Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Recalcitrant Pyoderma Gangrenosum: Clinical Burden and Unmet Needs
Authors Becker SL, Velasco R, Ortega-Loayza AG
Received 29 May 2023
Accepted for publication 29 July 2023
Published 9 August 2023 Volume 2023:16 Pages 2143—2152
DOI https://doi.org/10.2147/CCID.S381490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Sarah L Becker,1,* Rose Velasco,2,* Alex G Ortega-Loayza1
1Department of Dermatology, Oregon Health & Science University, Portland, OR, USA; 2University of Illinois College of Medicine, Chicago, IL, USA
*These authors contributed equally to this work
Correspondence: Alex G Ortega-Loayza, Department of Dermatology, Oregon Health & Science University, 3303 S Bond Ave Building 1, 16th Floor, Portland, OR, 97239, USA, Tel +1 503-418-3376, Fax +1 503-494-6968, Email [email protected]
Abstract: Pyoderma gangrenosum (PG) is a rare, autoinflammatory disease leading to aseptic ulcers which carries a significant disease burden and is often difficult to treat, with many patients failing first-line treatment and requiring additional therapies. Such cases are typically referred to in the literature as “recalcitrant”, “refractory”, or “resistant”, though little is known about the clinical characteristics of such cases. We performed a narrative literature review to characterize patient demographics and clinical course associated with difficult to treat pyoderma gangrenosum cases in order to identify trends to guide future clinical management and therapeutic innovation. We identified 148 cases with clinical manifestations and associated patient demographics stratified by ulcer and patient features. Consistent with previous work, a greater prevalence of PG was observed among female patients and those with a history of inflammatory bowel disease, however interestingly despite an aggressive course to their PG, few patients had comorbidities complicating their disease course. Additionally, despite the requirement of three or more treatments for most patients’ disease to resolve, the majority healed within the typical window observed in previous clinical studies with low rates of recurrence. Biologics were the most common medication patients were on at time of remission. Collectively, our results suggest a potential benefit for a reduced threshold for biologic initiation in PG patients and a need for standardization of language in the field to facilitate treatment outcomes comparisons and interventions.
Keywords: pyoderma gangrenosum, recalcitrant, refractory, resistant, biologics
Introduction
Pyoderma gangrenosum (PG) is a rare, autoinflammatory disease which causes characteristic ulcerations with aseptic neutrophilic infiltration into the skin. While PG is rare with an incidence of about 3–10 million cases annually, it is often difficult to treat and carries a significant disease burden with three times increased risk of mortality1 and a high morbidity due to delayed wound healing, scarring, and psychological effects from unpredictable clinical course and challenging management.2
The etiology of PG is currently unknown, though it is hypothesized to involve neutrophilic dysfunction and systemic inflammation in a genetically predisposed individual. PG affects patients of both sexes and of any age, however it is more common in middle-aged women, with the average age of onset 51.6 years3–5 and most cases occurring in those of Caucasian descent.5,6 Up to 50% of cases are associated with comorbidities, most commonly inflammatory bowel disease, autoimmune conditions like arthritis both rheumatologic and seronegative, and myeloproliferative disorders.7
PG has a heterogeneous disease presentation with numerous variants including ulcerative, bullous, pustular, vegetative, peristomal, and post-surgical. While there are emerging diagnostic frameworks, these have yet to be uniformly adopted and PG remains a clinical diagnosis and is frequently misdiagnosed.8 The disease’s rarity, coupled with its diversity of presentation and associated factors, have limited the availability of high-level evidence. There are very few randomized controlled trials investigating treatment of PG and so therapy remains based on case reports and small, cohort studies in addition to a few expert reviews.9 Therefore, there is a need for a systematic approach and therapeutic strategy for management of PG. Current standard of care includes systemic immunosuppressants as first line therapies and biologics are now becoming more popular but in clinical practice patients have varied responses to treatment and often require combination regimens.
