Back to Journals » Patient Preference and Adherence » Volume 14
Real-World Treatment Patterns of Disease Modifying Therapy (DMT) for Patients with Relapse-Remitting Multiple Sclerosis and Patient Satisfaction with Therapy: Results of the Non-Interventional SKARLET Study in Slovakia
Authors Turčáni P , Mašková J, Húska J
Received 24 March 2020
Accepted for publication 20 June 2020
Published 7 July 2020 Volume 2020:14 Pages 1129—1135
DOI https://doi.org/10.2147/PPA.S254427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Peter Turčáni,1 Jana Mašková,2 Jozef Húska3
1First Department of Neurology, Faculty of Medicine, Comenius University, Bratislava 81369, Slovak Republic; 2NEOX Clinical Research, Prague 1 110 00, Czech Republic; 3Sanofi-Aventis Slovakia, Bratislava 851 01, Slovak Republic
Correspondence: Jozef Húska Email [email protected]
Background: During long-term multiple sclerosis therapy, patient satisfaction with received treatment has considerable impact on treatment outcomes. Here we report the results of a non-interventional real-world study that mapped the treatment patterns of disease-modifying therapy (DMT) and assessed treatment satisfaction with DMT.
Patients and Methods: The SKARLET study was a non-interventional, cross-sectional study in Slovakia running from May 2016 to March 2017. Patients with relapsing-remitting multiple sclerosis on DMT for ≥ 3 months and ≤ 2 years (per local labelling) from 10 multiple sclerosis centers across Slovakia were included. The primary objective was to collect the Treatment Satisfaction Questionnaire for Medication version 9 (TSQM 9) score regarding perceived effectiveness, convenience and overall satisfaction with DMT.
Results: The following TSQM 9 scores (mean; 95% confidence interval) were reported from 415 patients: convenience (75.05; 73.49– 76.61), effectiveness (68.15; 66.56– 69.75) and global satisfaction scale (66.94; 65.26– 68.62). All three parameters of the TSQM 9 were analyzed by the route of DMT administration, with infusions best rated for effectiveness and global satisfaction in comparison to oral dosage and injections. For convenience, however, oral dosage forms were appraised highly (82.66; 80.59– 84.73) followed by infusions (74.40; 70.12– 78.69), while injections were rated as the worst (66.92; 64.81– 69.04). The difference of TSQM 9 scores according to the route of DMT administration is statistically significant for convenience (p < 0.001) and global satisfaction (p = 0.004), but not for effectiveness (p = 0.185).
Conclusion: In the present study, it was confirmed that patients find oral DMTs as most convenient; however, the infusion form of treatment outweighs oral DMTs in global satisfaction and effectiveness. The differences of TSQM 9 scores among DMT dosage forms were significant for convenience and global satisfaction. In conclusion, the results of this detailed survey increase our understanding of RRMS patient population characteristics and patient satisfaction with DMT treatment.
Keywords: multiple sclerosis, disease-modifying therapy, treatment satisfaction, treatment pattern, real-world
Introduction
In the management of multiple sclerosis (MS), a wide variety of new disease-modifying therapies (DMT) have been recently introduced what brings new opportunities for individualized therapy, where patients and healthcare providers must balance considerations of efficacy, side effects and long-term impact in a shared decision process.1 As a result, patients’ preferences become more important in decision-making.2 Since MS is a debilitating life-long condition seriously affecting the quality of life and the clinical outcome is directly related to patient adherence to treatment,3–8 high patient satisfaction with the chosen DMT plays a key role in successful MS management.
