(2):515–545. doi:10.1016/j.matt.2021.12.010
66. Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:(5696):666–9. doi:10.1126/science.1102896
67. Hernandez Y, Nicolosi V, Lotya M, et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol. 2008;3(9):563–568. doi:10.1038/nnano.2008.215
68. Liu L, Shen Z, Yi M, Zhang X, Ma S. A green, rapid and size-controlled production of high-quality graphene sheets by hydrodynamic forces. RSC Adv. 2014;4(69):36464–36470. doi:10.1039/C4RA05635C
69. Yi M, Shen Z. Kitchen blender for producing high-quality few-layer graphene. Carbon. 2014;78:622–626. doi:10.1016/j.carbon.2014.07.035
70. Stankovich S, Dikin DA, Piner RD, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45(7):1558–1565. doi:10.1016/j.carbon.2007.02.034
71. Naguib M, Mashtalir O, Carle J, et al. Two-dimensional transition metal carbides. ACS Nano. 2012;6(2):1322–1331. doi:10.1021/nn204153h
72. Sun Z, Liao T, Dou Y, et al. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat Commun. 2014;5:3813. doi:10.1038/ncomms4813
73. Shi W, Song S, Zhang H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem Soc Rev. 2013;42(13):5714–5743. doi:10.1039/c3cs60012b
74. Duan H, Yan N, Yu R, et al. Ultrathin rhodium nanosheets. Nat Commun. 2014;5:3093. doi:10.1038/ncomms4093
75. Tan C, Zhang H. Wet-chemical synthesis and applications of non-layer structured two-dimensional nanomaterials. Nat Commun. 2015;6:7873. doi:10.1038/ncomms8873
76. Badr HO, Montazeri K, El-Melegy T, et al. Scalable, inexpensive, one-pot, facile synthesis of crystalline two-dimensional birnessite flakes. Matter. 2022;5(7):2365–2381. doi:10.1016/j.matt.2022.05.038
77. Kim J-E, J-H O, Kotal M, et al. Self-assembly and morphological control of three-dimensional macroporous architectures built of two-dimensional materials. Nano Today. 2017;14:100–123. doi:10.1016/j.nantod.2017.04.008
78. Wu Z, Li Y, Liu J, et al. Colloidal self-assembly of catalytic copper nanoclusters into ultrathin ribbons. Angew Chem Int Ed. 2014;53(45):12196–12200. doi:10.1002/anie.201407390
79. Wu Z, Liu J, Li Y, et al. Self-Assembly of Nanoclusters into Mono-, Few-, and Multilayered Sheets via Dipole-Induced Asymmetric van der Waals Attraction. ACS Nano. 2015;9(6):6315–6323. doi:10.1021/acsnano.5b01823
80. Cheng W, He J, Yao T, et al. Half-unit-cell alpha-Fe2O3 semiconductor nanosheets with intrinsic and robust ferromagnetism. J Am Chem Soc. 2014;136(29):10393–10398. doi:10.1021/ja504088n
81. Yang Y, Jia L, Wang D, Zhou J. Advanced Strategies in Synthesis of Two-Dimensional Materials with Different Compositions and Phases. Small Methods. 2023;7(4):2201585. doi:10.1002/smtd.202201585
82. Bauer T, Zheng Z, Renn A, et al. Synthesis of free-standing, monolayered organometallic sheets at the air/water interface. Angew Chem Int Ed. 2011;50(34):7879–7884. doi:10.1002/anie.201100669
83. Xu L, Zhou X, Yu Y, et al. Surface-confined crystalline two-dimensional covalent organic frameworks via on-surface Schiff-base coupling. ACS Nano. 2013;7(9):8066–8073. doi:10.1021/nn403328h
84. Hong YL, Liu Z, Wang L, et al. Chemical vapor deposition of layered two-dimensional MoSi2N4 materials. Science. 2020;369(6504):670–674. doi:10.1126/science.abb7023
85. Xu X, Zhong T, Zuo N, et al. High-TC two-dimensional ferroelectric CuCrS2 grown via chemical vapor deposition. ACS Nano. 2022;16(5):8141–8149. doi:10.1021/acsnano.2c01470
86. Rubio-Giménez V, Arnauts G, Wang M, et al. Chemical vapor deposition and high-resolution patterning of a highly conductive two-dimensional coordination polymer film. J Am Chem Soc. 2023;145(1):152–159. doi:10.1021/jacs.2c09007
87. Chen C, Wu C, Yu J, et al. Photodynamic-based combinatorial cancer therapy strategies: tuning the properties of nanoplatform according to oncotherapy needs. Coord Chem Rev. 2022;461:214495. doi:10.1016/j.ccr.2022.214495
88. R-L G, Yan P-N, Liu Y, et al. Recent advances and clinical potential of near infrared photothermal conversion materials for photothermal hepatocellular carcinoma therapy. Adv Funct Mater. 2023:2301138. doi:10.1002/adfm.202301138
