Back to Journals » International Journal of Nanomedicine » Volume 19
Recent Advancements and Unexplored Biomedical Applications of Green Synthesized Ag and Au Nanoparticles: A Review
Authors Ahmad S , Ahmad S , Ali S, Esa M , Khan A, Yan H
Received 7 December 2023
Accepted for publication 12 March 2024
Published 3 April 2024 Volume 2024:19 Pages 3187—3215
DOI https://doi.org/10.2147/IJN.S453775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Shahbaz Ahmad,1 Shujaat Ahmad,2 Shujat Ali,3 Muhammad Esa,2 Ajmal Khan,1 Hai Yan1
1School of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing, 100083, People’s Republic of China; 2Department of Pharmacy, Shaheed Benazir Bhutto University, Sheringal Dir Upper Khyber Pakhtunkhwa, Pakistan; 3College of Electrical and Electronic Engineering, Wenzhou University, Wenzhou, 325035, People’s Republic of China
Correspondence: Hai Yan, School of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing, 100083, People’s Republic of China, Email [email protected] Shujaat Ahmad, Department of Pharmacy, Shaheed Benazir Bhutto University, Sheringal Dir Upper Khyber Pakhtunkhwa, Pakistan, Email [email protected]
Abstract: Green synthesis of silver (Ag) and gold (Au) nanoparticles (NPs) has acquired huge popularity owing to their potential applications in various fields. A large number of research articles exist in the literature describing the green synthesis of Ag and Au NPs for biomedical applications. However, these findings are scattered, making it time-consuming for researchers to locate promising advancements in Ag and Au NPs synthesis and their unexplored biomedical applications. Unlike other review articles, this systematic study not only highlights recent advancements in the green synthesis of Ag and Au NPs but also explores their potential unexplored biomedical applications. The article discusses the various synthesis approaches for the green synthesis of Ag and Au NPs highlighting the emerging developments and novel strategies. Then, the article reviews the important biomedical applications of green synthesized Ag and Au NPs by critically evaluating the expected advantages. To expose future research direction in the field, the article describes the unexplored biomedical applications of the NPs. Finally, the articles discuss the challenges and limitations in the green synthesis of Ag and Au NPs and their biomedical applications. This article will serve as a valuable reference for researchers, working on green synthesis of Ag and Au NPs for biomedical applications.
Keywords: green synthesis of nanoparticles, silver and gold nanoparticles, environmental-friendly, biomedical applications, unexplored potentials
Graphical Abstract:

Introduction
Silver (Ag) and gold (Au) nanoparticles (NPs) have attracted the most interest among the many types of NPs because of their outstanding qualities and diverse applications in biomedicine.1 Particularly, the green synthesis of Ag and Au NPs is a hot area of research.2 This approach uses natural resources including plants, microbes, and other biological components for NPs synthesis.3 Hence, compared to traditional methods, which frequently rely on potentially hazardous components and mechanical processes, this approach has many advantages. Overall, the green synthesis method offers a controlled, biocompatible, and sustainable approach to synthesize NPs with an extensive list of applications.4 In the literature, different synthesis approaches are followed for the green synthesis of NPs such as plant-mediated synthesis, microbial synthesis, bio-inspired synthesis, green chemistry approach; microwave-assisted synthesis and ultrasound-assisted synthesis approach (Table 1).
 |
Table 1 Various Green Synthesis Approaches Employed for the Production of Ag and Au NPs |
The green synthesis approach for Ag and Au NPs synthesis offers great promise for biological applications. These NPs exhibit unique physicochemical properties at the nanoscale, including high surface-to-volume ratios, improved reactivity, and adaptable optical characteristics. They are suitable for a range of biological applications, such as therapies, drug delivery, biosensing, and diagnostics as a result of their distinctive features.32 Promising novel possibilities for a range of biological applications have been made possible by the widespread use of eco-friendly procedures for the green synthesis of Ag and Au NPs.33 In addition, due to their exceptional qualities and capabilities, they serve as valuable tools for the purpose of diagnosis.34 Similarly, these NPs can also be functionalized with specific biomolecules, like antibodies or DNA probes, enabling them to more specifically bind to pathogens or disease markers, making it easier to recognize them.35 Furthermore, Ag and Au NPs developed by means of green synthesis have antibacterial and anticancer characteristics, making them interesting candidates for therapeutic applications.36 Ag NPs have been the focus of extensive research due to their remarkable antibacterial effects against a number of pathogens, including bacteria, viruses, and fungi.37 On the other hand, Au NPs have drawn interest due to their potential in photothermal therapy and targeted drug delivery.38 Due to their special characteristics, such as their small size and large surface area, green synthesized Ag and Au NPs have been found to offer potential useful tools in the field of drug delivery.39 Additionally, they can also be utilized as effective biosensors because of their excellent sensitivity, selectivity as well as their optical characteristics.40 Lastly, when incorporated into tissue engineering scaffolds or hydrogels, these NPs have unique advantages because they preserve the material’s structure and can change cellular behavior by encouraging cell adhesion, proliferation, and differentiation.41
In recent years, Ag and Au NPs have received a great deal of attention for their prospective applications in a variety of biomedical fields. Their individual applications in therapy, tissue engineering, regenerative medicine, and diagnostics have all been explored in various studies. The synthesis and characterization of Ag and Au NPs were the main focus of many earlier studies, but their potential use in biomedical fields was not fully explored. Furthermore, despite individual studies that have provided insightful information, there remains a lack of comprehensive review articles that compile existing literature, comprehend limitations, and provide recommendations for future research despite particular studies that have offered valuable information.42
This review article represents a significant contribution into the field. The article offers an up-to-date and comprehensive review of both Ag and Au NPs potential uses across multiple biomedical fields. It not only evaluates recent research but also indicates unexplored areas for further research, providing scientists and researchers an outline to follow. The article also discusses the classical methods of green synthesis, recent advances, and novel strategies for the green synthesis of Ag and Au NPs, advantages and disadvantages, along with unexplored biomedical applications and future directions.
The research on green synthesis of Ag and Au NPs is increasing day by day due to their unexpected properties and applications. The number of articles published on Ag and AuNPs are increasing continuously since 2010–2023 (Figures 1a and b). Moreover, the number of articles published annually on Ag NPs is more than that of Au NPs which reflects that research on Ag NPs are in more progress than Au NPs.
Methods for Green Synthesis of Ag and Au NPs
Ag and Au NPs are synthesized using environmentally friendly methods to improve environmental sustainability, reduce reliance on hazardous chemicals, and minimize energy consumption. Following are a few well-known green synthesis methods for Ag and Au NPs:
Plant Extracts Mediated Synthesis of Ag and Au NPs
Plant extract-mediated synthesis is a viable and sustainable approach for the green synthesis of Ag and Au NPs. In this technique, NPs are synthesized from a variety of plant components, including stems, leaves, seeds, and flowers, using bioactive chemicals as reducing and stabilizing agents. The utilization of plant extracts has several advantages, such as their easy availability, abundance, as well as the ability to modify the properties of NPs.43 The potential for nanoparticle production in stems, in particular the bark or interior tissues, has been studied. It has been demonstrated that many phytochemicals found within these plant components, especially tannins, alkaloids, and phenolic compounds, which substantially reduce metal ions and promote the development of well-defined Ag and Au NPs.44 Leaves are another very often used plant components for the synthesis of Ag and Au NPs. They include large amounts of bioactive compounds such polyphenols, terpenoids, and flavonoids, all of which have strong reducing properties. These compounds are necessary for reducing metal ions and controlling the synthesis of Ag and Au NPs.45
Similarly, potential has also been identified with the utilization of different plant seeds for green synthesis of NPs. In seed extracts, proteins, enzymes, and secondary metabolites act as reducing and capping agents to produce Ag and Au NPs. Ag and Au NPs with regulated sizes and shapes have been produced using seed extracts.33 Another source for production of Ag and Au NPs using plant extracts are flowers. Among the bioactive components included in samples extracted from flower petals and reproductive tissues that aid in reducing metal ions and stabilizing NPs are anthocyanins, flavonoids, and volatile oils. Ag and Au NPs with various morphologies and optical properties have been produced using flower extracts.46
Enzymes Mediated Synthesis of Ag and Au NPs
Enzyme-mediated synthesis is a highly effective and ecologically friendly method for synthesizing Ag and Au NPs. Enzymes can operate as biocatalysts in the reduction of metal ions to produce NPs when the reaction conditions are favorable.47 Because of their unique catalytic capabilities, enzymes can preferentially attach to metal ions and facilitate their reduction, resulting in the generation of NPs. Reductases, oxidases, and dehydrogenases are frequently used as enzymes in this process due to their ability to transport electrons and speed up the reduction of metal ions through the utilization of co-factors such Nicotinamide adenine dinucleotide (NADH) or Nicotinamide adenine dinucleotide phosphate (NADPH).48 The ability of enzyme-mediated synthesis to function at mild conditions, such as neutral pH and room temperature, is one of its advantages. Under typical lab conditions, this technique reduces the amount of energy required and provides simple synthesis. The mild reaction conditions also maintain the enzymes’ stability and activity, allowing for effective Ag and Au NPs production.49
Other benefits of enzyme-mediated synthesis include the exceptional selectivity of enzymes, which allows for exact control of NPs size, shape, and surface properties. Several studies in the literature support this mechanism. For instance, research by Wilner et al50 demonstrated the role of enzymes in templating the synthesis of AuNPs with defined shapes. Additionally, Schneidewind et al, work explored the enzymatic control of AgNPs size through a specific enzymatic reduction process. These findings collectively highlight the key role of enzymes in providing selectivity and precision in the synthesis of NPs, supporting the mentioned mechanism.51 Due to their biocompatibility and natural availability, enzymes are suitable for use in biological applications. Lack of hazardous compounds or residues during the synthesis process enhances the biocompatibility of the resulting NPs.52 The introduction of additional activities to the NPs is made possible by the adaptability of enzyme-mediated synthesis using various biomolecules and substrates. Enzymes can be developed or altered to include specific peptide sequences or ligands with targeting capabilities or increased stability in biological environments.53
Bio-Waste Materials Mediated Synthesis of Ag and Au NPs
Agricultural waste items such fruit peels, straw, husks, and stalks have attracted a lot of interest due to their affordability and sustainability as a source of bioactive compounds for the development of Ag and Au NPs.54 Proteins, phenolic compounds, polysaccharides, and organic acids that serve as reducing and stabilizing agents are just a few of the biomolecules found in these waste products. When biomolecules and metal ions interact, the metal ions are reduced and NPs are created.47,55 NPs are produced using the bioactive compounds that have been extracted using extraction methods from the waste materials. The affordability, cost-effectiveness, and ability to modify the properties of NPs are only a few benefits of this synthesis technique.56
Microorganisms Mediated Synthesis of Ag and Au NPs
Ag and Au NPs production using microorganism-mediated synthesis has been demonstrated to be an ecologically friendly process. This method makes use of microorganisms such bacteria, fungus, and yeast that effectively reduce metal ions and promote the creation of NPs.57,58 Since bacteria contain proteins or enzymes like nitrate reductase, which act as reducing agents and stabilise the NPs, they offer benefits such ease of cultivation, rapid growth, and the ability to be genetically modified to boost the production of NPs.59 Fungi, including yeasts and filamentous fungi, produce extracellular enzymes like reductases and cytochrome P450 enzymes, which effectively reduce metal ions and initiate nanoparticle synthesis. Fungi can tolerate a wide range of pH and temperature conditions and are capable of producing NPs in aqueous environments.60 Various yeast species, such as Saccharomyces cerevisiae, Candida species, and Pichia pastoris, possess reducing enzymes and metabolites that contribute in metal ion reduction ultimately resulting in the synthesis of NPs.61 High yields, scalability, and the possibility of intracellular synthesis within yeast cells are only a few advantages of yeast-mediated synthesis.62 The yield of Ag and Au NPs synthesis by microbial-mediated green synthesis can vary commonly based on multiple factors, including the specific microorganisms used, reaction conditions, and concentrations of reagents. The yield is often expressed in terms of the concentration (ppm) or mass of synthesized NPs obtained per unit volume or weight of the reaction mixture. For instance, the concentration of Ag and Au NPs was determined by Balakumaran, M. D.et al through ICP-AES analysis. Interestingly, A. terreus produced about 214 ppm of AgNPs/litre of culture filtrate when 01 mM silver nitrate was challenged and for AuNPs, it was 196 ppm.63 Lastly, it is important to keep in mind the biosafety factors, which include the choice of non-pathogenic microorganisms, assessment of metal ion sources for possible impurities, and thorough toxicity studies covering cytotoxicity, allergenicity, and long-term impacts.64
Mechanisms of Green Synthesis of Ag and Au NPs
Biomolecules-Mediated Reduction
Biomolecules from a variety of natural sources, such as plants, microorganisms, and biowaste products, play an active role in this process by acting as reducing agents. Proteins, enzymes, polysaccharides, phenolic compounds, and organic acids are examples of biomolecules that can reduce metal ions and then generate NPs as a result of their interaction. The precise biomolecules used in this procedure may vary depending on the synthesis technique employed.65
Bio-Reduction by Microorganisms
Microorganisms including bacteria, fungi, and yeast hold great promise for speeding nanoparticle creation because they include enzymes or proteins with inherent metal ion reduction capacities.66 For instance, nitrate reductase may be produced by bacteria, whereas cytochrome P450 enzymes and reductases are produced by fungi. The ability of these microorganisms to reduce helps with the transformation of metal ions into NPs.67
Phytochemical-Mediated Synthesis
During the synthesis of NPs, phytochemicals from plants, such as flavonoids, polyphenols, terpenoids, and alkaloids, serve as both reducing and stabilizing agents. These phytochemicals must first be extracted using the appropriate solvents in order to create plant extracts or infusions.33 The plant extracts are then mixed with metal ions, which causes their reduction and the creation of NPs68 (Figure 2).
Advantages and Disadvantages of Green Synthesis of Ag and Au NPs
Advantages of Green Synthesis of Ag and Au NPs
The biomedical application of green-synthesized Ag and Au NPs can be attributed to a combination of the inherent properties (antimicrobial properties and biocompatibility) of these materials and the specific characteristics they acquire through the green synthesis process ie better control over size and shape, ease of functionalization, and minimum toxicity.