Regrettably, some patients fail these treatments, with difficult to treat PG described in the literature as “recalcitrant”, “refractory”, or “recurrent”. However, such terms are often used interchangeably and without discretion, complicating their true clinical meaning and limiting the utility of assessing treatment response in such cases. To date, there is no consensus as to the order of treatment administration based on disease presentation and patient characteristics. To better understand patient characteristics associated with these terms, used as a proxy in the literature for difficult to treat PG, we performed a qualitative literature review of associated patient factors in cases described as “recalcitrant”, “refractory”, or “resistant”, with the primary objective of determining clinical burden, associated factors to guide management and future therapeutic directions based on the unmet needs.
Methods
An extensive narrative literature review was performed for each “recalcitrant”, “refractory”, and “resistant” PG case reports using PubMed. Search strings used were as follows: “recalcitrant pyoderma gangrenosum”, “refractory pyoderma gangrenosum”, and “resistant pyoderma gangrenosum”. Although we did not use specific criteria to define PG cases, we used the diagnosis provided by the authors and accepted in peer-reviewed studies. Two reviewers (S.L.B., R.V.) applied the study selection criteria independently to screen the titles and abstracts of all identified citations in a nonblinded manner and the full‐text articles of selected citations were then independently assessed based on previously reported associated features and characteristics of PG [Supplementary Table 1]. Data was compiled on patient demographics, ulcer characteristics, significant past medical history, treatments attempted, and outcomes, where healed was defined as complete re-epithelization following treatment. Once organized, frequencies were calculated via Microsoft Excel for each common term and all combined cases, to note trends distinguishing each PG definition. To review, cases described as “recalcitrant”, “refractory”, and “resistant” were grouped for analysis to aid in analysis and presentation of results though subgroups analysis was also performed.
Results
Demographics
148 total cases were reviewed; one case was labeled as both “refractory” and “resistant” (Table 1). The demographics of patients were similar throughout, with 74 cases (50.0%) within the 41 to 65-year-old age group and 87 cases (58.8%) of female sex, aligning with previous etiology reports. 107 cases (72.3%) were presented in dermatology-related journals followed by 12 cases in wound care journals (8.1%).
 |
Table 1 Demographics. Data Given as Numbers (Percentages) of Patients |
Presentation
Patient presentation was largely consistent with previous reports of PG. The majority of PG cases (73.0%) were defined as classical or ulcerative type, involving the lower extremity in 98 cases (68%) (Table 2). Only 11 (7.4%) patients presented with extracutaneous symptoms.
 |
Table 2 Ulcer Presentation. Data Given as Numbers (Percentages) of Patients |
Past Medical History
Overall, 44 cases (29.7%) had underlying inflammatory bowel disease; of these, 19 (43.2%) had Crohn’s disease, 19 (43.2%) with ulcerative colitis, and six (13.6%) unspecified (Table 3). The next common concomitant medical condition was arthritis in 24 cases (16.2%), of which 19 (79.2%) were of inflammatory subset. Blood disorders were seen in 10 cases (6.8%), of which six were monoclonal gammopathy of undetermined significance (60.0%). Among other unrelated comorbidities, diabetes and hypertension were the most prevalent, with 14 (9.5%) and 11 cases (7.4%), respectively.
 |
Table 3 Past Medical History. Data Given as Numbers (Percentages) of Patients |
Treatment
Of the 42 cases (28.4%) on systemic treatments, immunosuppressants were favored in 27 (64.3%), and 19 cases (45.2%) were prescribed a systemic antibiotic (Table 4). Other pre-diagnosis treatments included topical in 11 cases (7.4%), surgical in 9 cases (6.1%), and general wound care in 5 cases (3.4%).
 |
Table 4 Treatment. Data Given as Numbers (Percentages) of Patients |
Once diagnosed, treatment favored systemic immunosuppressants in 135 cases (91.2%), with 96 cases (64.9%) prescribed multiple at one time (Table 5). Biologic treatments were the next most common treatment used in 80 cases (54.1%); of those, 28 patients (40.6%) had an underlying IBD and 11 patients (15.9%) had arthritis. Other treatments included immunomodulators, topicals, surgical and infusion therapy and systemic antibiotics.
