Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 13
Real-World Treatment Patterns and Outcomes from an Electronic Medical Records Database for Patients with Rheumatoid Arthritis Treated with Repository Corticotropin Injection
Authors Hayes K , Panaccio MP, Houston P, Niewoehner J, Fahim M, Wan GJ , Dhillon B
Received 22 July 2021
Accepted for publication 11 September 2021
Published 15 October 2021 Volume 2021:13 Pages 315—323
DOI https://doi.org/10.2147/OARRR.S329766
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Kyle Hayes,1 Mary P Panaccio,1 Parul Houston,1 John Niewoehner,1 Mohammed Fahim,2 George J Wan,1 Bhavna Dhillon3
1Mallinckrodt Pharmaceuticals, Hampton, NJ, USA; 2KMK Consulting, Morristown, NJ, USA; 3United Rheumatology, Hauppauge, NY, USA
Correspondence: Kyle Hayes
Mallinckrodt Pharmaceuticals, Hampton, NJ, USA
Tel +1 314-393-3531
Email [email protected]
Purpose: Repository corticotropin injection (RCI; Acthar® Gel) is a naturally sourced mixture of adrenocorticotropic hormone analogs and other pituitary peptides that exerts anti-inflammatory and immunomodulatory properties via melanocortin receptors. RCI is approved as a short-term adjunctive therapy for rheumatoid arthritis (RA) and is typically used in patients with refractory RA. The objective of this study was to describe real-world outcomes of RA patients treated with RCI by retrospective analysis of an electronic medical records (EMR) database.
Patients and Methods: EMR data were obtained from the United Rheumatology-Normal Integrated Community Evidence (UR-NICETM) data repository for patients who used RCI for the treatment of RA. Demographics, comorbidities, disease history, medications, and laboratory evaluations 365 days prior to and 365 days after initiation of RCI were examined.
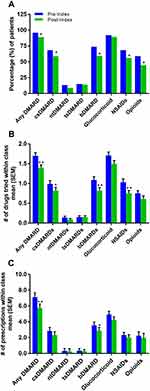
Results: The patient cohort was predominantly White females with a mean age of 60 years and high RA activity prior to RCI therapy. Clinical measures of disease severity indicated that patients had high RA activity before starting RCI therapy. Clinical Disease Activity Index (CDAI) scores were significantly reduced 365 days post-initiation of RCI. Swollen and tender joint counts and patient-reported outcomes, including Routine Assessment of Patient Index Data 3 (RAPID3), Physician Global Assessment, and patient assessment of pain severity were also significantly lower. The number of patients taking conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs), biologic (b) DMARDs, nonsteroidal anti-inflammatory drugs (NSAIDS), and opioids decreased, as did the number of drugs tried within each class for csDMARDs, bDMARDs, NSAIDs, and glucocorticoids.
Conclusions: These findings suggest that RCI significantly improves clinical outcomes of RA and decreases the need for concomitant medications for up to 1 year following initiation of therapy. The study provides valuable insights into the use of RCI and management of these difficult-to-treat RA patients during routine clinical practice.