In the real world, the DMT selection for an individual patient is affected by approved labelling, availability in a particular country, local treatment guidelines and/or reimbursement criteria. In Slovakia, DMT selection is mainly driven by the reimbursement criteria when the health-care payer approves a chosen DMT for an individual patient. At the time of data collection for this study, from May 2016 to March 2017, Slovakian reimbursement criteria considered interferons, glatiramer acetate, teriflunomide and dimethyl fumarate as equals for the initiation of relapsing-remitting multiple sclerosis (RRMS) treatment. A DMT switch was allowed for inadequate efficacy - no decrease of relapse rate, the presence of a Gd+T1 lesion or a significant increase of T2 lesions, or an increase in the Expanded Disability Status Scale (EDSS) score by ≥1 point while on treatment. Two DMTs from this 1st line group had to be introduced before escalation to DMTs with higher efficacy (fingolimod and natalizumab). According to reimbursement criteria, alemtuzumab was reserved for patients who failed three DMTs with different modes of action. Since 1 January 2016, dimethyl fumarate can be prescribed only to patients with a persistent 3-month EDSS change after relapse, regardless of treatment by corticosteroids or the presence of a Gd+ T1 lesion or infratentorial localization of lesions, or to patients who have failed two DMTs with different mechanisms of action. For rapidly evolved MS (two relapses within 12 months and the presence of a Gd+T1 lesion or a significant increase of T2 lesions), fingolimod and/or natalizumab or alemtuzumab (from 1 January 2017) were recommended as the first-choice treatment.
The primary objective of the SKARLET study was to assess the treatment satisfaction with DMTs in real-life setting using the TSQM 9 in patients with RRMS in routine clinical care in Slovakia. Here we also report the results of secondary objectives to characterize treatment patterns and relationships of DMT dosage form with TSQM scores.
Patients and Methods
Patient Population
The study was conducted in 10 out-patient centers specialized in the treatment of MS across Slovakia.
The study population consisted of adult patients (≥18 years of age) diagnosed with RRMS according to revised McDonald 2010 criteria9 who had been receiving DMT for ≥ 3 months and ≤ 2 years in real-life clinical practice, and in accordance with Summary of Product Characteristics and standard clinical practice. Patients had to be able to fulfil questionnaires independently, and sign and date informed consent before undergoing any study-specific procedure. Repeated participation in the SKARLET registry was not allowed.
Study Objectives
The primary objective of the study was to assess the treatment satisfaction with DMT in real-life settings using descriptive statistics of the TSQM 9 scores in patients with RRMS in routine clinical care in Slovakia. TSQM 9 was created from TSQM Version 1.4 but without the five items from the domain of side effects that has the potential to interfere with routine clinical care. TSQM-9 consists of three domains: the effectiveness scale (3 questions), the convenience scale (3 questions) and the global satisfaction scale (3 questions).10 Scores range from 0 to 100; higher scores indicate greater satisfaction. TSQM scores were assessed for all DMTs in total, separately for each route of administration (oral, injection, infusion), and separately for each DMT if used by more than 10 patients to avoid bias caused by a small number of patients in the relevant subgroup. Furthermore, the following endpoints were collected to characterize treatment patterns in DMTs for RRMS in the real-world: current DMT, any previous DMT, reason for discontinuing a previous DMT, the duration of a previous DMT, dosage form of the previous and current DMT, and EDSS11 at the start and end of each DMT.
Study Design/Data Collection
SKARLET was a non-interventional, cross-sectional study in Slovakia conducted from 2 May 2016 to 1 March 2017.
This registry was performed in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice,12 the Declaration of Helsinki,13 and any applicable local laws and regulations. Ethical committee of Faculty of Medicine, Comenius University and University Hospital Bratislava, Old Town Hospital approved the study before enrollment of the first patient.
Eligible patients who agreed to participate in the study and signed the informed consent form were enrolled on a consecutive basis during their routine office visits. During the only study-related visit (V1), they completed the TSQM 9 questionnaire, and answered questions about satisfaction with current therapy in comparison to the previous therapy (if treated with more than one DMT). Other required information was recorded from the medical documentation retrospectively. Data entered into the electronic Case Report Forms (eCRFs) were reviewed by data management for completeness and accuracy. In the case of any irregularity, additional queries were generated by the data manager to make any required corrections or additions. Furthermore, random data quality control was performed by a qualified site monitor for 5% of records on-site.