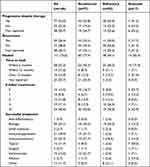 |
Table 5 Outcome. Data Given as Numbers (Percentages) of Patients |
Outcomes
77 cases (52.0%) showed progression of ulcer size despite treatment, and 39 cases (26.4%) had recurrence. Of the 111 cases (75.0%) that healed completely, 58 (52.3%) were within six months on treatment, however 54 cases (48.6%) had failed more than three treatment options in the process. Of those who healed, successful treatment modality was mostly seen on biologic medications in 39 cases (35.1%), systemic immunosuppression in 20 cases (18.0%), immunomodulators in 23 cases (20.7%), and intramuscular corticosteroids in 15 cases (13.5%).
In the 37 cases (25.0%) who did not heal, several had additional burdens of disease: large ulcer in 9 cases (24.3%), three or more ulcers in 8 cases (21.6%), underlying inflammatory bowel disease in 8 cases (21.6%), a positive microbiological culture in 7 cases (18.9%), diabetes and/or hypertension in 7 cases (18.9%), underlying arthritis in 5 cases (13.5%), ulcer with a depth to tendon or muscle in 4 cases (10.8%), obesity in 3 cases (8.1%), more than three months to diagnosis in 3 cases (8.1%), and age over 66 years in 3 cases (8.1%).
Discussion
In this retrospective review of 148 PG case studies described as “recalcitrant”, “refractory”, or “resistant”, we found most patients did not have significant prior medical history or risk factors predisposing them to a more severe course. Despite being labeled as having more aggressive manifestations of PG, most patients healed within a time frame that has been described previously (within 6 months) and commonly responded to biologics.
Consistent with previous work, a greater prevalence of PG was observed among female patients and most cases involved the lower extremity or trunk (when occurring as peristomal ulcers).1,6,10 Similarly, a history of inflammatory bowel disease was common with a prevalence of 13.6–31% among patients with a reported past medical history, a well-documented comorbidity in patients with PG.5,6
It is interesting that though only 7% of patients reported a past medical history of blood disorders, 55% had a diagnosis of monoclonal gammopathy of unknown significance (MGUS). This could potentially reflect a hesitancy for physicians to treat PG in these patients more aggressively due to concern for cancerous conversion leading to PG progression and recurrence.11 While the risks and benefits for each individual patient may alter treatment protocols, earlier, more aggressive PG treatment in patients with a history of MGUS might prevent PG progression thereby decreasing overall morbidity. It is also possible the increased relative incidence of MGUS among patients presented in this review points to yet undiscovered changes to the immune system with MGUS which predispose patients to PG.
Note that over 2/3 of cases did not receive immunosuppressive treatment before receiving the diagnosis of PG which might have contributed to the recalcitrant stage when later receiving appropriate treatment. Moreover, over a 1/3 of cases were prescribed a systemic antibiotic to aid treatment of their ulcer, aligning with previous studies,12 however only 14.2% of cases described a positive microbiological culture. This discrepancy may be due to misdiagnosis of PG, use of antimicrobial agents with anti-inflammatory properties, inadequate reporting of culture results, or prophylactic treatment of high-risk immunocompromised patients.13,14
Despite an aggressive PG course, few patients had significant reported past medical history, with many described as previously healthy prior to PG onset. As comorbidities affecting wound healing, such as venous stasis dermatitis, have been reported in association with PG with incidence as high as 16%,6 a lack of previous medical history may have delayed more aggressive treatment in these patients. Comorbid conditions present a particular challenge in PG patients as it can be difficult to ascertain the benefit of a therapy on PG itself versus its benefit in treating a comorbid condition, such as IBD or autoimmune disease, which may worsen PG symptoms and therefore mask the direct effect of a particular drug. For example, biologics are commonly used in the treatment of IBD but their effect on PG in a particular patient may or may not improve the patient’s PG, and if it does improve, it is often unclear if this is a direct effect of the biologic on the PG itself or due to the biologic’s improvement of the patient’s IBD, thereby improving their PG indirectly. However, many patients with difficult to treat PG did not have significant past medical history (eg IBD) which may reflect the current insurance landscape in which paradoxically the presence of a comorbidity can expedite insurance coverage for certain medications, such as biologics. This could worsen outcomes for patients previously healthy prior to PG onset, though additional research is needed to further investigate such associations due to numerous confounders such as social determinants of health which also influence health status and interactions with health systems.