Keywords: Acthar Gel, DMARDs, real-world evidence, rheumatoid arthritis, repository corticotropin injection, RCI, United Rheumatology database
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation of the joints and bone remodeling.1 RA poses a significant burden to the health care system.2 The goal for the management of RA is to achieve disease remission with a treat-to-target approach. Timely treatment of flares significantly impacts disease progression and outcomes.3 The American College of Rheumatology (ACR) recommends initiating treatment with synthetic disease-modifying antirheumatic drugs (DMARDs) and progressing to biologic DMARDs if disease activity remains high. Low-dose glucocorticoids can be considered as an adjunct therapy for patients with moderate to severe RA or for patients in whom DMARDs fail. According to the ACR, glucocorticoids should only be used at the lowest possible dose for the shortest possible duration.4 Despite treatment with DMARDs and glucocorticoids, many patients with RA continue to experience significant symptoms or flares.5
Repository corticotropin injection (RCI; Acthar® Gel) is a naturally sourced mixture of porcine-derived adrenocorticotropic hormone analogs and other pituitary peptides. RCI is US Food and Drug Administration–approved for use as an adjunctive therapy for short-term administration in RA. Detailed prescribing and safety information can be found in the prescribing label.6 Studies have demonstrated that RCI acts as an agonist at all 5 melanocortin receptors (MCRs) with a unique binding and activation profile compared to other MCR agonists.7 MC2R is found on adrenocortico cells and acts to stimulate glucocorticoid release; however, RCI shows its lowest full agonist activity at this receptor in vitro7 and results in lower endogenous cortisol release in vivo compared with other MC2R agonists.8 These and other published data indicate that RCI has a unique mechanism of action, distinct from glucocorticoids, and therefore may have different adverse effects than glucocorticoids; this has prompted a recent RCI label change to remove language that common adverse reactions for RCI are similar.6
Other MCR subtypes are located on immune cells where they function as anti-inflammatory and immunomodulatory receptors. MC1R, MC3R, and MC5R subtypes are found on macrophages, B lymphocytes, and T lymphocytes and mediate the anti-inflammatory and immunomodulatory properties of MCR agonists.7,9–11 RCI inhibits B lymphocyte proliferation and immunoglobulin production12 and inhibits inflammatory cytokine production from macrophages13 and T lymphocytes14 in vitro.
RCI is used in patients experiencing acute episodes or exacerbations of RA, as a late-line therapy to treat RA flares, as a bridge to new therapy, or as an add-on to prior therapy.6,15 Multiple published studies of RCI use in patients with refractory RA support its efficacy and safety including patient-reported improvements in pain, fatigue, and work productivity;16−20 however, there remains a need for real-world evidence for the use of RCI to treat RA. Namely, data on prescribing patterns, dosing, efficacy, and tolerability are limited. This study sought to expand the understanding of real-world utilization and effectiveness of RCI in patients with RA by using electronic medical record (EMR) data from a large rheumatology group practice network to inform appropriate use in clinical practice. The objectives of this study were to describe the demographics and clinical characteristics of patients treated with RCI for RA; describe treatment patterns including prior and/or current medications used to control RA; and assess changes in clinical outcomes, lab-based disease activity measures, and patient-reported outcomes (PROs) after RCI initiation.
Methods
Study Design
This was a descriptive, non-interventional, US-based retrospective analysis of EMR data of patients who used RCI for the treatment of RA from December 16, 2012, to June 5, 2020. Medical and treatment data including diagnoses, current and past medications, lab results, biometric data, and all visit information were from approximately 66 independent rheumatology practices in United Rheumatology’s physician network. The United Rheumatology-Normal Integrated Community Evidence (UR-NICETM) data repository was examined for the 365 days prior to and up to 365 days following the first prescription date for RCI treatment (RCI index date). The variables analyzed were demographics, comorbidities, disease history, medications, and laboratory evaluations pre- and post-initiation of RCI or at the RCI index date (Supplemental Tables 2–5).
Eligibility Criteria
Patients were included if they had been prescribed RCI between December 6, 2013, and June 5, 2019, with no evidence of prior RCI use, had ≥1 diagnosis of RA at any time prior to the index date, and had >1 record with any activity (diagnosis, medication, procedure, or biometric) in the database more than 180 days before and more than 180 days after the index date (Figure 1).
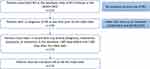 |
Figure 1 Study Flow Diagram. Abbreviation: RA, rheumatoid arthritis. |
Demographics, Clinical Characteristics, and Clinical Outcomes
Patient demographics and clinical characteristics were assessed using all available data on or before the index date. Demographics included age, sex, race, ethnicity, US geographic region, and insurance type. Clinical characteristics included time since RA diagnosis, weight, body mass index (BMI), and comorbidities.