Statistics
The analysis of collected data was based on descriptive statistics including absolute and relative frequencies of discrete variables. Continuous variables were described by: count, mean, standard deviation, median, minimum, maximum and 2-sided 95% confidence interval (CI) of the mean. Discrete variables were described by: count and percentages. Null hypotheses that there is no difference in TSQM 9 scores among different dosage forms of DMT were tested by independent samples Kruskal–Wallis Test at the level of significance of 0.05. Normal distribution of the data was excluded by Shapiro–Wilk test also at the level of significance of 0.05.
Results
Overall, 424 patients were screened and 417 were enrolled and evaluable for the primary analysis. This sample size corresponds to approximately 10% of the treated MS population in Slovakia.
Demographic and baseline disease characteristics of the study population are summarized in Table 1. The gender ratio (males: females) was 1:2 (32.6%:67.4%) with no major difference among individual drugs, except for glatiramer acetate with nearly 80% of female users. 72% of patients had an EDSS within the range 0–3.
 |
Table 1 Demographic and Baseline Disease Characteristics |
The distribution of current DMT treatment is summarized in Table 2.
 |
Table 2 Proportion of Patient by Current DMT |
Out of 417 patients, 185 (44.4%) were newly diagnosed patients on their first DMT. 232 patients (55.6%) had received a previous DMT. Overall, 367 DMT switches in total were reported in the SKARLET study. Reasons for discontinuing a previous DMT are summarized in Table 3. Disease activity accounted for 68% of DMT switches. The mean EDSS change during a previous treatment until a switch was 0.558 (95% CI: 0.480–0.636). According to single question asking patients with a DMT switch to evaluate the new DMT in comparison to the previous one, patients were generally satisfied with their treatment switch, with 82.6% patients more satisfied with the current treatment than the previous, 14.8% patients were equally satisfied with current and previous treatments, and 2.7% were more satisfied with the previous treatment than the current treatment.
 |
Table 3 Reasons for Discontinuing a Previous DMT |
The median duration of any previous DMT was 2 years (mean 33.6 months with 95% CI: 30.56–36.82 months).
When evaluating the last treatment switch with respect to the route of DMT administration, subsequent treatment using the oral form was the most frequent choice (61.2%), followed by switches between injectables (19.0%) and switches to infusions (16.8%) (Table 4).
 |
Table 4 DMT Switches from the Last Previous DMT to the Current DMT by Dosage Form |
Three parameters of TSQM 9, effectiveness, convenience and global satisfaction, were analyzed separately for all DMTs in total, for DMTs by the route of administration and separately for each DMT. When assessing all DMTs in total (Table 5), the highest score (mean; 95% CI) was reported for convenience (75.05; 73.49–76.61), followed by effectiveness (68.15; 66.56–69.75), with the lowest for global satisfaction (66.94; 65.26–68.62).
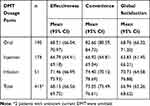 |
Table 5 TSQM 9 Scores of the Current DMT by Dosage Form – Effectiveness, Convenience and Global Satisfaction |
TSQM 9 parameters analyzed separately by route of DMT administration are summarized in Table 5, with infusions rated best for effectiveness and global satisfaction in comparison to oral dosage and injections. For convenience (mean; 95% CI), oral forms were appraised highly (82.66; 80.59–84.73), followed by infusions (74.40, 70.12–78.69), while injections were rated as the worst (66.92; 64.81–69.04).
The difference of TSQM 9 scores by the DMT route of administration is statistically significant for convenience (p < 0.0001) and global satisfaction (p = 0.004), but not for effectiveness (p = 0.185).