Polyvinylpyrrolidone which was commonly used as a capping or stabilizing agent in conventional synthesis of NPs. However, it can be non-biodegradable and may have potential long-term environmental impacts such as genotoxic effects as reported by Nymark, Penny et al.69 Cetyltrimethylammonium Bromide is used as a surfactant in some conventional synthesis methods. It can be associated with toxicity and environmental concerns to marine life.70 The green synthesis of Ag and Au NPs possesses a number of advantages over conventional approaches. First of all, it is an environmentally friendly approach that minimizes the use of hazardous chemicals and decreases the effects of NPs production on the environment.71 Secondly, by using abundant and renewable natural resources like plants, microorganisms, and bio-waste materials as ingredients for the production of NPs, encourages sustainability and renewability.72 Furthermore, considering that they use low-cost starting materials as well as simple reaction conditions, green synthesis approaches are inexpensive.73 Additionally, they work at mild reaction conditions, such neutral pH and ambient temperature, which saves energy while keeping laboratory procedures simple.74 With the use of green synthesis, NPs may possess their size, shape, and morphology precisely controlled, providing specific features that improve their performance in certain applications.75 Lastly, Ag and Au NPs synthesized through green approaches frequently exhibit excellent biocompatibility, making them suitable for various biomedical applications including drug delivery, imaging, and biosensing.76
Disadvantages of Green Synthesis of Ag and Au NPs
Green synthesis techniques may yield fewer NPs than conventional methods, which limits their potential for large-scale manufacturing.77 Green synthesis techniques usually require longer reaction times due to the relatively slow reduction process, which may delay the synthesis process.78 Additionally, as they include complex reaction processes and a number of factors that must be carefully optimized in order to produce the necessary nanoparticle characteristics, green synthesis approaches require substantial testing and knowledge.57 Moreover, it can be technically challenging and time-consuming to extract and purify bioactive compounds from natural sources, such as plants or microbes, in order to synthesize high-purity NPs. While green synthesis techniques enable precise control of some aspects, such as size, shape, and surface chemistry, it can be more challenging to achieve so for other features, such as crystallinity.79
Emerging Development and Novel Strategies for the Synthesis of Ag and Au NPs
In order to improve the effectiveness, scalability, and functionality of the synthesis of Ag and Au NPs, ongoing research and novel approaches are continuously being developed (Table 2).
 |
Table 2 Emerging Strategies for the Synthesis of Ag and Au NPs |
Microwave-Assisted Synthesis
Microwave irradiation is now able to be used to quickly and very efficiently produce NPs. It enables more rapid reaction kinetics, reduces the necessary reaction time, and improves the yield of NPs synthesis. Additionally, the shape and size of the resulting NPs may be precisely controlled using this technique, making it very attractive for large-scale production.80 Precursor ions, a reducing agent, and a stabilizing agent are first combined in a reaction mixture to produce Ag and Au NPs using a microwave.91 Microwave heating enables metal ions to reduce rapidly and uniformly, which promotes the development of small clusters or nuclei.92 As more metal ions are reduced and deposited onto the surfaces of these clusters, which will keep expanding, the stabilizing agent regulates growth and prevents agglomeration. Once the necessary size has been achieved, the reaction is stopped and the NPs are filtered to get rid of byproducts or unreacted substances. By altering the reaction conditions, size control can be achieved.93
Plant-Mediated Synthesis
Plant extracts or compounds derived from plants are utilized in plant-mediated synthesis to produce NPs. Utilizing a range of plant species, including medicinal herbs and herbal extracts, is a sustainable and environmentally friendly approach.94 Additionally, the phytochemicals found in plants are capable of imposing unique properties on the resulting NPs. In the process of producing Ag and Au NPs by plants, plant extracts can serve as both reducing and stabilizing agents. Selecting and harvesting plant material that contains bioactive compounds is the first step in the process. The metal ions are subsequently reduced and Ag and Au atoms are formed when the plant extract is combined with precursor ions of Ag and Au. These components act as nucleation sites during the development of NPs. At the precise point of their production, the NPs are stabilized by the bioactive components in the plant extract. To remove any impurities, the synthesized NPs are inspected and thoroughly cleaned.32,95
One-Pot Synthesis
The production of Ag and Au NPs in a single reaction vessel is a faster and more efficient approach known as “one-pot synthesis.” Preparing a reaction mixture comprising a stabilizing agent, a reducing agent, and Ag and Au precursors is the first step in the process. Metal ions must first be reduced by the reducing agent to generate Ag and Au atoms, which act as the nucleation sites for the production of NPs. The stabilizing agent controls the development process to prevent agglomeration and ensure the stability of NPs. The resultant NPs are next scrutinized and cleaned of any impurities.96,97
Bio-Inspired Synthesis
Bio-inspired synthesis uses biological and natural processes to mimic the synthesis of NPs. Examples of biomolecules that can be modified to serve as catalysts or templates for the production of NPs include peptides, DNA, and proteins.98 The size, shape, and surface chemistry of the NPs can be carefully controlled to produce customized properties and enhanced functioning.83 Bio-inspired synthesis of Ag and Au NPs draws inspiration from nature to create NPs using biological entities or processes. In this method, natural sources like plants, microbes, or biomolecules are chosen and their special qualities such as reducing or stabilizing agents are used to promote the production of NPs. The bio-inspired source interacts with the Ag and Au precursor ions, reducing them and consequently producing NPs as a result. The specific chemicals or enzymes present in the bio-inspired source act as a nucleation, growth, and stabilization guidance for the process. The resulting NPs can be further modified for specific uses in a variety of areas after being characterized. A sustainable and ecologically friendly way to create NPs, bio-inspired synthesis offers the potential for modified nanoparticle properties and a wide range of applications.99,100
Electrochemical Synthesis
For the production of NPs, a variety of electrochemical technologies may be employed. By carefully controlling the electrode voltage and amount of current, NPs can be generated directly on conductive surfaces or in solutions. Due to the precise control it allows over nanoparticle size, shape, and composition, electrochemical synthesis is feasible for industrial-scale production.85 Ag and Au NPs may be produced electrochemically in an electrochemical cell where metal ions are reduced at the cathode to produce NPs.101 An electrolyte solution including an Ag and Au salt is first developed, then the electrochemical cell is assembled. By generating a potential difference between the anode and the cathode, which also triggers the nucleation and growth of NPs on the cathode surface, metal ion reduction is accelerated. Nanoparticle development can be regulated and stabilized by adding chemicals to the electrolyte. The resultant NPs are assessed and processed to remove impurities.102
Templated Synthesis
In templated synthesis, the synthesis of NPs is controlled by the application of templates or scaffolds. These replica templates could be biological, self-assembling, or made of porous substances. Templated synthesis, allowing for control over nanoparticle size, shape, and dispersion, enables the production of complex nanostructures with desired functionality.86 Templated synthesis of Ag and Au NPs involves using templates or frameworks to guide the formation and control the properties of the NPs. Choosing a template and modifying its surface is the first step in the process. The template is then treated with metal ions, which proceed through reduction and growth to produce NPs. After synthesis, the template is removed in order to isolate the NPs. The characterized templated NPs can be used for a number of biomedical applications.103
Micro-Fluidic Synthesis
Micro-fluidic platforms provide exceptional control over reaction conditions and mixing dynamics at the micro scale.104 Quick mixing, constant heat transfer, and faster mass transfer are advantages of this technique, which improves control over the synthesis of NPs. Microfluidic synthesis can produce monodisperse NPs with excellent uniformity.88 In order to accurately regulate the flow, mixing, and reaction conditions for nanoparticle synthesis, Ag and Au NPs are synthesized utilizing microfluidic devices. First, the precursor solutions are added to the microfluidic apparatus, where they mix and react to produce NPs. Through the enclosed microfluidic environment, nucleation, growth, and nanoparticle properties are better controlled. Once the synthesized NPs have been gathered and purified, stabilizing agents may be used to prevent agglomeration.105
Hybrid and Composite NPs
Hybrid and composite NPs incorporate valuable components or combine various materials to improve efficiency. These NPs’ synergistic actions, improved stability, or multifunctionality make them particularly versatile. Energy storage, sensing, and catalysis are among the uses for hybrid and composite NPs.86 Ag and Au NPs are combined with other materials to create hybrid and composite NPs that possess novel properties and functionalities. The required components are integrated throughout the synthesis process using techniques like surface functionalization, co-reduction synthesis, or core-shell synthesis. These techniques enable the systematic incorporation of different components into the nanoparticle structure. The resulting NPs are carefully scrutinized and modified for specific applications including energy conversion, sensing, imaging, and drug delivery. The incorporation of various materials provides a wide range of alternatives because of the synergistic effects and unique combination of properties exhibited by hybrid and composite NPs. This opens up new possibilities for innovative applications in various industries.90
Biomedical Applications of Green Synthesized NPs
Green synthesized NPs have displayed potential in various biomedical fields such as drug delivery, tissue engineering, diagnostics, cancer therapy, ophthalmology, and dental care (Figure 3).
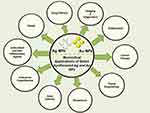 |
Figure 3 Biomedical Applications of Green Synthesized Ag and Au NPs. |
Drug Delivery Systems
Green synthesized NPs can be modified with ligands or antibodies that specifically target diseased cells or tissues. This targeted accumulation improves the efficacy of drug delivery, minimizes off-target effects, and enhances therapeutic outcomes106 (Table 3). Additionally, they can be engineered for carrying drugs on their surface or within their structure. Drug release can be modulated through modification of nanoparticle composition, size, and surface properties, resulting in sustained and controlled release profiles that extend therapeutic effects and minimize the frequency of drug administration.107 Furthermore, Green synthesized NPs protect drugs against environmental factors including light, heat, and moisture attributable to their modified surface characteristics and stabilizing agents. The integrity of the drugs is improved, and the shelf life is extended.108 Green synthesized NPs, particularly those made from bioactive chemicals or plant extracts, increase the solubility and bioavailability of natural or herbal medicines. As a result, they can be effectively utilized as a source of delivery and used in the field of drug delivery sciences.109
 |
Table 3 Summarized Overview of Biomedical Applications of Green Synthesized NPs |
Furthermore, they can also be designed as multifunctional platforms that can deliver multiple drugs simultaneously. This provides avenues for combination therapy, so that multiple drugs or therapeutic agents can be administered together to effectively target numerous pathways or disease sites.110 In addition, these NPs also have imaging properties, acting as contrast materials in MRI, CT, or fluorescence imaging procedures, which facilitate non-invasive visualization and monitoring of drug delivery mechanisms, disease progression, and therapy response. They are able to combine therapeutic and diagnostic features to develop theranostic platforms. These technologies enable simultaneous drug delivery and imaging, enabling real-time drug distribution monitoring, personalized therapy, and treatment efficacy evaluation.136
Saha et al physically attached a number of antibiotics, including ampicillin, kanamycin, and streptomycin, to non-functionalized spherical AuNPs with a diameter of roughly 14 nm. Consequently, compared to their free form, conjugated antibiotics demonstrated greater stability and bacterial growth suppression.137 Methotrexate, a folic acid analog, is primarily utilized as a cytotoxic anti-cancer drug. It was conjugated to 13 nm colloidal Au NPs to interfere with the metabolism of folate in cancer cells. According to the study reports, the quantity of AuNP-conjugated methotrexate in tumor cells was higher than that of free methotrexate after the drug’s carboxylic groups were able to interact with the surface of Au NPs during the overnight incubation. Similarly, compared to free methotrexate, the conjugated version had a cytotoxic effect that was 07 times greater in Lewis lung cancer animal models.138
Imaging and Diagnostics
In the field of healthcare, Green synthesized NPs have a number of uses as potential imaging and diagnostic tools. They can be utilized in medical imaging procedures as contrast agents. These NPs improve the visibility of particular tissues or structures through modifying their surface properties, assisting in the precise identification and characterization of diseases.139 They are useful tools for fluorescence-based imaging since they may be engineered to exhibit fluorescence qualities. These NPs emit light at particular wavelengths that make it possible to see cellular functions and spot alterations brought on by disease.140 These NPs generate acoustic waves when exposed to laser light, which may be recognized and turned into high-resolution images to make it easier to see biological structures.112
Additionally, ligands or antibodies can be used to functionalize Green synthesized NPs to make biosensors and diagnostic procedures. Through their interactions with target molecules like disease indicators, these NPs enable the sensitive and accurate identification of diseases using a variety of diagnostic procedures. Similarly, combining therapeutic and diagnostic functions play a role in theranostics. In order to optimize patient outcomes, medical professionals can evaluate therapy response, modify doses, and personalize medicines with the use of these NPs.141 Furthermore, Green synthesized NPs have been incorporated into portable diagnostic tools, allowing for rapid on-site testing. At the point of care, these technologies quickly and accurately diagnose patients by identifying specific disease markers or infections in samples.142
Lee et al used the hepatitis B virus (HBV) core protein as a scaffold for developing ultrasmall AuNPs with a particle size of 1.4 nm. Both subcutaneous and deep-tissue tumors in living mice were significantly affected by these AuNPs in terms of MRI and magnetic hyperthermia therapy.143 The absorption cross-section of AuNPs is thousands of times greater than that of popular chemical dyes like indocyanine green or rhodamine-6G. AuNPs have been effectively employed as molecularly targeted contrast agents for PAI in animal models due to their exceptional performance. Larger diameter AuNPs, however, are not well eliminated by the kidney, which restricts their use in clinical settings.144 Consequently, the development of particular ultrasmall AuNPs for PAI is required. AuNPs with particle diameters of roughly 5 nm and 40 nm were coupled to a monoclonal anti-EGFR (epidermal growth factor receptor; EGFR) antibody by Sokolov et al and utilized to identify cancer cells. The findings demonstrated that the 5 nm AuNPs retained the same PA signal as the 40 nm AuNPs and exhibited high near-infrared absorption. Due to their incredibly small diameter, the 5 nm AuNPs surprisingly demonstrated exceptional tissue penetration and in vivo clearance ability. These findings showed that ultrasmall AuNPs would be a practical in vivo solution for sensitive PAI of cancer cells.145