Changes in clinical outcomes were determined by comparison of RA disease activity, symptoms, and PROs prior to and following RCI initiation. Clinical measures were initially evaluated within 7 days prior to or following the index date and compared to the last recorded values in the 365-day post-index period. RA disease activity was determined by Clinical Disease Activity Index (CDAI) scores (0–2.8, remission; 2.9–10.0, low activity; 10.1–22.0, moderate activity; 22.1–76.0, high activity),21 levels of C-reactive protein, and erythrocyte sedimentation rate (ESR). The presence of symptoms was evaluated by the number of tender and swollen joints. PROs were assessed by Routine Assessment of Patient Index Data 3 (RAPID3), pain (visual analog scale [VAS], 0–10 [ 3.4, mild pain; 3.5–7.4, moderate pain;
3.4, mild pain; 3.5–7.4, moderate pain;  7.5, severe pain]),22 and quality of life (Health Assessment Questionnaire [HAQ]). RAPID3 serves as a quick indicator of disease without using clinical measures but may overestimate severity compared to CDAI and Disease Activity Score in 28 joints with ESR (DAS28-ESR).23 We used the modified RAPID3 scale to simplify the patients’ RAPID3 cumulative score to a weighted score (0–1.0, near remission; 1.3–2.0, low severity; 2.3–4.0, moderate severity; 4.3–10.0, high severity).24 RCI effectiveness was determined by mean changes in clinical measures between the index date and 365 days post–index date. Comparison of index and post-index clinical values only included patients who had both measures in their EMRs.
7.5, severe pain]),22 and quality of life (Health Assessment Questionnaire [HAQ]). RAPID3 serves as a quick indicator of disease without using clinical measures but may overestimate severity compared to CDAI and Disease Activity Score in 28 joints with ESR (DAS28-ESR).23 We used the modified RAPID3 scale to simplify the patients’ RAPID3 cumulative score to a weighted score (0–1.0, near remission; 1.3–2.0, low severity; 2.3–4.0, moderate severity; 4.3–10.0, high severity).24 RCI effectiveness was determined by mean changes in clinical measures between the index date and 365 days post–index date. Comparison of index and post-index clinical values only included patients who had both measures in their EMRs.
RA Treatment Patterns
Treatment information obtained from EMR data was used to evaluate patient use of standard RA medication 365 days prior to (pre-index) and 365 days after (post-index) initiation of RCI. Medications were classified as conventional synthetic DMARDs (csDMARDs), nontraditional DMARDs (ntDMARDs), biologic DMARDs (bDMARDs), or targeted synthetic (tsDMARDs). Use of glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids was also assessed. Specific RA-related medication used in this analysis can be found in Supplemental Table 1. As there were limited data available regarding dosage of medication, we focused our analysis on changes in RA treatment patterns by comparing the frequency of patients prescribed a specific class of drugs or by the number of drugs tried within each class from pre-index to post-index. The year of RCI initiation, number of RCI prescriptions (by unique date), and the duration of RCI treatment, measured as time from date of first RCI prescription to last RCI prescription in the patient’s EMR, were assessed. All prescription data are based on the assumption that prescriptions identified in the patient’s chart were actually filled by the patient.
Statistical Analysis
Descriptive statistics were used for this analysis. Demographic and baseline characteristics are presented as frequency and percentage of the total study population. Mean and standard deviation are shown for continuous variables. Shapiro–Wilk test was used to assess normality. Sample proportions were compared with McNemar test and means were compared with paired t test for normally distributed data or non-normal distributions with n>30. Wilcoxon signed-rank test was used to compare medians in non-normally distributed data with n<30.
Results
Patient Profiles at or Prior to RCI Initiation
Demographics, Clinical Characteristics, and Treatments
Of the 114 patients in the UR-NICETM database who met the study inclusion criteria, the majority were White (55.3%) women (80.7%) aged approximately 60 years (60.1±11.0 years) living in the South (57%). Most patients had commercial insurance (49.1%) or Medicare (49.1%) (Table 1). The mean time since diagnosis of RA was 1038±943 days. Osteoarthritis (28.1%), osteoporosis (26.3%), overweight (78.3%), and hypertension (16.7%) were the most prevalent comorbidities. The average BMI was 32.0±8.6 kg/m2, placing the majority of patients in the obese category (58.5%) (Table 1). Within 365 days prior to RCI initiation, most patients were treated with csDMARDs (68.4%), bDMARDs (73.7%), glucocorticoids (92.1%), NSAIDs (68.4%), and/or opioids (58.8%) (Table 1).