Furthermore, we evaluated TSQM-9 scores separately for each DMT (Table 6). To avoid bias caused by a small number of patients, we included only DMTs used by more than 10 patients. The greatest difference among DMTs was for convenience, with a range from 85.12 (teriflunomide) to 65.36 (interferon beta-1b). Global satisfaction ranged from 71.25 (natalizumab) to 62.18 (interferon beta-1b) and effectiveness from 71.95 (natalizumab) to 65.41 (interferon beta-1a subcutaneous).
 |
Table 6 TSQM 9 Scores for Each Current DMT and in Total |
Discussion
The rapid increase in the number of disease-modifying drugs for MS makes treatment decisions in all phases of the disease very complex. Treatment satisfaction for medication is a patient-reported outcome that gives valuable information on both the patient’s perception of current treatment and the differentiation among alternative treatments. In the past decade, several papers have reported on MS patient treatment satisfaction with DMT in various countries.14–20 Since a patient’s treatment experience is to a certain extent influenced by the local availability of particular drugs, country-specific data are needed. To the best of our knowledge, the study presented here is the first report dealing with treatment satisfaction in the Slovak MS population.
The SKARLET study patient population is similar to populations of MS patients described in previous studies,14,21,22 where MS predominantly affects females and young people. In the SKARLET study, there were more females than males (67.4% women) and the average age was 38.85 years, in comparison to the Polish study21 with 69.7% women and an average age of 37.8 years, or an American study22 with 71.6% women and an average age of 48.9 years. The EDSS and duration of MS are also comparable – SKARLET study: EDSS 2.6, duration of MS 5.5 years; Polish study:21 EDSS 3.2, duration 14.5 years.
TSQM as the primary parameter was used in the study of Glanz et al,16 who separately evaluated different DMTs (intramuscular interferon beta 1a - IFNβ-1a IM, subcutaneous interferon beta 1a - IFNβ-1a SC, glatiramer acetate, and natalizumab) in patients with RRMS. Among DMTs, overall treatment satisfaction was similar, but there were differences in terms of satisfaction with effectiveness, side effects, and convenience. Natalizumab-treated participants reported greater satisfaction with the ability of the medication to treat or prevent their condition than IFNβ-1a IM-treated participants. In terms of convenience, the natalizumab group reported significantly higher overall convenience scores than the IFNβ-1a IM group and greater satisfaction with the ease of use of the medication than the other groups. These findings are in accordance with the SKARLET study results. However, the SKARLET study included also oral DMTs aside from infusions and injections.
A similar study was done by Haase et al,14 for patients with clinically isolated syndrome (CIS) and RRMS who used a first-line DMT (IFNβ-1a IM, IFNβ-1a SC, IFNβ-1b SC, glatiramer acetate) administered by injections in Germany. Differences between current medications were found for convenience scores but not for effectiveness, satisfaction or adherence. Higher global satisfaction and effectiveness were associated with fewer relapses, longer duration of medication, lower disability score and the absence of several side effects.
When compared with the conventional injectable therapies, the oral teriflunomide achieved substantially higher satisfaction on TSQM in the TENERE study,23 since all scores were substantially higher (depending on the dose) with effectiveness rated at 63–67 points, convenience at 88–90 points and global satisfaction at 68–69 points. In the SKARLET study, teriflunomide was rated with a mean 67.15 points for effectiveness, 85.12 points for convenience and 69.58 points for global satisfaction.
Treatment satisfaction reported by patients receiving teriflunomide for 48 weeks was the primary endpoint of the Teri-PRO study.17 The following TSQM scores were reported for the whole study population (1001 patients): global satisfaction 68.2, effectiveness 66.3 and convenience 90.4.
Satisfaction with fingolimod was evaluated by Hanson et al,15 with the following results: convenience (71.7), effectiveness (70.1) and global satisfaction (68.9); in total, 310 patients were included in the study. In the SKARLET study, fingolimod was rated with a mean 69.64 points for effectiveness, 81.70 points for convenience and 68.47 points for global satisfaction.
In the publication on the EPOC study24 that compared the switch from glatiramer acetate or beta interferons to fingolimod versus staying on the injectable DMT, no absolute TSQM values were reported. However, patients switched to oral therapy improved on the effectiveness scale depending on the original injectable DMT by 12–18 points and on the convenience scale by 38–44 points (values for global satisfaction were not given). In the SKARLET study, the difference in the mean scale for effectiveness between oral and injection DMT was 1.72 points and 15.74 for convenience.