Antibacterial Agents
Green-synthesized NPs, including Ag NPs, possess inherent antibacterial properties. They have the ability to damage bacterial cell structures, inhibit bacterial growth. In a study, Escherichia coli strains were subjected to 10:1 reaction mixture mediated green synthesized Au NPs of Macadamia nut shell extract, upon antibacterial assessment the zone of inhibition was increased 2 times in comparison to crude extract (Figure 4).146,147 In addition, Ag and Au NPs are capable of eliminating bacteria because of their capacity to penetrate bacterial cell walls, interact with internal components, produce reactive oxygen species (ROS), impair membrane integrity, and inhibit vital cellular functions.148 Additionally, both Gram-positive and Gram-negative bacteria are susceptible to the antibacterial effects of green synthesized NPs, because they have a broad-spectrum antibacterial coverage. Due to their effectiveness towards a variety of bacterial species, including frequently encountered pathogenic strains, they are effective for treating bacterial infections.149 Additionally, they exhibit synergistic effects when used in combination with conventional antibiotics. Combination therapy minimizes the dose of antibiotics required, reduces the incidence of antibiotic resistance, and improves antibacterial activity.114
 |
Figure 4 A representative petri dish sample of sterile disc and plant extract alone (a), Escherichia coli subjected to 10:1 reaction mixture mediated green synthesized Au NPs (b), and (c) indicative outcomes for the antibacterial assessment (The zone of inhibition was increased 2X). Notes: Reprinted from Dang H, Fawcett D, Poinern GEJ. Green synthesis of gold nanoparticles from waste macadamia nut shells and their antimicrobial activity against Escherichia coli and Staphylococcus epidermis. International Journal Of research in medical sciences. 2019;7(4):1171–1177. Creative Commons.146 |
The “Bacterial biofilms” are a significant problem in antibacterial drug resistance and represent significant challenge in the treatment of bacterial infections due to antibiotic resistance.150 Green synthesized NPs have demonstrated potential in preventing the formation of new biofilms and destroying those that already exist, making bacteria more sensitive to antibacterial treatments. For instance, the biofilm production by L. Monocytogenes and S. marcescens was inhibited by Au NPs synthesized from extract of Trachyspermum ammi (Figure 5).151 Furthermore, these NPs can be incorporated into a number of delivery systems, such as hydrogels, coatings, and NPs loaded with conventional antibiotics. These delivery methods enable NPs and antibiotics to be delivered for an extended period of time at the infection site, enhancing their antibacterial properties and reducing the frequency of administration.152 When it comes to toxicity studies, these NPs exhibit lower toxicity to mammalian cells compared to conventional antibacterial agents, making them potentially safer for therapeutic use.153
Flavonoid glycerin was utilized by Alsamhary et al as a capping and reducing agent during the synthesis of AuNPs. Furthermore, an evaluation was conducted on the antibacterial activity of synthetic AuNPs against opportunistic bacterial pathogens responsible for respiratory tract infections. Research on bacteria has verified that AuNPs have broad-spectrum antibacterial action against both Gram-positive and Gram-negative tested bacteria.154 A novel method of developing AuNPs with glucosamine functionalization was described by Govindaraju et al. During the study, a laser and UV light were applied to AuNPs. The findings showed that compared to pure AuNPs, functionalized AuNPs and AuNPs exposed to UV and laser light demonstrated enhanced antibacterial properties.155 According to Bajaj et al, free metal nanoparticles and natural peptides with AuNPs at the end exhibited lower antibacterial and antifungal effects than those with AuNPs at the end. It was shown that the antibacterial effect of AuNPs encapsulated with the cationic peptide (1-His1-Arg-OMe) surpassed that of conventional antibiotics.156 Additionally, a family of nonantibiotic drugs including mercaptopyrimidine-coated AuNPs have been shown to have bactericidal and broad-spectrum antibacterial effects on multidrug resistant bacteria and superbugs.157
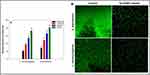 |
Figure 5 (A) Effect of sub-MICs of TA-AuNPs on biofilm formation. *Denotes significance atp ≤ 0.05 and **Denotes significance atp ≤0.005. (B) Confocal laser scanning microscopic images of L. Monocytogenes and S. marcescens biofilm in the absence and presence of 0.5× MICs of TA-AuNPs (Gold NPs synthesized from extract of Trachyspermum ammi). The biofilm production was inhibited by TA-AuNPs. Notes: Reprinted from Perveen K, Husain FM, Qais FA, et al. Microwave-assisted rapid green synthesis of gold nanoparticles using seed extract of Trachyspermum ammi: ROS mediated biofilm inhibition and anticancer activity. Biomolecules. 2021;11(2):197. Creative Commons.151 |
AuNPs based on polyvalent amino sugars were presented by Yang et al as therapeutic option for wounds infected with super bacteria. Polyvalent amino sugar-based AuNPs were produced by modifying AuNPs with d-glucosamine (GluN). Au-GluN not only has exceptional super bacterial infection wound healing properties but also does not damage red blood cells outside of the body.158 A number of studies have demonstrated that AuNPs, cefotaxime, and ciprofloxacin work against all strains of Salmonella. Conventional antibiotics encourage the production of reactive oxygen species, which kills bacteria, but AuNPs disrupt the equilibrium of cations. Salmonella cells undergo apoptosis as a result of this mechanism.159 Long-term resistance to MDR bacteria is an urgent issue in healthcare, and Li et al found that cationic and hydrophobic functionalized AuNPs can successfully inhibit the growth of 11 clinical MDR isolates, including Gram-negative and Gram-positive bacteria, providing a promising strategy for antibiotic resistance.160 Dextrose-coated AuNPs (D-AuNPs) were the subject of a study by Badwaik et al on their antibacterial properties. The research demonstrated the effectiveness of D-AuNPs against both Gram-positive and Gram-negative bacteria, as well as their bacteriostatic and bactericidal properties.161 AuNPs with chitosan, ethylene glycol chitosan, and poly(γglutamic acid) caps were developed by Inbaraj et al, researchers also assessed and described the particles’ catalytic and antibacterial properties. Each of the three artificially functionalized AuNPs exhibits a characteristic antibacterial action.162 Wang et al developed stable daptomycin Au nanoflowers in moderate lab conditions by using daptomycin (Dap) micelles as a template and reducing agent. Under near-infrared light illumination at 808 nm, Dap-AuNPs suppressed the growth of bacterial strains and cancers.163
Cancer Therapy
In the field of cancer therapy, Green synthesized NPs demonstrate great promise as innovative approaches to addressing cancer-related issues. They can be modified to bind specifically to cancer cells or tumor markers using certain ligands or antibodies. With this targeted strategy, chemotherapy drugs can be delivered precisely, enhancing their accumulation at the tumor site while reducing their toxicity to healthy tissues.164 Additionally, Green synthesized NPs enhance the stability, solubility, and bioavailability of anticancer drugs by encapsulating it. This avoids challenges related to conventional chemotherapy via enhancing drug efficacy, extended drug release pattern, and improved therapeutic outcomes.165 Moreover, they can act as imaging agents to help with early cancer detection, precise tumor location, and tracking effectiveness of the therapy.113 These NPs preferentially absorb and convert light spectrum into heat when exposed to near-infrared light, which leads to localized hyperthermia and the destruction of cancer cells while minimizing harm to healthy tissues.166
Green synthesized NPs offer a viable route for anticancer hyperthermia, offering cutting-edge strategies to treat cancer by localized heating.167 Gold or carbon-based NPs are two examples of NPs made via green synthesis that have special photothermal characteristics.112,168,169 These NPs selectively absorb near-infrared light when exposed, transforming it into heat and producing reactive oxygen species (ROS) that can specifically destroy cancer cells while causing minimal amounts of damage to healthy surrounding tissues, enhancing the effectiveness of anticancer treatments (Figure 6).170 They can also be designed to release heat in a regulated manner, allowing for exact control of the elevation in temperature within the tumor area. Similarly, they can also be used in combination with chemotherapy or immunotherapy to achieve synergistic effects. As a result of their relative safety and ecologically friendly nature, Green synthesized NPs exhibit a lower impact on healthy cells and tissues.171
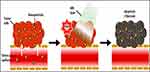 |
Figure 6 When exposed to near-infrared light (NIR laser), Au NPs selectively absorb and convert light into heat, resulting in localized hyperthermia and generation of ROS resulting in selective apoptosis/necrosis of cancer cells. Notes: Reprinted from Kim HS, Lee DY. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers. 2018;10(9):961. Creative Commons.170 |
AuNPs with a size range of 8–15 nm have the potential to be produced in large quantities using the hydroethanolic extract of Brazilian Red Propolis and its components. AuNPs exhibited a dose dependent cytotoxic action in T24 and PC-3 cells. The compounds with the highest in vitro cytotoxic impact were AuNP dichloromethane and AuNP extract. Furthermore, pathways connected to apoptosis promoted the cytotoxicity of biogenic nanoparticles. A surface plasmon resonance (SPR) band was visible in AuNPs at 535 nm.172 AuNPs and carboxymethylcellulose were combined to create a nanocomposite (ACMC-AuNPs). CMC-AuNPs showed the lowest IC50 values (2.56 ± 0.19 μg/mL) in MCF-7 cells, indicating a potent cytotoxic action. In cells treated with CMC-AuNPs, the percentage of overall apoptosis was 38.81 ± 1.99%, while the control group had 0.61 ± 0.022%. The results lead to conclude that these CMC-AuNPs’ anticancer effect is caused by increasing caspase-8 and −9 activity and lowering VEGFR-2 levels, which in turn trigger necrosis and apoptosis in breast cancer cells. In accordance with cell cycle, CMC-AuNPs also stopped the cell cycle at the G1/G0 phase.173
Tissue Engineering
Green synthesized NPs demonstrate promise as tissue engineering tools, offering innovative approaches to improve tissue regeneration and repair. They have the potential to be used in tissue engineering as controlled drug delivery platforms. They may contain growth factors, cytokines, or drugs that will release locally in a sustained and controlled manner to promote tissue regeneration and regulate the healing process. In order to direct cellular behavior and tissue development, they can also be functionalized with bioactive compounds or growth factors. In addition to minimizing their adverse effects on cells and tissues and promoting better integration with the host tissue, they disintegrate over time, avoiding the need for removal. The green synthesis method makes use of elements from the natural world; these NPs also include built-in bioactive qualities like anti-oxidant, anti-inflammatory, antibacterial, or angiogenic activities. These features assist tissue engineering by improving vascularization, promoting regeneration, and lowering inflammation and infection susceptibility.174 Green-synthesized NPs can be used as imaging agents to track and monitor tissue engineering structures. They might utilize imaging probes to offer real-time monitoring of implanted scaffolds, cell behavior, and tissue growth. As a result, the regeneration process may be enhanced and tissue engineering techniques’ efficacy may be assessed.175
Yi et al proposed that AuNPs may interact mechanically with proteins in the cytoplasm and extracellular matrix to activate the p38 MAPK signaling pathway, which in turn causes the up-regulation of osteogenic genes and the down-regulation of adipogenic genes. This mechanism explains how AuNPs induce osteogenic differentiation.176 On the other hand, Zhang et al proposed that AuNPs raised the degree of ERK phosphorylation/total ERK and that the ERK/MAPK pathway is activated during the differentiation and mineralization of primary osteoblasts.177 AuNPs at 20 nm are more efficient than those at 40 nm. In addition to promoting skeletal muscle regeneration and repair, activation of this pathway can increase myoblast adhesion and proliferation.178 Mahmoud et al examined the effects of several nanostructures on bone marrow-derived mesenchymal stem cells (BM-MSCs) in order to determine their capacity to induce osteogenic development. They studied Hydroxyapatite NPs, AuNPs, Chitosan NPs, Gold/hydroxyapatite NPs, and Chitosan/hydroxyapatite NPs. AuNPs and Au/HA-NPs have the most significant impact among all.179,180 AuNPs improved the bone development of adipose-derived stem cells (ADSCs) at 30 and 50 nm in size, according to Ko et al.181 Li et al discovered that GNP size and shape influenced the osteogenic differentiation of hMSCs by controlling Yes-associated protein (YAP) activation through a comparison of multiple GNP forms and sizes.182 There were concentration-dependent relationships found in the study of superparamagnetic iron oxide (SPIO)-Au core-shell nanoparticles for stimulating osteogenic differentiation of MC3T3-E1 cells.183
Biosensors and Diagnostics
With the development of green-synthesized NPs, biosensors and diagnostics have encountered an enormous evolution, providing new possibilities for the precise and sensitive detection of various analytes. Green synthesized NPs have special physicochemical characteristics that raise the sensitivity of biosensors. With excellent precision and sensitivity, they can detect analytes at low concentrations due to their large surface area to volume ratio, adjustable optical qualities, and significant signal amplification abilities. Biorecognition components like antibodies, enzymes, DNA, or aptamers can be used to functionalize them. These components allow for the selective binding and recognition of target analytes, resulting in highly specialized biosensors that are capable of precisely identifying and measuring a variety of biomolecules, pathogens, or chemical substances. The aforementioned parts enable the selective binding and recognition of target analytes, providing highly specialized biosensors that are capable of precisely identifying and measuring a variety of biomolecules, pathogens, or chemical substances.184 Additionally, they enable non-invasive visualization and monitoring of biological processes, disease progression, and therapy response because of their unique optical and magnetic features. They can be incorporated into sensor platforms for continuous monitoring of the quality of the air, water, or soil, assisting in preserving the environment and protecting public health.113,185
For the purpose of identifying multiple allergy protein types on a single strip, the multiplex lateral flow immunoassay offers a viable platform. The red and blue colors were represented by the SPR bands of spherical and desert rose-like AuNPs, which were detected at 525 nm and 620 nm, respectively.186 AuNPs were functionalized with two distinct capture proteins, and each form was responsible for identifying a single allergen. Spherical and desert rose-like AuNPs could, respectively, reveal the presence of casein and hazelnut proteins in biscuits and hazelnut flowers using the multi-chromatic lateral flow test probes. Compared to the traditional enzyme-linked immunosorbent assay and polymerase chain reaction (PCR), this approach was both quicker and less expensive. Finding the allergic DNA is an additional strategy for treating food allergies. Hybridization chain reaction was used by researchers since it did not require expensive equipment or enzymes.187 AuNPs-based techniques have the potential to offer a simple and easy detection method. For instance, Ochratoxin A (a toxic mycotoxin) detection using semi-quantitative and visual methods based on Au nanorod color shift has been described.188 Free antibodies that would not bind to the test line were occupied if zearalenone was present in the sample. In the scenario that zearalenone was not present, unbound antibodies would attach to the antigen coated on the test line. In this instance, red conjugation would occur on the test line due to functionalized AuNPs’ affinity for free antibodies. In contrast, when zearalenone was present, it did not exhibit any color. Zearalenone detection’s limit of detection was 5 ng L− 1. In order to easily detect zearalenone and aflatoxin B1 simultaneously, two types of identifiable Au nanoclusters were linked by aflatoxin B1 aptamer and zearalenone aptamer, respectively.189