Even with use of three or more treatments, most patients healed within the typical window observed in clinical trials15,16 with low rates of recurrence. Biologics were the most common medication patients were on at the time of healing, though most patients were maintained on multiple therapies concurrently and therefore the precise added benefit of a biologic cannot be quantified from these results. There are numerous reports in the literature of the benefit of biologics in treatment-resistant cases of PG, with adalimumab and infliximab being perhaps the most well-documented.16–18 Of note, while such agents continue to be used off-label in the United States, Japan has recently approved adalimumab for use in PG which could be seen as an indication of the growing consensus regarding the benefit of biologics in PG.19 Therefore, the observed benefit from biologics we observed in our review might argue for a lower threshold to begin a biologic in PG patients, such as a patient who has failed two or more previous treatments. The reinforced utility of biologics also advances a need for a decreased threshold for prior authorizations even without comorbidities given the observed clinical benefit for PG patients.20
Our review also emphasizes the need for standardization of language within the field. The terms “recalcitrant”, “refractory”, and “resistant” were sometimes used interchangeably even within the same paper without a clear meaning of their respective clinical significance. Differences in classification of difficult to treat PG may simply reflect semantic variance between geographic region, physician background, or journal type rather than reflect clinically distinct disease manifestations. The indiscriminate use of such similar terms may obscure real clinical differences between presentations and there is benefit to defining the clinical course for each subgroup to determine if there are differences which could better guide management.
Furthermore, standardization of language in the field would help mitigate misdiagnosis, thereby reducing time to diagnosis and treatment and hopefully resulting in improved outcomes and decreased burden of disease. PG remains largely a clinical diagnosis despite the emergence of diagnostic criteria which have yet to be broadly adopted, resulting in misdiagnosis.21 Due to the lack of diagnostic criteria or lack of reporting of diagnostic reasoning outlined in this review such trends cannot be identified herein, though the authors acknowledge differences in diagnostic standards influence patient outcomes and increased adherence to diagnostic criteria have been described to improve patient outcomes due to decreased misdiagnosis and optimized management.10 The use of standardized language and diagnostic criteria would improve both research methodology but more importantly patient outcomes. Efforts to standardize PG research, such as work by the C3 UPGRADE (Understanding Pyoderma Gangrenosum: Review and Analysis of Disease Effects) initiative19 are a crucial step though more work is needed for such initiatives to be more universally adopted.
Limitations for this study include, notably, publication bias which may artificially inflate the number of cases reported as healed. Previous reports of PG treatment efficacy have estimated the percentage healed as closer to 47% in a study comparing ulcer healing in response to cyclosporine or prednisone after six months15 while an analysis of patients in a registry from our institution found a healing rate of only 38% over nine months in response to various treatments.22 Therefore, the true healing rate in difficult to treat cases of PG may be lower than we identified. While gaps in reported data were minimized as much as possible through coding and analysis, a significant percentage of measured outcomes were not reported in the reviewed cases and therefore could not be analyzed.