A recently published study on treatment satisfaction with injectable DMT in Spain19 reported an effectiveness score of 66.8, convenience 72.5 and global satisfaction 68.8. These scores for convenience and global satisfaction are slightly higher than in the SKARLET study (convenience 66.9, global satisfaction 63.8), but this is in line with our assumptions on country-specific differences.
There are several limitations associated with this study. First, the sample size was relatively small, which could limit the generalizability of the results. Second, the injectable DMTs were not distinguished by intramuscular versus subcutaneous administration or by administration with an auto-injector pen. However, the strength of the study is the implementation of oral and infusion administration forms of DMTs, as opposed to for injectable DMTs there is little information published on these two types of DMT. Third, there are little data on the psychometric qualities of the TSQM, and it has not been validated for use with MS patients.16 Fourth, we did not consider different characteristics of populations treated with injectables, orals or infusions when especially disease activity had to be much higher in patients receiving infusions. Also information given to patient by physician probably highly influences how patients evaluate their treatment. However, it is satisfaction as subjectively perceived by a patient that strongly correlates with treatment adherence and outcome and TSQM represents an efficient tool to measure it. Fifth, the study did not include any measures of adherence and its correlation with treatment satisfaction. Last but not least, we limited DMT duration of ≤ 2 years in our study population as we aimed to capture current trends in MS treatment. As a result, we omitted patients who take their DMT longer and are probably stable and satisfied with their treatment.
In conclusion, the results of this detailed survey increase our understanding of RRMS patient population characteristics, patient satisfaction with DMT treatment and treatment practice of MS specialists in Slovakia. This study confirmed that patients find oral DMT to be most convenient. However, the infusion form of treatment outweighed oral DMTs in global satisfaction and effectiveness, as both DMTs administered by infusions (alemtuzumab and natalizumab) are highly effective MS therapy options. For the future, longitudinal comparative studies are needed to confirm the current findings, measure long-term satisfaction, deeply investigate factors that influence patient satisfaction and assess patient preferences across a range of treatment options.
Acknowledgments
The authors had unrestricted access to study data, were involved in the evaluation and interpretation of the study results and reviewing and approval of the manuscript. The authors would like to thank all study teams for their devotion and effort during participation in the SKARLET study at the specialized MS centers in: Nemocnica Staré Mesto, Bratislava; Martin; Nitra; Nemocnica Ružinov, Bratislava; Nemocnica akad. L. Dérera, Bratislava; Trnava; Banska Bystrica; Ružomberok; Trenčín and Nemocnica svätého Michala, Bratislava.
Disclosure
Peter Turčáni is a member of Advisory Boards in Bayer, Biogen, Boehringer, Eli Lilly, GlaxoSmithKline, Lundbeck, Merck, Novartis, Pfizer, Roche, Teva and Sanofi Genzyme. He also received honoraria for lectures and research projects from Bayer, Biogen, Boehringer, Ebewe, Eli Lilly, GlaxoSmithKline, Janssen, Lundbeck, Merck, Mundipharma, Novartis, Novonordisk, Pfizer, Roche, Sanofi Genzyme, Servier and Teva. Jana Mašková is an employee of NEOX Clinical Research – clinical research organization that provided the following services funded by the sponsor: administration, data management, site monitoring, statistical analyses and medical writing. Jozef Húska is an employee of Sanofi-aventis Slovakia. The authors report no other conflicts of interest in this work.