L-proline-modified Au nanoclusters, which emit blue light, and bovine serum albumin-modified Au nanoclusters, which emit red light can be used as detectors for aflatoxin. According to Di Nardo et al, aflatoxin B1 and type-B fumonisins can be detected immediately using a multiplex lateral flow immunoassay that is based on a single test line labeled with both antigens. Aflatoxin B1 and type-B fumonisins, respectively, were targeted by two different types of AuNPs. After synthesizing the nanoparticles using a sodium citrate-induced tetrachloroauric acid reduction, red spherical AuNPs with a mean diameter of around 30 nm and an SPR band at 525 nm were coupled with an aflatoxin-specific antibody. Antibody specific to type-B fumonisins was used to connect blue desert rose-like nanoparticles. These nanoparticles, which have an SPR band at 620 nm and a mean diameter of roughly 75 nm, were synthesized using a seed-mediated growth technique. Aflatoxin B1 would competitively bind to red AuNPs upon their addition, preventing them from adhering to the test line. The test line displayed blue nanoparticles that resembled desert roses. Blue desert rose-like nanoparticles were unable to conjugate to the test line in samples contaminated with type-B fumonisins. The test line had a red appearance. A color loss on the test line would occur if aflatoxin B1 and type-B fumonisins were present at the same time. On the other hand, in the event that neither of the pollutants was present, the test line would be purple. Additionally, this method’s validity was put to the test. In 30 blank samples, there were no false positive results, and in 60 fortified samples, there were no false negative results. Furthermore, every positive sample was accurately identified. Through a red-green-blue data evaluation, the relative standard deviation was found to range between 1.5% and 5.9%. It might be quickly, easily, and affordably applied to cereal-based foods.190 Moreover, an another study by Valentini and Pompa states that Listeria monocytogenes can be taken up by many antibody-coated AuNPs, resulting in the formation of dimers, trimers, or multi-aggregates of AuNPs. Using dynamic light scattering, the amount of Listeria monocytogenes could be measured; 35 colony-forming units (CFU) mL− 1 were found in PBS, while 22 CFU g− 1 were found in samples of lettuce. Gastrointestinal disorders can be brought on by the spore-forming, Gram-positive bacterium Bacillus cereus, which is present in both food and soil. Although gel electrophoresis-based detection and the PCR assay are frequently employed, they are both time-consuming and labor-intensive.191 AuNPs and asymmetric PCR were utilized to find the living cells’ ssDNA in order to accurately determine the concentration of Bacillus cereus.192
Gene Delivery
In terms of safety, biocompatibility, and sustainability, green synthesized NPs have been regarded as a viable and creative method for gene delivery. These NPs can be modified to effectively transfer genetic material into target cells due to their biocompatible nature, indicating that they are less likely to trigger adverse consequences or provoke immune responses.193 Additionally, surface modifications are occasionally used to facilitate effective cellular absorption and the release of transferred genes within the cells.194 Furthermore, adding specific ligands and targeting molecules to their surfaces facilitates the transport of genes to the desired site. It significantly improves the specificity and efficacy of gene transfer by making it possible to deliver genes selectively to appropriate cells. Targeted gene delivery improves the therapeutic potential of gene-based treatments and minimizes off-target effects.195
A drug delivery nanoplatform mediated by AuNPs was developed to co-deliver doxorubicin and siRNA targeting polo-like kinase 1 (PLK1). Polyethyleneimine was applied to AuNPs to help PLK1 assemble on the surface. A pH-sensitive linker containing a thiol group at one terminal end was used to load doxorubicin onto nanoparticles for controlled release. In a cancer therapy model, the lowered IC50 value clearly showed the synergistic effect of combination drugs and gene administration over individual delivery.196 A gold nanovehicle that is highly effective in delivering doxorubicin hydrochloride and siRNAs in combination with chemotherapy and photothermal treatment was developed using dsDNA and MMP-2 cleavable peptides, modified with AS1411 aptamer. According to the results, targeted gene silencing and tumor inhibition were achieved with this combined treatment. A synergistic effect that promoted the eradication of long-lived tumors and an enhanced survival rate of mice were noted after nearly a month of treatment with Au-siRNA-PAA-AS1411 nanoparticles loaded with DOX, given once every three days. In comparison to the passive genetic therapy group, which showed a 30% tumor inhibition ratio and a 0% survival rate, the combined genetic, chemotherapeutic, and photothermal treatment group demonstrated more than 90% tumor inhibition ratio (tumor signal) and an approximately 67% survival rate.197
Antioxidant and Anti-Inflammatory Agents
Green synthesis techniques use sustainable production techniques and natural resources like plant extracts or biological components.198 This aspect aligns with eco-friendly values and promotes the development of environmentally conscious antioxidant and anti-inflammatory agents. By providing novel applications as antioxidant and anti-inflammatory agents, Green synthesized NPs shows potential for managing oxidative stress and inflammation.199 These NPs can scavenge free radicals and reactive oxygen species, minimizing oxidative stress and the cellular damage linked to many diseases. They also control the amount of cytokines, chemokine’s, and other inflammatory mediators released, which lowers inflammation and its adverse effect.200
Studies have demonstrated that AuNPs have antioxidant applications, since they can efficiently scavenge DPPH free radicals in a shorter period of time. This may be the result of AuNPs’ capacity to neutralize DPPH free radicals by donating electrons or hydrogen ions.201 Green synthesized AuNPs were reported to have a greater capacity to scavenge free radicals than an extract of the same plant that did not contain nanoparticles.202 Dipankar and Murugan also reported an association of a similar nature. This might be the result of antioxidants (such polyphenols) in the plant extract adhering to the AuNPs’ surface. This might be the result of antioxidants (such polyphenols) in the plant extract adhering to the AuNPs’ surface.203 In addition to their ability to scavenge free radicals like DPPH, AuNPs have also been shown to scavenge ABTS and nitric oxide. This makes them a viable option for the cosmetics industry, where they can be employed in the formulation of anti-aging creams and sunscreens.204 It further raises the likelihood that AuNPs will be extremely useful in the key fight against cancer and liver illnesses as an antioxidant agent.
Dental Applications
Green synthesized NPs indicate promise in a variety of dental applications, providing revolutionary options for dental health and treatment operations. Utilizing biocompatible and non-toxic ingredients, green synthesis techniques guarantee the safety and compatibility of NPs with oral tissues.205 These NPs have antimicrobial activity against bacteria, fungi, and viruses which cause tooth decay, periodontal disease, and oral infections in humans.206 This antimicrobial activity effectively inhibits microbial growth. Dental materials like resin composites and dental cement benefit from the addition of Green synthesized NPs since it increases their mechanical qualities, durability, and longevity while lowering the possibility of subsequent cavities or restoration failure.207
Caries is a dental condition where the enamel is broken away by the action of acidogenic organisms such as lactobacillus and Streptococcus mutans (S. mutans). Either salivary malfunction or a diet high in carbohydrates are the causes.208 Because of their nanosize, AuNPs have a greater surface area that facilitates increased interaction with both organic and inorganic molecules. Hernández Sierra et al assessed the effects of zinc oxide nanoparticles (ZnPs), AgNPs, and AuNPs on S. mutans, as well as their bacteriostatic and bactericidal properties. AgNPs (0.0976 to 100 μg/mL), AuNPs (0.192 to 197 μg/mL), and ZnPs (3.90 to 4000 μg/mL) were the three nanoparticle concentrations that were studied. Using the liquid microdilution approach, the minimum inhibitory concentration (MIC) was determined. The findings demonstrated that, compared to AuNPs and ZnPs, AgNPs exhibited better antibacterial action at lower concentrations.209 Park et al investigated the impact on S. mutans at low plasma temperatures using AuNPs (30 nm). Bacterial viability stains and colony-forming unit counts (CFU) were used to calculate the survival rate. The study’s findings demonstrated that the addition of AuNPs enhanced S. mutans’ ability to be killed by low-temperature plasma. This may be the result of AuNPs increasing plasma energy and stress toward S. mutans’ cell walls.210 Elgamily et al evaluated the antibacterial activity of Chlorhexidine gluconate-CHX (0.2%) and NanoCare (AuNPs and AgNPs) against S. mutans. Both NanoCare and CHX disinfection shown a considerable reduction in the number of bacteria when tested against Gram positive cariogenic bacteria (S. mutans), while CHX demonstrated a significantly greater rate of bacterial inhibition. This could be because NanoCare’s gold ions, which are released from AuNPs, reduce the disinfectant’s antibacterial efficacy. Nonetheless, it demonstrated that, in contrast to conventional agents, the innovative addition of AuNPs to the cavity disinfectant might enhance the material’s antibacterial activity and reduce the incidence of secondary caries.211
Advantages of Biomedical Applications of Green Synthesized Ag and Au NPs
Ag and Au NPs synthesized using green synthesis approach provide a number of benefits over conventional NPs in biomedical applications. Utilizing plant resources, natural extracts, or other ecologically friendly techniques, green synthesis techniques reduce the consumption of hazardous chemicals and encourage sustainability.43,212 Secondly, Ag and Au NPs synthesized by green synthesis approach frequently demonstrate excellent biocompatibility, indicating their compatibility with biological systems.213 In comparison to their counterparts made using conventional synthesis methods, NPs synthesized via green synthesis techniques frequently use non-toxic or low-toxic ingredients.214,215 Au NPs synthesized through green synthesis approach possess better stability, allowing them to hold onto their characteristics and effectiveness for longer periods of time. For NPs to be reliable and effective in biological applications, this stability is essential.2,216 Green-synthesized Ag and Au NPs also offer the advantage of tunable properties.217
Ag and Au NPs, particularly those synthesized via a green synthesis procedure, exhibit antibacterial properties. Due to their ability for inhibiting the growth of many bacteria and pathogens, these NPs are helpful for applications such as wound healing, infection control, and antibacterial coatings.218 Additionally, they can also be functionalized with biomolecules like antibodies, peptides, or enzymes to improve their selectivity and targeting.122,219 Furthermore, they can be used in a variety of scenarios, including cancer therapy, wound healing, biosensing, tissue engineering, and diagnostics.220,221
Unexplored Biomedical Applications and Future Directions
As previously mentioned, green-synthesized Ag and Au NPs have demonstrated significant potential in a variety of biological applications, but there are still a number of unexplored possibilities (Figure 7). In order to further advance the fields of biomedical engineering and medicine, these disciplines present interesting future applications for environmentally friendly synthesized Ag and Au NPs.
 |
Figure 7 Unexplored biomedical applications of Green Synthesized Ag and Au NPs. |
Neurological Disorders
Green synthesized Ag and Au NPs could be effective in treating neurological disorders. Due to their ability to target neural pathways and to cross the blood-brain barrier (BBB), drugs can be precisely administered to the brain while using them.222 Future research in this domain might concentrate on using these environmentally friendly NPs as drug delivery systems for neurological diseases like Alzheimer’s, ALS, Huntington’s disease, Motor Neuron disease, and Parkinson’s disorders. In addition, future studies should also focus on utilizing green synthesized Ag and Au NPs in order to uncover the effects of these NPs in reducing the oxidative stress and inflammation linked to neurodegenerative conditions.
Immunotherapy
Green synthesized Ag and Au NPs are potentially helpful in immunotherapy, particularly for the treatment of cancer. These NPs may function as vaccine or immunostimulatory drug delivery systems, enhancing the immune response to tumors and enhancing therapeutic outcomes. Their unique properties allow for the targeted delivery of drugs, which could improve the accuracy and potency of immunotherapeutic treatments.223,224 Future studies should focus on co-delivery of Ag and Au NPs along with immuno-stimulatory drugs and vaccinations for promoting therapeutic results.
Regenerative Medicine
Green-synthesized Ag and Au NPs could help in the development of regenerative medicine procedures. For the purpose of promoting cellular development, differentiation, and tissue regeneration, they can be included into the scaffolds or matrices used in tissue engineering.224 Future research in this area must combine tissue engineering scaffolds and matrices with Ag and Au NPs to explore their regeneration potential. Future studies might look into their ability to modify the stem cell microenvironment and promote stem cell proliferation and differentiation.
Ophthalmology
The use of Ag and Au NPs produced by green synthesis in ophthalmology is an area that needs more research. These NPs may be assessed for the delivery of specific drugs to the eye as well as for the treatment of eye infections, glaucoma, macular degeneration, and other ocular diseases.225,226 Future studies may also enable imaging and diagnosis of many ocular diseases using the optical properties of Ag and Au NPs.
Antibacterial Assessment Through Inhibition of Biofilm
Ag and Au NPs produced by green synthesis have shown promise in inhibiting biofilm formation and disrupting existing biofilms, rendering bacteria more susceptible to antibacterial agents.150 However, there are many bacterial strands showing resistance to antibiotics due to formation of biofilm around their colonies. Future research should concentrate on using green synthesized Ag and Au NPs to combat different bacterial strains that form biofilms, including Methicillin-resistant Staphylococcus aureus (MRSA), Extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, Pseudomonas aeruginosa, Vancomycin-resistant Enterococcus (VRE) and Candida biofilms. In addition, Green synthesized Ag and Au NPs should also be researched as drug delivery vehicles for the delivery of conventional antibiotics.
Potential Impact of Green Synthesized Ag and Au NPs on Cellular and Molecular Processes
There are several ways that Ag and Au NPs might enter the human body, but the most common ones are through the skin, intravenous injection, oral delivery, and inhalation. Ag and Au NPs come into touch with a variety of biological components, including proteins, lipids, polysaccharides, and nucleic acids, once they are within the body. The distribution of these NPs in various organs or tissues can be influenced by the interaction between ubiquitous proteins and NPs, forming protein corona.227 The inherent characteristics of proteins and Ag/Au NPs may alter as a result of their interaction.228 Significantly, the contact can cause a number of physiological alterations, such as changes in bound protein structure, complement activation, blood clotting, and protein aggregation.229
A comprehensive examination into the intricate mechanisms regulating the interplay between green-synthesized Ag/Au NPs and molecular and cellular activities is a multidisciplinary challenge. The first emphasis will be centered on figuring out the exact mechanisms by which these nanoparticles enter cells, exploring mechanisms like endocytosis or direct membrane penetration. Moreover, the intracellular environment should then be examined, with a focus on the location within cellular compartments or organelles. Beyond the point of cellular entry, researchers should study the mechanisms and the manner in which vital cellular processes are altered, focusing for how this affects metabolism, homeostasis, proliferation, and differentiation.