Finally, management of PG remains challenging due to the diverse presentation of the disease and heterogeneity in treatment response to standard therapeutics, with many patients presenting with difficult to treat PG labeled as “refractory”, “recalcitrant”, or “resistant”. Our literature review did not identify specific risk factors predisposing patients to such a challenging disease course, however most patients did heal within a clinically acceptable period and commonly responded to biologics. We also propose treatment of such cases may be impeded by imprecise diagnosis and description by relying on dated terminology which does not carry clinical significance. A better understanding of patient characteristics associated with difficult to treat cases is needed to better stratify risk and provide more targeted, earlier intervention.23 Additionally, widespread usage of diagnostic criteria and standardization of language within the field would further facilitate early diagnosis and classification of severity and improve patient outcomes with timely intervention and management. Future directions will include molecular and genetic analysis in a controlled clinical setting (eg, clinical trial) to determine the true impact of new therapeutics in the healing of PG patients as well as continued research into the mechanisms of biologics in this patient population to better understand benefits and predict patient response to specific agents.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Langan SM, Groves RW, Card TR, et al. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Investig Dermatol. 2012;132(9):2166–2170. doi:10.1038/jid.2012.130
2. Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18(3):355–372. doi:10.1007/s40257-017-0251-7
3. Ruocco E, Sangiuliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23(9):1008–1017. doi:10.1111/j.1468-3083.2009.03199.x
4. Wollina U. Pyoderma gangrenosum--a review. Orphanet J Rare Dis. 2007;2:19. doi:10.1186/1750-1172-2-19
5. Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154(4):409–413. doi:10.1001/jamadermatol.2017.5978
6. Orfaly VE, Reese AM, Friedman M, et al. Pyoderma gangrenosum study pilot registry: the first step to a better understanding. Wound Repair Regen. 2022;30(3):334–337. doi:10.1111/wrr.13005
7. Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73(4):691–698. doi:10.1016/j.jaad.2015.06.021
8. Haag C, Hansen T, Hajar T, et al. Comparison of three diagnostic frameworks for pyoderma gangrenosum. J Invest Dermatol. 2021;141(1):59–63. doi:10.1016/j.jid.2020.04.019
9. Almeida IR, Coltro PS, Gonçalves HOC, et al. The role of negative pressure wound therapy (NPWT) on the treatment of pyoderma gangrenosum: a systematic review and personal experience. Wound Repair Regen. 2021;29(3):486–494. doi:10.1111/wrr.12910
10. Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sex-adjusted population analysis. J Am Acad Dermatol. 2020;83(2):425–429. doi:10.1016/j.jaad.2019.08.001
11. Machan A, Azendour H, Frikh R, et al. The dilemma of treating pyoderma gangrenosum associated with monoclonal gammopathy of undetermined significance. Dermatol Online J. 2020;26(5). doi:10.5070/D3265048791
12. Reese AM, Gupta AS, Latour E, et al. Clinical characteristics and misdiagnosis of pyoderma gangrenosum of the head and neck: a retrospective study. J Am Acad Dermatol. 2022;87(5):1130–1133.
13. Chow RK, Ho VC. Treatment of pyoderma gangrenosum. J Am Acad Dermatol. 1996;34(6):1047–1060. doi:10.1016/S0190-9622(96)90285-6
14. Quist SR, Kraas L. Treatment options for pyoderma gangrenosum. J Dtsch Dermatol Ges. 2017;15(1):34–40.
15. Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958. doi:10.1136/bmj.h2958
16. Yamasaki K, Yamanaka K, Zhao Y, et al. Adalimumab in Japanese patients with active ulcers of pyoderma gangrenosum: twenty-six-week Phase 3 open-label study. J Dermatol. 2020;47(12):1383–1390. doi:10.1111/1346-8138.15533
17. Herberger K, Dissemond J, Brüggestrat S, Sorbe C, Augustin M. Biologics and immunoglobulins in the treatment of pyoderma gangrenosum - analysis of 52 patients. J Dtsch Dermatol Ges. 2019;17(1):32–41.
18. Agarwal A, Andrews JM. Systematic review: IBD-associated pyoderma gangrenosum in the biologic era, the response to therapy. Aliment Pharmacol Ther. 2013;38(6):563–572. doi:10.1111/apt.12431
19. Mital R, Gray A, Minta A, et al. Novel and off-label biologic use in the management of hidradenitis suppurativa, pyoderma gangrenosum, lichen planus, and seborrheic dermatitis: a narrative review. Dermatol Ther. 2023;13(1):77–94. doi:10.1007/s13555-022-00860-5
20. Maronese CA, Pimentel MA, Li MM, et al. Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol. 2022;23(5):615–634. doi:10.1007/s40257-022-00699-8
21. Weenig RH, Davis MDP, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347(18):1412–1418. doi:10.1056/NEJMoa013383
22. Orfaly VE, Kovalenko I, Tolkachjov SN, et al. Tofacitinib for the treatment of refractory pyoderma gangrenosum. Clin Exp Dermatol. 2021;46(6):1082–1085. doi:10.1111/ced.14683
23. Malachowski SJ, Latour E, Ortega-Loayza AG. Clinical and laboratory factors associated with wound healing in patients with pyoderma gangrenosum: a retrospective study. Wounds. 2022;34(6):178–184. doi:10.25270/wnds/2022.0408
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