References
1. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25:215–237. doi:10.1111/ene.13536
2. Mendel R, Traul-Mattausch E, Frey D, et al. Do physicians’ recommendations pull patients away from their preferred treatment options? Health Expect. 2012;15(1):23–31. doi:10.1111/j.1369-7625.2010.00658.x
3. Albrecht G, Hoogstraten J. Satisfaction as a determinant of compliance. Community Dent Oral Epidemiol. 1998;26:139–146. doi:10.1111/j.1600-0528.1998.tb01940.x
4. Hirji I, Gupta S, Goren A, et al. Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient’s perspective. Health Qual Life Outcomes. 2013;11:167. doi:10.1186/1477-7525-11-167
5. Turk-Adawi K, Oldridge N, Tarima S, Stason W, Shepard D. Cardiac rehabilitation patient and organizational factors: what keeps patients in programs? J Am Heart Assoc. 2013;2:e000418. doi:10.1161/JAHA.113.000418
6. Chrystyn H, Small M, Milligan G, Higgins V, Gil E, Estruch J. Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108:358–365. doi:10.1016/j.rmed.2013.09.021
7. Wong W, Chow Y, Chen P, Wong S, Fielding R. A longitudinal analysis on pain treatment satisfaction among Chinese patients with chronic pain: predictors and association with medical adherence, disability, and quality of life. Qual Life Res. 2015;24:2087–2097. doi:10.1007/s11136-015-0955-1
8. Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30:89–100. doi:10.2165/11533330-000000000-00000
9. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):
10. Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. doi:10.1186/1477-7525-7-36
11. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale EDSS. Neurology. 1983;33(11):1444–1452. doi:10.1212/WNL.33.11.1444
12. International Conference on Harmonisation Expert Working Group. ICH harmonized tripartite guideline guideline for good clinical practice E6(R1); 2016. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf.
13. World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects; 2016. Available from: http://www.wma.net/en/30publications/10policies/b3/17c.pdf.
14. Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing–remitting multiple sclerosis: the THEPA-MS survey. Ther Adv Neurol Disord. 2016;9:250–263. doi:10.1177/1756285616634247
15. Hanson K, Agashivala N, Stringer S, Balantac Z, Brandes D. A cross-sectional survey of patient satisfaction and subjective experiences of treatment with fingolimod. Patient Prefer Adherence. 2013;309. doi:10.2147/PPA.S41992
16. Glanz BI, Musallam A, Rintell DJ, Chitnis T, Weiner HL, Healy BC. Treatment Satisfaction in Multiple Sclerosis. Int J MS Care. 2014;16:68–75. doi:10.7224/1537-2073.2013-021
17. Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in relapsing forms of MS: real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord. 2017;17:107–115. doi:10.1016/j.msard.2017.07.006
18. Eagle T, Stuart F, Chua AS, et al. Treatment satisfaction across injectable, infusion, and oral disease-modifying therapies for multiple sclerosis. Mult Scler Relat Disord. 2017;18:196–201. doi:10.1016/j.msard.2017.10.002
19. Fernández O, Duran E, Ayuso T, Hernández L, Bonaventura I, Forner M, on behalf of the STICK Study Investigators Group. Treatment satisfaction with injectable disease-modifying therapies in patients with relapsing-remitting multiple sclerosis (the STICK study). PLoS One. 2017;12:e0185766. doi:10.1371/journal.pone.0185766.
20. Mékiès C, Heinzlef O, Jenny B, Ramelli A-L, Clavelou P. Treatment satisfaction and quality of life in patients treated with fingolimod. Patient Prefer Adherence. 2018;12:899–907. doi:10.2147/PPA.S144021
21. Brola W, Sobolewski P, Fudala M, et al. Self-reported quality of life in multiple sclerosis patients: preliminary results based on the Polish MS Registry. Patient Prefer Adherence. 2016;10:1647–1656. doi:10.2147/PPA.S109520
22. Gross HJ, Watson C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: a cross-sectional US survey. Neuropsychiatr Dis Treat. 2017;13:1349–1357. doi:10.2147/NDT.S132079
23. Vermersch P, Hobart J, Dive-Pouletty C, Bozzi S, Hass S, Coyle PK. Measuring treatment satisfaction in MS: is the treatment satisfaction questionnaire for medication fit for purpose? Mult Scler J. 2017;23:604–613. doi:10.1177/1352458516657441
24. Calkwood J, Cree B, Crayton H, et al. Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: post hoc analyses of the EPOC trial. BMC Neurol. 2014;14. doi:10.1186/s12883-014-0220-1.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