Challenges and Limitations in Green Synthesized Ag and Au NPs for Biomedical Applications
There are a number of issues that need to be resolved before Ag and Au NPs synthesized through green synthesis approach could potentially be utilized successfully in biological applications. Firstly, natural extracts or plant materials used in green synthesis processes might cause differences in the characteristics of individual batches of NPs. For large-scale manufacturing and commercialization, it is essential to achieve repeatable nanoparticle characteristics and consistent synthesis techniques.230 Secondly, Green synthesis techniques may not always provide for exact control of the NPs’ size and form. To maximize their qualities and guarantee reliable performance in biomedical applications, homogeneity in particle size and shape is essential.231 The first step in the extraction of natural compounds (NCs) include sample preparation, procedure selection, and a thorough review of the literature. Researchers are primarily concerned with reducing the interference of undesired compounds that might coextract with the targeted compounds during the extraction of NCs from biological materials. While many extraction techniques have been developed in addition to the current classical extraction approach (conventional extraction methodology), scientists are still working to create a single, universal technique for removing NCs from biological materials. A major hurdle in green synthesis methodologies is the technical complexity of extracting and purifying bioactive chemicals from natural sources. By addressing these complexities in our discussion, we aim to offer a more nuanced understanding of the practical obstacles associated with the utilization of green synthesis methods, fostering a comprehensive view for researchers and practitioners in the field.232 Furthermore, under certain conditions, such as changes in pH or temperature, green-synthesized Ag and Au NPs may aggregate or become unstable For their biological uses, it is essential that they remain stable over long periods of time and under physiological settings.233 Moreover, additional surface alterations or functionalization may be necessary to improve the stability, biocompatibility, and targeting abilities of green NPs. To customize NPs for particular biological applications, effective and reliable technologies for surface modification must be developed.234,235 Green synthesized NPs are often thought to be less harmful than conventionally synthesized NPs, however further investigations on their long-term toxicity and biocompatibilities are still required. It’s important to assess how they affect biological systems and any potential negative effects in the long term.236,237 Last but not the least, it can be difficult to scale up the production of Ag and Au NPs produced through green synthesis approach to fulfill the needs of biomedical applications. The development of scalable and affordable production techniques that maintain the fundamental characteristics of NPs is essential for their widespread applications.238
Conclusion
The utilization of Ag and Au NPs made through green synthesis in biomedical applications is extremely promising. These NPs have several benefits, including biocompatibility, reduced toxicity, improved stability, adaptable characteristics, antibacterial activity, surface functionalization, therapeutic efficacy, and imaging capabilities. In a number of domains, including drug delivery, tissue engineering, diagnostics, cancer treatment, ophthalmology, and dental care, these NPs have already demonstrated promising results. However, employing Ag and Au NPs synthesized through green synthesis has drawbacks and limitations. Future studies should focus on overcoming limitations and paying attention to the underexplored biomedical uses of Green Synthesized Ag and Au NPs.
In conclusion, Ag and Au NPs produced via green synthesis method offer potential uses in the realms of science and biomedicine. These have a variety of uses, distinctive characteristics, eco-friendly synthesis approaches, and serve as valuable nanotools to be utilized in healthcare and medicine. Even if there are still challenges and limitations, more research, collaboration, and technological advancements will clear the way for their successful integration. The ability of green synthesized NPs to overcome these challenges and explore unknown biomedical spaces will transform biomedical research, improve healthcare outcomes, and bring novel treatment options in near future.
Acknowledgments
The authors would like to acknowledge the University of Science and Technology Beijing, China for supporting this work.
Funding
This study was funded by the National Key Research and Development Program of China (2022YFE0118800).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Singh A, Dhau JS. Advanced Nanomaterials in Biomedicine: benefits and Challenges. In: Advanced Functional Nanoparticles” Boon or Bane” for Environment Remediation Applications: Combating Environmental Issues. Springer; 2023:263–278.
2. Malik AQ, TuG M, Kumar D, et al. A review on the green synthesis of nanoparticles, their biological applications, and photocatalytic efficiency against environmental toxins. Environ Sci Pollut Res. 2023;2023:1–28.
3. Nadaf SJ, Jadhav NR, Naikwadi HS, et al. Green synthesis of gold and silver nanoparticles: updates on research, patents, and future prospects. OpenNano. 2022;2022:100076.
4. Ardila‐Fierro KJ, Hernández JG. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem. 2021;14(10):2145–2162. doi:10.1002/cssc.202100478
5. Alfryyan N, Kordy MG, Abdel-Gabbar M, Soliman HA, Shaban M. Characterization of the biosynthesized intracellular and extracellular plasmonic silver nanoparticles using Bacillus cereus and their catalytic reduction of methylene blue. Sci Rep. 2022;12(1):12495. doi:10.1038/s41598-022-16029-1
6. Ibrahim S, Ahmad Z, Manzoor MZ, Mujahid M, Faheem Z, Adnan A. Optimization for biogenic microbial synthesis of silver nanoparticles through response surface methodology, characterization, their antimicrobial, antioxidant, and catalytic potential. Sci Rep. 2021;11(1):770. doi:10.1038/s41598-020-80805-0
7. Fouad H, Hongjie L, Yanmei D, et al. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif Cells Nanomed Biotechnol. 2017;45(7):1369–1378. doi:10.1080/21691401.2016.1241793
8. Gan L, Zhang S, Zhang Y, He S, Tian Y. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep Biochem Biotechnol. 2018;48(7):582–588. doi:10.1080/10826068.2018.1476880
9. Ranjani A, Gopinath PM, Ananth S, et al. Multidimensional dose–response toxicity exploration of silver nanoparticles from Nocardiopsis flavascens RD30. Appl Nanosci. 2018;8:699–713. doi:10.1007/s13204-018-0824-7
10. Ahmed E, Kalathil S, Shi L, Alharbi O, Wang P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J Saudi Chem Soc. 2018;22(8):919–929. doi:10.1016/j.jscs.2018.02.002
11. Bing W, Sun H, Wang F, Song Y, Ren J. Hydrogen-producing hyperthermophilic bacteria synthesized size-controllable fine gold nanoparticles with excellence for eradicating biofilm and antibacterial applications. J Mat Chem B. 2018;6(28):4602–4609. doi:10.1039/C8TB00549D
12. Jafari M, Rokhbakhsh-Zamin F, Shakibaie M, et al. Cytotoxic and antibacterial activities of biologically synthesized gold nanoparticles assisted by Micrococcus yunnanensis strain J2. Biocatal Agric Biotechnol. 2018;15:245–253. doi:10.1016/j.bcab.2018.06.014
13. Husen A, Siddiqi KS. Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol. 2014;12(1):1–10. doi:10.1186/s12951-014-0028-6
14. Maliszewska I. Microbial mediated synthesis of gold nanoparticles: preparation, characterization and cytotoxicity studies. Dig J Nanomat Biostruc. 2013;8:3.
15. Sarkar J, Ray S, Chattopadhyay D, Laskar A, Acharya K. Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Bioprocess Biosyst Eng. 2012;35:637–643. doi:10.1007/s00449-011-0646-4
16. Pradeepa M, Harini K, Ruckmani K, Geetha N. Extracellular bio-inspired synthesis of silver nanoparticles using raspberry leaf extract against human pathogens. Int J Pharm Sci Rev Res. 2014;25(2):160–165.
17. Alfarraj NS, Tarroum M, Al-Qurainy F, et al. Biosynthesis of silver nanoparticles and exploring their potential of reducing the contamination of the in vitro culture media and inducing the callus growth of Rumex nervosus explants. Molecules. 2023;28(9):3666. doi:10.3390/molecules28093666
18. El-Desouky N, Shoueir KR, El-Mehasseb I, El-Kemary M. Bio-inspired green manufacturing of plasmonic silver nanoparticles/degussa using Banana Waste Peduncles: photocatalytic, antimicrobial, and cytotoxicity evaluation. J Mater Res Technol. 2021;10:671–686. doi:10.1016/j.jmrt.2020.12.035
19. Swarnavalli GCJ, Dinakaran S, Raman N, Jegadeesh R, Pereira C. Bio inspired synthesis of monodispersed silver nano particles using Sapindus emarginatus pericarp extract–Study of antibacterial efficacy. J Saudi Chem Soc. 2017;21(2):172–179. doi:10.1016/j.jscs.2015.03.004
20. Asiya S, Pal K, Kralj S, El-Sayyad G, de Souza F, Narayanan T. Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications. Mater Today Chem. 2020;17:100327. doi:10.1016/j.mtchem.2020.100327
21. Ahmad S, Ahmad H, Khan I, et al. Green synthesis of gold nanaoparticles using delphinium chitralense tuber extracts, their characterization and enzyme inhibitory potential. Braz J Biol. 2022; 82:e257622.
22. Nguyen THA, Nguyen V-C, Phan TNH, et al. Novel biogenic silver and gold nanoparticles for multifunctional applications: green synthesis, catalytic and antibacterial activity, and colorimetric detection of Fe (III) ions. Chemosphere. 2022;287:132271. doi:10.1016/j.chemosphere.2021.132271
23. Constantin M, Răut I, Suica-Bunghez R, et al. Ganoderma lucidum-mediated green synthesis of silver nanoparticles with antimicrobial activity. Materials. 2023;16(12):4261. doi:10.3390/ma16124261
24. González-Ballesteros N, Rodríguez-Argüelles M, Lastra-Valdor M, et al. Synthesis of silver and gold nanoparticles by Sargassum muticum biomolecules and evaluation of their antioxidant activity and antibacterial properties. J Nanostruct Chem. 2020;10:317–330. doi:10.1007/s40097-020-00352-y
25. Filip GA, Moldovan B, Baldea I, et al. UV-light mediated green synthesis of silver and gold nanoparticles using Cornelian cherry fruit extract and their comparative effects in experimental inflammation. J Photochem Photobiol B Biol. 2019;191:26–37. doi:10.1016/j.jphotobiol.2018.12.006
26. Punnoose MS, Bijimol D, Mathew B. Microwave assisted green synthesis of gold nanoparticles for catalytic degradation of environmental pollutants. Environ Nanotechnol Monit Manage. 2021;16:100525. doi:10.1016/j.enmm.2021.100525
27. Roy A, Mohanta B. Microwave-assisted green synthesis of Gold nanoparticles and its catalytic activity. Int J Nano Dimension. 2019;10(4):404–412.
28. Kaplan Ö, Tosun NG, Özgür A, et al. Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: characterization, anticancer, antimicrobial and wound healing activities. J Drug Delivery Sci Technol. 2021;64:102641. doi:10.1016/j.jddst.2021.102641
29. Vandarkuzhali SAA, Karthikeyan G, Pachamuthu M. Microwave assisted biosynthesis of Borassus flabellifer fruit mediated silver and gold nanoparticles for dye reduction, antibacterial and anticancer activity. J Environ Chem Eng. 2021;9(6):106411. doi:10.1016/j.jece.2021.106411
30. Strapasson GB, Assis M, Backes CW, Corrêa SA, Longo E, Weibel DE. Microwave assisted synthesis of silver nanoparticles and its application in sustainable photocatalytic hydrogen evolution. Int J Hydrogen Energy. 2021;46(69):34264–34275. doi:10.1016/j.ijhydene.2021.07.237
31. Raghavendra N, Hublikar LV, Patil S, Bhat P. Microwave assisted biosynthesis of silver nanoparticles using banana leaves extract: phytochemical, spectral characterization, and anticancer activity studies. J Water Environ Nanotechnol. 2021;6(1):49–61.
32. Shreyash N, Bajpai S, Khan MA, Vijay Y, Tiwary SK, Sonker M. Green synthesis of nanoparticles and their biomedical applications: a review. ACS Appl Nano Mater. 2021;4(11):11428–11457. doi:10.1021/acsanm.1c02946
33. Habeeb Rahuman HB, Dhandapani R, Narayanan S, et al. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 2022;16(4):115–144. doi:10.1049/nbt2.12078
34. Ngcobo S, Silwana B, Maqhashu K, Matoetoe MC. Bentonite nanoclay optoelectrochemical property improvement through bimetallic silver and gold nanoparticles. J Nanotechnol. 2022;2022:1–9. doi:10.1155/2022/3693938
35. Oliveira BB, Ferreira D, Fernandes AR, Baptista PV. Engineering gold nanoparticles for molecular diagnostics and biosensing. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(1):e1836. doi:10.1002/wnan.1836
36. Al Saqr A, Khafagy E-S, Alalaiwe A, et al. Synthesis of gold nanoparticles by using green machinery: characterization and in vitro toxicity. Nanomaterials. 2021;11(3):808. doi:10.3390/nano11030808
37. Kowalczyk P, Szymczak M, Maciejewska M, et al. All that glitters is not silver—a new look at microbiological and medical applications of silver nanoparticles. Int J Mol Sci. 2021;22(2):854. doi:10.3390/ijms22020854
38. Beik J, Asadi M, Khoei S, et al. Simulation-guided photothermal therapy using MRI-traceable iron oxide-gold nanoparticle. J Photochem Photobiol B Biol. 2019;199:111599. doi:10.1016/j.jphotobiol.2019.111599
39. Uthappa U, Kigga M, Sriram G, et al. Facile green synthetic approach of bio inspired polydopamine coated diatoms as a drug vehicle for controlled drug release and active catalyst for dye degradation. Microporous Mesoporous Mater. 2019;288:109572. doi:10.1016/j.micromeso.2019.109572
40. Fritea L, Banica F, Costea TO, et al. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio) sensors with biomedical applications. Materials. 2021;14(21):6319. doi:10.3390/ma14216319
41. Nezhad-Mokhtari P, Akrami-Hasan-Kohal M, Ghorbani M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int J Biol Macromol. 2020;154:198–205. doi:10.1016/j.ijbiomac.2020.03.112
42. Andrea R, Nora I, Dora IA, et al. Green silver and gold nanoparticles: biological synthesis approaches and potentials for biomedical applications. Molecules 2021;26 (4): 844. doi: 10.3390/molecules26040844
43. Castillo-Henríquez L, Alfaro-Aguilar K, Ugalde-álvarez J, Vega-Fernández L, Montes de Oca-Vásquez G, Vega-Baudrit JR. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials. 2020;10(9):1763. doi:10.3390/nano10091763
44. Ritu VKK, Das A, Chandra P, Perara R, Sathiyanarayanan Y. Phytochemical-based synthesis of silver nanoparticle: mechanism and potential applications. BioNanoScience. 2023;1–22. doi:10.1007/s12668-023-01116-y
45. Nguyen NTT, Nguyen LM, Nguyen TTT, Nguyen TT, Nguyen DTC, Tran TV. Formation, antimicrobial activity, and biomedical performance of plant-based nanoparticles: a review. Environ Chem Lett. 2022;20(4):2531–2571. doi:10.1007/s10311-022-01425-w
46. Nath S, Shyanti RK, Pathak B. Plant-Mediated Synthesis of Silver and Gold Nanoparticles for Antibacterial and Anticancer Applications. Green Nanopart. 2020;2020:163–186.
47. Reddy KV, Sree NRS, Kumar PS, Ranjit P. Microbial Enzymes in the Biosynthesis of Metal Nanoparticles. In: Ecological Interplays in Microbial Enzymology. Springer; 2022:329–350.
48. Sheldon RA, Brady D. Streamlining design, engineering, and applications of enzymes for sustainable biocatalysis. ACS Sustainable Chem Eng. 2021;9(24):8032–8052. doi:10.1021/acssuschemeng.1c01742
49. Arib C, Spadavecchia J, de La Chapelle ML. Enzyme mediated synthesis of hybrid polyedric gold nanoparticles. Sci Rep. 2021;11(1):3208. doi:10.1038/s41598-021-81751-1
50. Willner I, Baron R, Willner B. Growing metal nanoparticles by enzymes. Adv Mater. 2006;18(9):1109–1120. doi:10.1002/adma.200501865
51. Schneidewind H, Schüler T, Strelau KK, et al. The morphology of silver nanoparticles prepared by enzyme-induced reduction. Beilstein J Nanotechnol. 2012;3(1):404–414. doi:10.3762/bjnano.3.47
52. Ekeoma BC, Ekeoma LN, Yusuf M, et al. Recent advances in the biocatalytic mitigation of emerging pollutants: a comprehensive review. J Biotechnol. 2023;369:14–34. doi:10.1016/j.jbiotec.2023.05.003
53. Pathak J, Ahmed H, Singh DK, Pandey A, Singh SP, Sinha RP. Recent developments in green synthesis of metal nanoparticles utilizing cyanobacterial cell factories. Nanomat Plant Alg Microorg. 2019;2019:237–265.