89. Ouyang J, Zhang L, Li L, et al. Cryogenic exfoliation of 2D stanene nanosheets for cancer theranostics. Nanomicro Lett. 2021;13(1):90. doi:10.1007/s40820-021-00619-1
90. Yin H, Zhou B, Zhao C, et al. 2D core/shell‐structured mesoporous silicene@Silica for targeted and synergistic NIR‐II‐induced photothermal ablation and hypoxia‐activated chemotherapy of tumors. Adv Funct Mater. 2021;31(24). doi:10.1002/adfm.202102043
91. Zhou G, Li M. Biodegradable copper telluride nanosheets for redox-homeostasis breaking-assisted chemodynamic cancer therapy boosted by mild-photothermal effect. Chem Eng J. 2022;450:138348. doi:10.1016/j.cej.2022.138348
92. Wang X, Wang X, Yue Q, et al. Liquid exfoliation of TiN nanodots as novel sonosensitizers for photothermal-enhanced sonodynamic therapy against cancer. Nano Today. 2021;39:101170. doi:10.1016/j.nantod.2021.101170
93. Yin H, Sun L, Pu Y, et al. Ultrasound-Controlled CRISPR/Cas9 system augments sonodynamic therapy of hepatocellular carcinoma. ACS Cent Sci. 2021;7(12):2049–2062. doi:10.1021/acscentsci.1c01143
94. Dong Y, Dong S, Liu B, et al. 2D piezoelectric Bi2 MoO6 nanoribbons for GSH-enhanced sonodynamic therapy. Adv Mater. 2021;33(51). doi:10.1002/adma.202106838
95. Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130(33):10876–10877. doi:10.1021/ja803688x
96. Zhang Y, Fang J, Ye S, et al. A hydrogen sulphide-responsive and depleting nanoplatform for cancer photodynamic therapy. Nat Commun. 2022;13(1):1685. doi:10.1038/s41467-022-29284-7
97. Lu H, Zada S, Tang S, et al. Artificial photoactive chlorophyll conjugated vanadium carbide nanostructure for synergistic photothermal/photodynamic therapy of cancer. J Nanobiotechnology. 2022;20(1):121. doi:10.1186/s12951-022-01331-x
98. Zhang Q, Guo Q, Chen Q, et al. Highly Efficient 2D NIR-II Photothermal Agent with Fenton Catalytic Activity for Cancer Synergistic Photothermal-Chemodynamic Therapy. Adv Sci. 2020;7(7):1902576. doi:10.1002/advs.201902576
99. Pan Y, Zhu Y, Xu C, et al. Biomimetic yolk–shell nanocatalysts for activatable dual-modal-image-guided triple-augmented chemodynamic therapy of cancer. ACS Nano. 2022;16(11):19038–19052. doi:10.1021/acsnano.2c08077
100. Murali A, Lokhande G, Deo KA, et al. Emerging 2D nanomaterials for biomedical applications. Mater Today. 2021;50:276–302. doi:10.1016/j.mattod.2021.04.020
101. Fang X, Wu X, Li Z, et al. Biomimetic Anti-PD-1 Peptide-Loaded 2D FePSe3 nanosheets for efficient photothermal and enhanced immune therapy with multimodal MR/PA/thermal imaging. Adv Sci. 2021;8(2):2003041. doi:10.1002/advs.202003041
102. Duan H, Chang M, Lin H, et al. Two-dimensional silicene photodynamic tumor-targeting nanomedicine. Mater Today Bio. 2022;16:100393. doi:10.1016/j.mtbio.2022.100393
103. Liu Y, Li X, Shi Y, et al. Two-dimensional intermetallic PtBi/Pt core/shell nanoplates overcome tumor hypoxia for enhanced cancer therapy. Nanoscale. 2021;13(33):14245–14253. doi:10.1039/d1nr02561a
104. Tang L, Li J, Pan T, et al. Versatile carbon nanoplatforms for cancer treatment and diagnosis: strategies, applications and future perspectives. Theranostics. 2022;12(5):2290–2321. doi:10.7150/thno.69628
105. Ni N, Zhang X, Ma Y, et al. Biodegradable two-dimensional nanomaterials for cancer theranostics. Coord Chem Rev. 2022;458:214415. doi:10.1016/j.ccr.2022.214415
106. Liu Y, Li J, Chen M, et al. Palladium-based nanomaterials for cancer imaging and therapy. Theranostics. 2020;10(22):10057–10074. doi:10.7150/thno.45990
107. He E, Qiu R, Cao X, et al. Elucidating toxicodynamic differences at the molecular scale between ZnO Nanoparticles and ZnCl 2 in enchytraeus crypticus via nontargeted metabolomics. Environ Sci Technol. 2020;54(6):3487–3498. doi:10.1021/acs.est.0c00663
108. Liao C, Jin Y, Li Y, et al. Interactions of zinc oxide nanostructures with mammalian cells: cytotoxicity and photocatalytic toxicity. Int J Mol Sci. 2020;21(17):6305. doi:10.3390/ijms21176305
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