54. Ali S, Chen X, Shah MA, et al. The avenue of fruit wastes to worth for synthesis of silver and gold nanoparticles and their antimicrobial application against foodborne pathogens: a review. Food Chem. 2021;359:129912. doi:10.1016/j.foodchem.2021.129912
55. Sanket S, Das SK. Role of enzymes in synthesis of nanoparticles. Bioprosp Enzy Indus. 2021;2021:139–153.
56. Pechyen C, Ponsanti K, Tangnorawich B, Ngernyuang N. Waste fruit peel–Mediated green synthesis of biocompatible gold nanoparticles. J Mater Res Technol. 2021;14:2982–2991. doi:10.1016/j.jmrt.2021.08.111
57. Dikshit PK, Kumar J, Das AK, et al. Green synthesis of metallic nanoparticles: applications and limitations. Catalysts. 2021;11(8):902. doi:10.3390/catal11080902
58. Ghosh S, Ahmad R, Zeyaullah M, Khare SK. Microbial nano-factories: synthesis and biomedical applications. Front Chem. 2021;9:626834. doi:10.3389/fchem.2021.626834
59. Elbahnasawy MA, Shehabeldine AM, Khattab AM, Amin BH, Hashem AH. Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: characterization and anticandidal activity. J Drug Delivery Sci Technol. 2021;62:102401. doi:10.1016/j.jddst.2021.102401
60. Jeevanandam J, Kiew SF, Boakye-Ansah S, et al. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale. 2022;14(7):2534–2571. doi:10.1039/d1nr08144f
61. Mohamed HI, Fawzi EM, Abd-Elsalam KA, Ashry NA, Basit A. Endophytic fungi-derived biogenic nanoparticles: mechanisms and applications. In: Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications. Elsevier; 2023:361–391.
62. Patil MP, Kim G-D. Microorganism-mediated functionalization of nanoparticles for different applications. Functionalized Nanomaterials I. 2020;2020:279–298.
63. Balakumaran M, Ramachandran R, Balashanmugam P, Mukeshkumar D, Kalaichelvan P. Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol Res. 2016;182:8–20. doi:10.1016/j.micres.2015.09.009
64. Noga M, Milan J, Frydrych A, Jurowski K. Toxicological aspects, safety assessment, and green toxicology of silver nanoparticles (AgNPs)—critical review: state of the art. Int J Mol Sci. 2023;24(6):5133. doi:10.3390/ijms24065133
65. Selvinsimpson S, Chen Y. Microbial-based synthesis of nanoparticles to remove different pollutants from wastewater. In: Environmental Applications of Microbial Nanotechnology. Elsevier; 2023:167–181.
66. Rana A, Yadav K, Jagadevan S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: mechanism, application and toxicity. J Cleaner Prod. 2020;272:122880.
67. Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S. Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int J Mol Sci. 2018;19(12):4100. doi:10.3390/ijms19124100
68. Rónavári A, Igaz N, Adamecz DI, et al. Green silver and gold nanoparticles: biological synthesis approaches and potentials for biomedical applications. Molecules. 2021;26(4):844. doi:10.3390/molecules26040844
69. Nymark P, Catalán J, Suhonen S, et al. Genotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in BEAS 2B cells. Toxicology. 2013;313(1):38–48. doi:10.1016/j.tox.2012.09.014
70. Kaczerewska O, Martins R, Figueiredo J, Loureiro S, Tedim J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J Hazard Mater. 2020;392:122299. doi:10.1016/j.jhazmat.2020.122299
71. Jiang Y, Zhou P, Zhang P, et al. Green synthesis of metal-based nanoparticles for sustainable agriculture. Environ Pollut. 2022;309:119755. doi:10.1016/j.envpol.2022.119755
72. Zhang Y, Poon K, Masonsong GSP, Ramaswamy Y, Singh G. Sustainable Nanomaterials for Biomedical Applications. Pharmaceutics. 2023;15(3):922. doi:10.3390/pharmaceutics15030922
73. Gebreslassie YT, Gebretnsae HG. Green and cost-effective synthesis of tin oxide nanoparticles: a review on the synthesis methodologies, mechanism of formation, and their potential applications. Nanoscale Res Lett. 2021;16(1):97. doi:10.1186/s11671-021-03555-6
74. Alprol AE, Mansour AT, El-Beltagi HS, Ashour M. Algal extracts for green synthesis of zinc oxide nanoparticles: promising approach for algae bioremediation. Materials. 2023;16(7):2819. doi:10.3390/ma16072819
75. Mughal B, Zaidi SZJ, Zhang X, Hassan SU. Biogenic nanoparticles: synthesis, characterisation and applications. Appl Sci. 2021;11(6):2598. doi:10.3390/app11062598
76. Khan SA, Shahid S, Hanif S, Almoallim HS, Alharbi SA, Sellami H. Green synthesis of chromium oxide nanoparticles for antibacterial, antioxidant anticancer, and biocompatibility activities. Int J Mol Sci. 2021;22(2):502. doi:10.3390/ijms22020502
77. Mohammadinejad R, Shavandi A, Raie DS, et al. Plant molecular farming: production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019;21(8):1845–1865. doi:10.1039/C9GC00335E
78. Zheng Z, Nguyen HL, Hanikel N, et al. High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air. Nature Protoc. 2023;18(1):136–156. doi:10.1038/s41596-022-00756-w
79. Pearce AK, Wilks TR, Arno MC, O’Reilly RK. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nature Rev Chemist. 2021;5(1):21–45. doi:10.1038/s41570-020-00232-7
80. Chin CD-W, Treadwell LJ, Wiley JB. Microwave synthetic routes for shape-controlled catalyst nanoparticles and nanocomposites. Molecules. 2021;26(12):3647. doi:10.3390/molecules26123647
81. Sen M. Green Synthesis: introduction, Mechanism, and Effective Parameters. Bioinsp Green Synth Nanostruct. 2023;2023:1–24.
82. Heuson E, Dumeignil F. The various levels of integration of chemo-and bio-catalysis towards hybrid catalysis. Catal Sci Technol. 2020;10(21):7082–7100. doi:10.1039/D0CY00696C
83. Cho NH, Guerrero-Martínez A, Ma J, et al. Bioinspired chiral inorganic nanomaterials. Nature Rev Bioeng. 2023;1(2):88–106.
84. Yaraki MT, Zahed Nasab S, Zare I, et al. Biomimetic metallic nanostructures for biomedical applications, catalysis, and beyond. Ind Eng Chem Res. 2022;61(22):7547–7593. doi:10.1021/acs.iecr.2c00285
85. McDarby SP, Wang CJ, King ME, Personick ML. An Integrated Electrochemistry Approach to the Design and Synthesis of Polyhedral Noble Metal Nanoparticles. J Am Chem Soc. 2020;142(51):21322–21335. doi:10.1021/jacs.0c07987
86. Ha M, Kim J-H, You M, Li Q, Fan C, Nam J-M. Multicomponent plasmonic nanoparticles: from heterostructured nanoparticles to colloidal composite nanostructures. Chem Rev. 2019;119(24):12208–12278. doi:10.1021/acs.chemrev.9b00234
87. Patwardhan SV, Staniland SS. Bioinspired ‘Green’synthesis of Nanomaterials. Green Nanomaterials: From Bioinspired Synthesis to Sustainable Manufacturing of Inorganic Nanomaterials. IOP Publishing; 2019.
88. Sohrabi S, Moraveji MK, Keshavarz moraveji M. Droplet microfluidics: fundamentals and its advanced applications. RSC Adv. 2020;10(46):27560–27574. doi:10.1039/D0RA04566G
89. Niculescu A-G, Chircov C, Bîrcă AC, Grumezescu AM. Nanomaterials synthesis through microfluidic methods: an updated overview. Nanomaterials. 2021;11(4):864. doi:10.3390/nano11040864
90. Lee EM, Lee J, Kim Y, et al. Hybrid Composite of Silver Nanoparticle–Porous Silicon Microparticles as an Image-Guided Localization Agent for Computed Tomography Scan of the Lungs. ACS Biomater Sci Eng. 2020;6(8):4390–4396. doi:10.1021/acsbiomaterials.0c00611
91. Hallot G, Cagan V, Laurent S, Gomez C, Port M. A greener chemistry process using microwaves in continuous flow to synthesize metallic bismuth nanoparticles. ACS Sustainable Chem Eng. 2021;9(28):9177–9187. doi:10.1021/acssuschemeng.1c00396
92. Kumari KA, Reddy GB, Mittapalli V Microwave assisted synthesis of gold nanoparticles with Phyla nodiflora (L.) Greene leaves extract and its studies of catalytic reduction of organic pollutants. Mater Today. 2020;27:1449–1454.
93. Manno R, Sebastian V, Irusta S, Mallada R, Santamaria J. Ultra-small silver nanoparticles immobilized in mesoporous SBA-15. Microwave-assisted synthesis and catalytic activity in the 4-nitrophenol reduction. Catal. Today. 2021;362:81–89. doi:10.1016/j.cattod.2020.04.018
94. Waghchaure RH, Adole VA. Biosynthesis of metal and metal oxide nanoparticles using various parts of plants for antibacterial, antifungal and anticancer activity: a review. J Indian Chem Soc. 2023;100:100987. doi:10.1016/j.jics.2023.100987
95. Nithin B, Bhuyar P, Maniam GP, Rahim MHA, Govindan N. Environment friendly approach for plant mediated green biosynthesis of gold nanoparticles and their modern applications in biomedical aspects—an updated report. BioNanoScience. 2023;2023:1–24.
96. Joseph D, Baskaran R, Yang SG, Huh YS, Han Y-K. Multifunctional spiky branched gold-silver nanostars with near-infrared and short-wavelength infrared localized surface plasmon resonances. J Colloid Interface Sci. 2019;542:308–316. doi:10.1016/j.jcis.2019.01.132
97. Kumar I, Mondal M, Meyappan V, Sakthivel N. Green one-pot synthesis of gold nanoparticles using Sansevieria roxburghiana leaf extract for the catalytic degradation of toxic organic pollutants. Mater Res Bull. 2019;117:18–27. doi:10.1016/j.materresbull.2019.04.029
98. Johnson AP, Sabu C, Nivitha K, et al. Bioinspired and biomimetic micro-and nanostructures in biomedicine. J Control Release. 2022;343:724–754. doi:10.1016/j.jconrel.2022.02.013
99. Sundaram T, Indu B, Reddy CS, et al. Bio Inspired Silver Nanoparticle Synthesis from Fish Liver Oil and Its Antibacterial Activity Against Shrimp Pathogen. IOP Publishing; 2020:012166.
100. Ali S, Iqbal M, Naseer A, et al. State of the art of gold (Au) nanoparticles synthesis via green routes and applications: a review. Environ Nanotechnol Monit Manage. 2021;16:100511.
101. Leon-Fernandez L, Caballero-Ortiz A, Martinez-Mora O, Fransaer J, Dominguez-Benetton X. Mechanism and kinetics of gold recovery and Au nanoparticle synthesis by Gas-Diffusion Electrocrystallization (GDEx). Electrochim Acta. 2023;460:142592. doi:10.1016/j.electacta.2023.142592
102. Wu H, Liu Z, Xu L, Wang X, Chen Q, Ostrikov KK. The Ag+ reduction process in a plasma electrochemical system tuned by the pH value. J Electrochem Soc. 2021;168(12):123508. doi:10.1149/1945-7111/ac41f5
103. Goswami S, Noh H, Redfern LR, et al. Pore-templated growth of catalytically active gold nanoparticles within a metal–organic framework. Chem Mater. 2019;31(5):1485–1490. doi:10.1021/acs.chemmater.8b04983
104. Yao F, Zhu P, Chen J, et al. Synthesis of nanoparticles via microfluidic devices and integrated applications. Mikrochim Acta. 2023;190(7):256. doi:10.1007/s00604-023-05838-4
105. Kung C-T, Gao H, Lee C-Y, et al. Microfluidic synthesis control technology and its application in drug delivery, bioimaging, biosensing, environmental analysis and cell analysis. Chem Eng J. 2020;399:125748.
106. Afshari AR, Sanati M, Mollazadeh H, Kesharwani P, Johnston TP, Sahebkar A. Nanoparticle-Based Drug Delivery Systems in Cancer: A Focus on Inflammatory Pathways. Elsevier; 2022.
107. Azadpour A, Hajrasouliha S, Khaleghi S. Green synthesized-silver nanoparticles coated with targeted chitosan nanoparticles for smart drug delivery. J Drug Delivery Sci Technol. 2022;74:103554. doi:10.1016/j.jddst.2022.103554
108. Wang X, Yuan L, Deng H, Zhang Z. Structural characterization and stability study of green synthesized starch stabilized silver nanoparticles loaded with isoorientin. Food Chem. 2021;338:127807. doi:10.1016/j.foodchem.2020.127807
109. Shady NH, Khattab AR, Ahmed S, et al. Hepatitis c virus ns3 protease and helicase inhibitors from red sea sponge (Amphimedon) species in green synthesized silver nanoparticles assisted by in silico modeling and metabolic profiling. Int j Nanomed. 2020;Volume 15:3377–3389. doi:10.2147/IJN.S233766
110. Chen Z, Wang Y, Yang Y, Yang X, Zhang X. Multifunctional sensing platform based on green-synthesized silver nanostructure and microcrack architecture. Chem Eng J. 2021;403:126388. doi:10.1016/j.cej.2020.126388
111. Barbinta-Patrascu M. Biogenic nanosilver from Cornus mas fruits as multifunctional eco-friendly platform:“green” development and biophysical characterization. J Optoelectron Adv Mater. 2020. 22:523–528.
112. Bharadwaj KK, Rabha B, Pati S, et al. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules. 2021;26(21):6389. doi:10.3390/molecules26216389
113. Sargazi S, Laraib U, Er S, et al. Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials. 2022;12(7):1102. doi:10.3390/nano12071102
114. Yassin MT, Mostafa AA-F, Al-Askar AA, Al-Otibi FO. Synergistic antibacterial activity of green synthesized silver nanomaterials with colistin antibiotic against multidrug-resistant bacterial pathogens. Crystals. 2022;12(8):1057. doi:10.3390/cryst12081057
115. Pammi S, Padavala VS, Karumuri TSK, et al. Wound healing synergy in Wistar albino rats via green synthesized nanoparticles and topical antibiotic neomycin. OpenNano. 2023;11:100135. doi:10.1016/j.onano.2023.100135
116. Qu J, Yang J, Chen M, Zhai A. Anti-human gastric cancer study of gold nanoparticles synthesized using Alhagi maurorum. Inorg Chem Commun. 2023;151:109859. doi:10.1016/j.inoche.2022.109859
117. Jadoun S, Arif R, Jangid NK, Meena RK. Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett. 2021;19:355–374. doi:10.1007/s10311-020-01074-x
118. Ramírez-Rodríguez GB, Dal Sasso G, Carmona FJ, et al. Engineering biomimetic calcium phosphate nanoparticles: a green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl Bio Mater. 2020;3(3):1344–1353. doi:10.1021/acsabm.9b00937
119. Li S, Tan L, Meng X. Nanoscale metal‐organic frameworks: synthesis, biocompatibility, imaging applications, and thermal and dynamic therapy of tumors. Adv Funct Mater. 2020;30(13):1908924. doi:10.1002/adfm.201908924
120. Imran M, Ehrhardt CJ, Bertino MF, Shah MR, Yadavalli VK. Chitosan stabilized silver nanoparticles for the electrochemical detection of lipopolysaccharide: a facile biosensing approach for gram-negative bacteria. Micromachines. 2020;11(4):413. doi:10.3390/mi11040413
121. Xu L, Bai X, Bhunia AK. Current state of development of biosensors and their application in foodborne pathogen detection. Journal of Food Protection. 2021;84(7):1213–1227. doi:10.4315/JFP-20-464
122. Amina SJ, Guo B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int j Nanomed. 2020;15:9823–9857. doi:10.2147/IJN.S279094
123. Desai MP, Paiva-Santos AC, Nimbalkar MS, Sonawane KD, Patil PS, Pawar KD. Iron tolerant Bacillus badius mediated bimetallic magnetic iron oxide and gold nanoparticles as Doxorubicin carrier and for hyperthermia treatment. J Drug Delivery Sci Technol. 2023;81:104214. doi:10.1016/j.jddst.2023.104214
124. Patil T, Gambhir R, Vibhute A, Tiwari AP. Gold nanoparticles: synthesis methods, functionalization and biological applications. Journal of Cluster Science. 2023;34(2):705–725. doi:10.1007/s10876-022-02287-6
125. Abed AS, Khalaf YH, Mohammed AM. Green synthesis of gold nanoparticles as an effective opportunity for cancer treatment. Res Chem. 2023;5:100848. doi:10.1016/j.rechem.2023.100848
126. Rezaeian A, Amini SM, Najafabadi MRH, Farsangi ZJ, Samadian H. Plasmonic hyperthermia or radiofrequency electric field hyperthermia of cancerous cells through green-synthesized curcumin-coated gold nanoparticles. Lasers Med Sci. 2022;37(2):1333–1341. doi:10.1007/s10103-021-03399-7
127. El-Borady OM, Ayat MS, Shabrawy MA, Millet P. Green synthesis of gold nanoparticles using Parsley leaves extract and their applications as an alternative catalytic, antioxidant, anticancer, and antibacterial agents. Adv Powder Technol. 2020;31(10):4390–4400. doi:10.1016/j.apt.2020.09.017
128. Majeed M, Hakeem KR, Rehman RU. Synergistic effect of plant extract coupled silver nanoparticles in various therapeutic applications-present insights and bottlenecks. Chemosphere. 2022;288:132527. doi:10.1016/j.chemosphere.2021.132527
129. Shahid H, Arooj I, Zafar S. Honey-mediated synthesis of Cr2O3 nanoparticles and their potent anti-bacterial, anti-oxidant and anti-inflammatory activities. Arabian J Chem. 2023;16:104544. doi:10.1016/j.arabjc.2023.104544
130. Faisal S, Ullah R, Alotaibi A, Zafar S, Rizwan M, Tariq MH. Biofabrication of silver nanoparticles employing biomolecules of Paraclostridium benzoelyticum strain: its characterization and their in-vitro antibacterial, anti-aging, anti-cancer and other biomedical applications. Microsc Res Tech. 2023;86:846–861. doi:10.1002/jemt.24362
131. Nandhini SN, Sisubalan N, Vijayan A, et al. Recent advances in green synthesized nanoparticles for bactericidal and wound healing applications. Heliyon. 2023;9:e13128. doi:10.1016/j.heliyon.2023.e13128
132. Patil TP, Vibhute AA, Patil SL, Dongale TD, Tiwari AP. Green synthesis of gold nanoparticles via Capsicum annum fruit extract: characterization, antiangiogenic, antioxidant and anti-inflammatory activities. Appl Surf Sci Adv. 2023;13:100372. doi:10.1016/j.apsadv.2023.100372
133. Yazdanian M, Rostamzadeh P, Rahbar M, et al. The potential application of green-synthesized metal nanoparticles in dentistry: a comprehensive review. Bioinorg Chem Applic. 2022;2022:1–27. doi:10.1155/2022/2311910
134. Rodrigues MC, Rolim WR, Viana MM, et al. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J Dent. 2020;96:103327. doi:10.1016/j.jdent.2020.103327
135. Ahmed O, Sibuyi NRS, Fadaka AO, et al. Plant extract-synthesized silver nanoparticles for application in dental therapy. Pharmaceutics. 2022;14(2):380. doi:10.3390/pharmaceutics14020380
136. Muddapur UM, Alshehri S, Ghoneim MM, et al. Plant-based synthesis of gold nanoparticles and theranostic applications: a review. Molecules. 2022;27(4):1391. doi:10.3390/molecules27041391
137. Chen Y-H, Tsai C-Y, Huang P-Y, et al. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol Pharmaceut. 2007;4(5):713–722. doi:10.1021/mp060132k
138. Saha B, Bhattacharya J, Mukherjee A, et al. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res Lett. 2007;2:614–622. doi:10.1007/s11671-007-9104-2
139. Kharey P, Goel M, Husain Z, et al. Green synthesis of biocompatible superparamagnetic iron oxide-gold composite nanoparticles for magnetic resonance imaging, hyperthermia and photothermal therapeutic applications. Mater Chem Phys. 2023;293:126859. doi:10.1016/j.matchemphys.2022.126859
140. Pavithra N, Johri S, Varshney R, Ramamurthy PC Dopamine fluorescent sensor based on green synthesized copper oxide nanoparticles and tyrosinase. IEEE J Flexib Elect. 2023;2023:1
141. Adhikary T, Hossain CM, Basak P. Optimization of the process parameters to develop green‐synthesized nanostructures with a special interest in cancer theranostics. Bioinsp Green Synth Nanostruct. 2023;2023:43–64.
142. Sohrabi H, Majidi MR, Khaki P, Jahanban‐Esfahlan A, de la Guardia M, Mokhtarzadeh A. State of the art: lateral flow assays toward the point‐of‐care foodborne pathogenic bacteria detection in food samples. Compr Rev Food Sci Food Saf. 2022;21(2):1868–1912. doi:10.1111/1541-4337.12913
143. Kwon KC, Jo E, Kwon YW, et al. Superparamagnetic gold nanoparticles synthesized on protein particle scaffolds for cancer theragnosis. Adv Mater. 2017;29(38):1701146. doi:10.1002/adma.201701146
144. Luke GP, Myers JN, Emelianov SY, Sokolov KV. Sentinel lymph node biopsy revisited: ultrasound-guided photoacoustic detection of micrometastases using molecularly targeted plasmonic nanosensors. Cancer Res. 2014;74(19):5397–5408. doi:10.1158/0008-5472.CAN-14-0796
145. Han S, Bouchard R, Sokolov KV. Molecular photoacoustic imaging with ultra-small gold nanoparticles. Biomed Opt Express. 2019;10(7):3472–3483. doi:10.1364/BOE.10.003472
146. Dang H, Fawcett D, Poinern GEJ. Green synthesis of gold nanoparticles from waste macadamia nut shells and their antimicrobial activity against Escherichia coli and Staphylococcus epidermis. Int J Res Med Sci. 2019;7(4):1171. doi:10.18203/2320-6012.ijrms20191320
147. Jiang M, Li S, Ming P, et al. Rational design of porous structure-based sodium alginate/chitosan sponges loaded with green synthesized hybrid antibacterial agents for infected wound healing. Int J Biol Macromol. 2023;237:123944. doi:10.1016/j.ijbiomac.2023.123944
148. Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, et al. Cellulose-graphene oxide nanocomposites encapsulated with green synthesized silver nanoparticles as an effective antibacterial agent. Mater Today Commun. 2023;35:105652. doi:10.1016/j.mtcomm.2023.105652
149. Farizan AF, Yusoff HM, Badar N, et al. Green synthesis of magnesium oxide nanoparticles using mariposa christia vespertilionis leaves extract and its antimicrobial study toward S. aureus and E. coli. Arab J Sci Eng. 2023;48(6):7373–7386. doi:10.1007/s13369-022-07282-7
150. Hetta HF, Ramadan YN, Al-Harbi AI, et al. Nanotechnology as a promising approach to combat multidrug resistant bacteria: a comprehensive review and future perspectives. Biomedicines. 2023;11(2):413. doi:10.3390/biomedicines11020413
151. Perveen K, Husain FM, Qais FA, et al. Microwave-assisted rapid green synthesis of gold nanoparticles using seed extract of Trachyspermum ammi: ROS mediated biofilm inhibition and anticancer activity. Biomolecules. 2021;11(2):197. doi:10.3390/biom11020197
152. Pourmadadi M, Yazdian F, Koulivand A, Rahmani E. Green synthesized polyvinylpyrrolidone/titanium dioxide hydrogel nanocomposite modified with agarose macromolecules for sustained and pH-responsive release of anticancer drug. Int J Biol Macromol. 2023;240:124345. doi:10.1016/j.ijbiomac.2023.124345
153. Rais A, Varshney I, Kumar S, Kumar V, Prasad T. Green Nanotechnology: applications in Medicine. In: Innovations in Green Nanoscience and Nanotechnology. CRC Press; 2022:167–207.
154. Alsamhary K, Al-Enazi N, Alshehri WA, Ameen F. Gold nanoparticles synthesised by flavonoid tricetin as a potential antibacterial nanomedicine to treat respiratory infections causing opportunistic bacterial pathogens. Microb Pathogenesis. 2020;139:103928. doi:10.1016/j.micpath.2019.103928
155. Govindaraju S, Ramasamy M, Baskaran R, Ahn SJ, Yun K. Ultraviolet light and laser irradiation enhances the antibacterial activity of glucosamine-functionalized gold nanoparticles. Int j Nanomed. 2015;10(sup1):67–78. doi:10.2147/IJN.S88318
156. Bajaj M, Pandey SK, Nain T, et al. Stabilized cationic dipeptide capped gold/silver nanohybrids: towards enhanced antibacterial and antifungal efficacy. Colloids Surf B. 2017;158:397–407. doi:10.1016/j.colsurfb.2017.07.009
157. Zheng Y, Wei M, Wu H, Li F, Ling D. Antibacterial metal nanoclusters. J Nanobiotechnol. 2022;20(1):328. doi:10.1186/s12951-022-01538-y
158. Yang X, Wei Q, Shao H, Jiang X. Multivalent aminosaccharide-based gold nanoparticles as narrow-spectrum antibiotics in vivo. ACS Appl Mater Interfaces. 2019;11(8):7725–7730. doi:10.1021/acsami.8b19658
159. Lee B, Lee DG. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis‐like death. J Appl Microbiol. 2019;127(3):701–712. doi:10.1111/jam.14357
160. Li X, Robinson SM, Gupta A, et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS nano. 2014;8(10):10682–10686. doi:10.1021/nn5042625
161. Badwaik VD, Vangala LM, Pender DS, et al. Size-dependent antimicrobial properties of sugar-encapsulated gold nanoparticles synthesized by a green method. Nanoscale Res Lett. 2012;7:1–11. doi:10.1186/1556-276X-7-623
162. Inbaraj BS, Chen B-Y, Liao C-W, Chen B-H. Green synthesis, characterization and evaluation of catalytic and antibacterial activities of chitosan, glycol chitosan and poly (γ-glutamic acid) capped gold nanoparticles. Int J Biol Macromol. 2020;161:1484–1495. doi:10.1016/j.ijbiomac.2020.07.244
163. Wang J, Zhang J, Liu K, et al. Synthesis of gold nanoflowers stabilized with amphiphilic daptomycin for enhanced photothermal antitumor and antibacterial effects. Int J Pharm. 2020;580:119231. doi:10.1016/j.ijpharm.2020.119231
164. Awad NS, Salkho NM, Abuwatfa WH, Paul V, AlSawaftah NM, Husseini GA. Tumor vasculature vs tumor cell targeting: understanding the latest trends in using functional nanoparticles for cancer treatment. OpenNano. 2023;11:100136. doi:10.1016/j.onano.2023.100136
165. Yadav P, Ambudkar SV, Rajendra Prasad N. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J Nanobiotechnol. 2022;20(1):1–35. doi:10.1186/s12951-021-01184-w
166. Mosleh-Shirazi S, Kasaee SR, Dehghani F, et al. Investigation through the anticancer properties of green synthesized spinel ferrite nanoparticles in present and absent of laser photothermal effect. Ceram Int. 2023;49(7):11293–11301. doi:10.1016/j.ceramint.2022.11.329
167. Andleeb A, Andleeb A, Asghar S, et al. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers. 2021;13(11):2818. doi:10.3390/cancers13112818
168. Gao Q, Zhang J, Gao J, Zhang Z, Zhu H, Wang D. Gold nanoparticles in cancer theranostics. Front Bioeng Biotechnol. 2021;9:647905. doi:10.3389/fbioe.2021.647905
169. Yin W, Pan F, Zhu J, et al. Nanotechnology and nanomedicine: a promising avenue for lung cancer diagnosis and therapy. Engineering. 2021;7(11):1577–1585. doi:10.1016/j.eng.2020.04.017
170. Kim HS, Lee DY. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers. 2018;10(9):961. doi:10.3390/polym10090961
171. Stabile J, Najafali D, Cheema Y, et al. Engineering gold nanoparticles for photothermal therapy, surgery, and imaging. In: Nanoparticles for Biomedical Applications. Elsevier; 2020:175–193.
172. Botteon C, Silva L, Ccana-Ccapatinta G, et al. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci Rep. 2021;11(1):1974. doi:10.1038/s41598-021-81281-w
173. Doghish AS, Hashem AH, Shehabeldine AM, Sallam -A-AM, El-Sayyad GS, Salem SS. Nanocomposite based on gold nanoparticles and carboxymethyl cellulose: synthesis, characterization, antimicrobial, and anticancer activities. J Drug Delivery Sci Technol. 2022;77:103874. doi:10.1016/j.jddst.2022.103874
174. Fathi-Achachelouei M, Knopf-Marques H, Ribeiro da Silva CE, et al. Use of nanoparticles in tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2019;7:113. doi:10.3389/fbioe.2019.00113
175. Jagadeesh P, Rangappa SM, Siengchin S. Advanced characterization techniques for nanostructured materials in biomedical applications. Adv Ind Eng Polym Res. 2023;2023:1.
176. Yi C, Liu D, Fong -C-C, Zhang J, Yang M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS nano. 2010;4(11):6439–6448. doi:10.1021/nn101373r
177. Zhang D, Liu D, Zhang J, Fong C, Yang M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater Sci Eng C. 2014;42:70–77. doi:10.1016/j.msec.2014.04.042
178. Ge J, Liu K, Niu W, et al. Gold and gold-silver alloy nanoparticles enhance the myogenic differentiation of myoblasts through p38 MAPK signaling pathway and promote in vivo skeletal muscle regeneration. Biomaterials. 2018;175:19–29. doi:10.1016/j.biomaterials.2018.05.027
179. Heo DN, W-K K, Moon H-J, et al. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS nano. 2014;8(12):12049–12062. doi:10.1021/nn504329u
180. Conners CM, Bhethanabotla VR, Gupta VK. Concentration‐dependent effects of alendronate and pamidronate functionalized gold nanoparticles on osteoclast and osteoblast viability. J Biomed Mater Res Part B. 2017;105(1):21–29. doi:10.1002/jbm.b.33527
181. W-K K, Heo DN, Moon H-J, et al. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J Colloid Interface Sci. 2015;438:68–76. doi:10.1016/j.jcis.2014.08.058
182. Li J, Zhang J, Wang X, Kawazoe N, Chen G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016;8(15):7992–8007. doi:10.1039/C5NR08808A
183. Yuan M, Wang Y, Qin YX. SPIO‐Au core–shell nanoparticles for promoting osteogenic differentiation of MC3T3‐E1 cells: concentration‐dependence study. J Biomed Mater Res Part A. 2017;105(12):3350–3359. doi:10.1002/jbm.a.36200
184. Ali S, Chen X, Ahmad S, et al. Morphology-controllable bimetallic gold nanostructures for mercury detection: recent developments, challenges and prospects. Arabian J Chem;2023. 104997. doi:10.1016/j.arabjc.2023.104997
185. Noah NM, Ndangili PM. Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sen Internat. 2022;3:100166. doi:10.1016/j.sintl.2022.100166
186. Anfossi L, Di Nardo F, Russo A, et al. Silver and gold nanoparticles as multi-chromatic lateral flow assay probes for the detection of food allergens. Anal Bioanal Chem. 2019;411:1905–1913. doi:10.1007/s00216-018-1451-6
187. Yuan D, Fang X, Liu Y, Kong J, Chen Q. A hybridization chain reaction coupled with gold nanoparticles for allergen gene detection in peanut, soybean and sesame DNAs. Analyst. 2019;144(12):3886–3891. doi:10.1039/C9AN00394K
188. Tian F, Zhou J, Fu R, et al. Multicolor colorimetric detection of ochratoxin A via structure-switching aptamer and enzyme-induced metallization of gold nanorods. Food Chem. 2020;320:126607. doi:10.1016/j.foodchem.2020.126607
189. Khan IM, Niazi S, Yu Y, et al. Aptamer induced multicolored AuNCs-WS2 “turn on” FRET nano platform for dual-color simultaneous detection of aflatoxinB1 and zearalenone. Anal Chem. 2019;91(21):14085–14092. doi:10.1021/acs.analchem.9b03880
190. Di Nardo F, Alladio E, Baggiani C, et al. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta. 2019;192:288–294. doi:10.1016/j.talanta.2018.09.037
191. Valentini P, Pompa PP. A universal polymerase chain reaction developer. Angew Chem Int Ed. 2016;55(6):2157–2160. doi:10.1002/anie.201511010
192. Li F, Li F, Yang G, Aguilar ZP, Lai W, Xu H. Asymmetric polymerase chain assay combined with propidium monoazide treatment and unmodified gold nanoparticles for colorimetric detection of viable emetic Bacillus cereus in milk. Sensors and Actuat B Chem. 2018;255:1455–1461. doi:10.1016/j.snb.2017.08.154
193. Trigueros SB, Domènech E, Toulis V, Marfany G. In vitro gene delivery in retinal pigment epithelium cells by plasmid DNA-wrapped gold nanoparticles. Genes. 2019;10(4):289. doi:10.3390/genes10040289
194. Sarkar K, Banerjee SL, Kundu PP, Madras G, Chatterjee K. Biofunctionalized surface-modified silver nanoparticles for gene delivery. J Mat Chem B. 2015;3(26):5266–5276. doi:10.1039/C5TB00614G
195. Kumar S, Diwan A, Singh P, et al. Functionalized gold nanostructures: promising gene delivery vehicles in cancer treatment. RSC Adv. 2019;9(41):23894–23907. doi:10.1039/C9RA03608C
196. Shrestha B, Wang L, Zhang H, Hung CY, Tang L. Gold nanoparticles mediated drug-gene combinational therapy for breast cancer treatment. Int j Nanomed. 2020;Volume 15:8109–8119. doi:10.2147/IJN.S258625
197. Yang Y, Han Y, Sun Q, et al. Au-siRNA@ aptamer nanocages as a high-efficiency drug and gene delivery system for targeted lung cancer therapy. J Nanobiotechnol. 2021;19(1):1–14. doi:10.1186/s12951-020-00759-3
198. Verma V, Al-Dossari M, Singh J, Rawat M, Kordy MG, Shaban M. A review on green synthesis of TiO2 NPs: photocatalysis and antimicrobial applications. Polymers. 2022;14(7):1444. doi:10.3390/polym14071444
199. Al-Ramamneh EA-DM, Ghrair AM, Shakya AK, et al. Efficacy of Sterculia diversifolia leaf extracts: volatile compounds, antioxidant and anti-Inflammatory activity, and green synthesis of potential antibacterial silver nanoparticles. Plants. 2022;11(19):2492. doi:10.3390/plants11192492
200. Nahari MH, Al Ali A, Asiri A, et al. Green synthesis and characterization of iron nanoparticles synthesized from aqueous leaf extract of vitex leucoxylon and its biomedical applications. Nanomaterials. 2022;12(14):2404. doi:10.3390/nano12142404
201. Ganaie SU, Abbasi T, Abbasi SA. Utilization of the terrestrial weed A ntigonon leptopus in the rapid and green synthesis of stable gold nanoparticles with shape/size control. Environ Prog Sustainable Energy. 2016;35(1):20–33. doi:10.1002/ep.12181
202. Patra JK, Baek K-H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int j Nanomed. 2015;10:7253–7264. doi:10.2147/IJN.S95483
203. Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B. 2012;98:112–119. doi:10.1016/j.colsurfb.2012.04.006
204. Royer M, Prado M, García-Pérez ME, Diouf PN, Stevanovic T. Study of nutraceutical, nutricosmetics and cosmeceutical potentials of polyphenolic bark extracts from Canadian forest species. PharmaNutrition. 2013;1(4):158–167. doi:10.1016/j.phanu.2013.05.001
205. Mahmood RI, Mohammed-Salih HS, Ghazi A, Abdulbaqi HJ, Al-Obaidi JR. Exploring the potential of copper oxide biogenic synthesis: a review article on the biomedical and dental implementations. Arab Gulf J Sci Res. 2023. doi:10.1108/AGJSR-12-2022-0315
206. Mohandoss S, Murugaboopathy V, Haricharan PB, et al. Ulvan as a reducing agent for the green synthesis of silver nanoparticles: a novel mouthwash. Inorganics. 2023;11(1):5. doi:10.3390/inorganics11010005
207. Bin-Jardan LI, Almadani DI, Almutairi LS, et al. Inorganic Compounds as Remineralizing Fillers in Dental Restorative Materials: narrative Review. Int J Mol Sci. 2023;24(9):8295. doi:10.3390/ijms24098295
208. Qiu W, Zhou Y, Li Z, et al. Application of antibiotics/antimicrobial agents on dental caries. Biomed Res Int. 2020;2020:1–11. doi:10.1155/2020/5658212
209. Hernández-Sierra JF, Ruiz F, Pena DCC, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomed Nanotechnol Biol Med. 2008;4(3):237–240. doi:10.1016/j.nano.2008.04.005
210. Park SR, Lee HW, Hong JW, et al. Enhancement of the killing effect of low-temperature plasma on Streptococcus mutans by combined treatment with gold nanoparticles. J Nanobiotechnol. 2014;12:1–8. doi:10.1186/s12951-014-0029-5
211. Elgamily HM, El-Sayed HS, Abdelnabi A. The antibacterial effect of two cavity disinfectants against one of cariogenic pathogen: an in vitro comparative study. Contemp Clin Dent. 2018;9(3):457. doi:10.4103/ccd.ccd_308_18
212. Khan SA, Shahid S, Lee C-S. Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules. 2020;10(6):835. doi:10.3390/biom10060835
213. Ifijen IH, Maliki M, Udokpoh NU, Odiachi IJ, Atoe B. A Concise Review of the Antibacterial Action of Gold Nanoparticles Against Various Bacteria. Springer; 2023:655–664.
214. Nair GM, Sajini T, Mathew B. Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications. Talanta Open. 2022;5:100080. doi:10.1016/j.talo.2021.100080
215. Dubadi R, Huang SD, Jaroniec M. Mechanochemical synthesis of nanoparticles for potential antimicrobial applications. Materials. 2023;16(4):1460. doi:10.3390/ma16041460
216. Kumar A, Choudhary A, Kaur H, Mehta S, Husen A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J Nanobiotechnol. 2021;19(1):256. doi:10.1186/s12951-021-00996-0
217. Hassan H, Sharma P, Hasan MR, Singh S, Thakur D, Narang J. Gold nanomaterials–The golden approach from synthesis to applications. Mater Sci Energy Technol. 2022;5:375–390. doi:10.1016/j.mset.2022.09.004
218. Rabiee N, Ahmadi S, Akhavan O, Luque R. Silver and gold nanoparticles for antimicrobial purposes against multi-drug resistance bacteria. Materials. 2022;15(5):1799. doi:10.3390/ma15051799
219. Lee KX, Shameli K, Yew YP, et al. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int j Nanomed. 2020;Volume 15:275–300. doi:10.2147/IJN.S233789
220. Sharma P, Kaushal A, Kaushal A. Green nanoparticle formation toward wound healing, and its application in drug delivery approaches. Eur J Med Chem Rep. 2022;6:100088. doi:10.1016/j.ejmcr.2022.100088
221. Londhe S, Haque S, Patra CR. Silver and gold nanoparticles: potential cancer theranostic applications, recent development, challenges, and future perspectives. In: Gold and Silver Nanoparticles. Elsevier; 2023:247–290.
222. Strużyńska L, Dąbrowska-Bouta B, Sulkowski G. Developmental neurotoxicity of silver nanoparticles: the current state of knowledge and future directions. Nanotoxicology. 2022;16(4):500–525. doi:10.1080/17435390.2022.2105172
223. Chauhan A, Khan T, Omri A. Design and encapsulation of immunomodulators onto gold nanoparticles in cancer immunotherapy. Int J Mol Sci. 2021;22(15):8037. doi:10.3390/ijms22158037
224. Almeida JPM, Figueroa ER, Drezek RA. Gold nanoparticle mediated cancer immunotherapy. Nanomed Nanotechnol Biol Med. 2014;10(3):503–514. doi:10.1016/j.nano.2013.09.011
225. Siddique S, Chow JC. Gold nanoparticles for drug delivery and cancer therapy. Appl Sci. 2020;10(11):3824. doi:10.3390/app10113824
226. Kanwar R, Rathee J, Salunke DB, Mehta SK. Green nanotechnology-driven drug delivery assemblies. ACS omega. 2019;4(5):8804–8815. doi:10.1021/acsomega.9b00304
227. Li H, Wang Y, Tang Q, et al. The protein Corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021;129:57–72. doi:10.1016/j.actbio.2021.05.019
228. Ryu H-J, Lee WK, Kim YH, Lee J-S. Interfacial interactions of SERS-active noble metal nanostructures with functional ligands for diagnostic analysis of protein cancer markers. Mikrochim Acta. 2021;188:1–26. doi:10.1007/s00604-021-04807-z
229. Mehrizi TZ, Ardestani MS, Kafiabad SA. A review of the use of metallic nanoparticles as a novel approach for overcoming the stability challenges of blood products: a narrative review from 2011–2021. Current Drug Deliv. 2023;20(3):261–280. doi:10.2174/1567201819666220513092020
230. Saleh TA, Fadillah G. Green synthesis protocols, toxicity, and recent progress in nanomaterial-based for environmental chemical sensors applications. Trend Environm Analy Chem. 2023;2023:e00204.
231. Fan J, Cheng Y, Sun M. Functionalized gold nanoparticles: synthesis, properties and biomedical applications. Chem Rec. 2020;20(12):1474–1504. doi:10.1002/tcr.202000087
232. Srivastava N, Singh A, Kumari P, et al. Advances in extraction technologies: isolation and purification of bioactive compounds from biological materials. In Natural Bioactive Compounds. Elsevier; 2021:409–433.
233. Chen X, Xue Z, Ji J, et al. Hedysarum polysaccharides mediated green synthesis of gold nanoparticles and study of its characteristic, analytical merit, catalytic activity. Mater Res Bull. 2021;133:111070. doi:10.1016/j.materresbull.2020.111070
234. Asariha M, Kiaie SH, Izadi SH, Pirhayati F, Fouladi M, Gholamhosseinpour M. Extended-release of doxorubicin through green surface modification of gold nanoparticles: in vitro and in ovo assessment. BMC Chemistry. 2022;16(1):110. doi:10.1186/s13065-022-00895-x
235. Khan MA, Singh D, Ahmad A, Siddique HR. Revisiting inorganic nanoparticles as promising therapeutic agents: a paradigm shift in oncological theranostics. Eur J Pharm Sci. 2021;164:105892. doi:10.1016/j.ejps.2021.105892
236. Oladipo AO, Lebelo SL, Msagati TA. Nanocarrier design–function relationship: the prodigious role of properties in regulating biocompatibility for drug delivery applications. Chem Biol Interact. 2023;377:110466. doi:10.1016/j.cbi.2023.110466
237. Abbasi R, Shineh G, Mobaraki M, Doughty S, Tayebi L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: a review. J Nanopart Res. 2023;25(3):43. doi:10.1007/s11051-023-05690-w
238. Gupta D, Thakur A, Gupta TK, Gupta TK. Green and sustainable synthesis of nanomaterials: recent advancements and limitations. Environ Res. 2023;231:116316. doi:10.1016/j.envres.2023.116316
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.