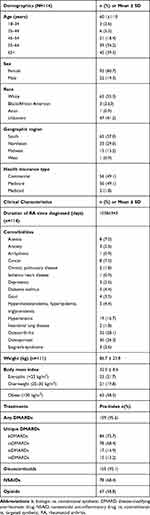 |
Table 1 Demographics, Clinical Characteristics, and Treatments |
Disease Activity and PROs
At the time of RCI initiation (index date), CDAI (29.7±16.4) and RAPID3 (6.1±2.2) scores indicated that patients had high RA disease activity at baseline. The number of tender joints (9.8±7.9) was higher than the number of swollen joints (6.9±6.6). Patients’ level of pain (VAS, 0–10) averaged 6.7±2.4 out of 10, and they rated their ability to perform daily activities (HAQ, 0–10) 4.2±2.6 out of 10 (Table 2).
 |
Table 2 RA Disease Activity and PROs at Baseline |
RCI Treatment Patterns
The majority of patients had only one RCI prescription (54.4%), while 10.5% of patients had 5 or more (Figure 2). Among patients with at least 2 RCI prescriptions (45.7%), the average duration from RCI initiation to the last RCI prescription was 152±117 days.
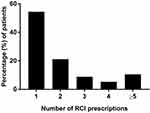 |
Figure 2 Repository Corticotropin Injection (RCI) Use in the Study Sample. Percentage of patients receiving at least one RCI prescription as counted by unique date. |
Change in Clinical Outcomes Post-RCI Initiation
Disease Activity and PROs
A clinically meaningful reduction in CDAI scores (–9.7±16.9, p=0.0101) 365 days post-RCI index represented a change from high RA activity to moderate RA activity. Swollen and tender joint counts significantly decreased by –1.1±2.8 (p=0.0116) and –3.3±8.0 (p=0.0128), respectively. PROs including RAPID3 (–1.1±1.9, p=0.0036), physician assessment of global health (–1.3±2.4, p=0.0214), and patient assessment of pain severity (–1.1±2.8, p=0.0056) were also significantly lower post–RCI index (Table 2). Although pain scores remained in the moderate range, the reduction is considered clinically meaningful for patients with RA.26
Change in RA Treatment Patterns Post-RCI Initiation
The number of patients with RA treated with csDMARDs (–9.6%, p<0.05), bDMARDs (–14%, p<0.05), NSAIDs (–12.3%, p<0.05), and/or opioids (–14%, p<0.05) was significantly reduced after initiation of RCI (Figure 3 and Supplemental Table 1). The average number of drugs tried within class from pre-index to post-index was significantly lowered for csDMARDs, bDMARDs, glucocorticoids, and NSAIDs. The average number of prescriptions within class from pre-index to post-index was significantly decreased for bDMARDs (Figure 3).
Discussion
The majority of patients were White females averaging 60 years of age, consistent with disease prevalence in the general population.27 The most common comorbidities were osteoarthritis, osteoporosis, hypertension, and obesity, a well-documented risk factor for RA.28 Additional real-world outcome studies show that osteoarthritis is a prevalent comorbidity in patients with RA treated with RCI.15,29,30 In addition, the presence of more tender than swollen joints may reflect the coexistence of osteoarthritis in this patient population.31 Clinical measures of disease severity, including CDAI and number of tender or swollen joints, indicated that patients had high RA activity prior to starting RCI therapy. We observed statistically significant reductions in these clinical measures as well as PROs (RAPID3 and pain assessment). Changes in CDAI and pain assessment met the minimal clinically important difference representing reduced severity of RA for up to 365 days after RCI initiation.
There was a statistically significant reduction in the number of patients being treated with certain classes of RA drugs (csDMARDs, bDMARDs, NSAIDS, and opioids). The number of prescriptions for bDMARDs were also decreased following RCI, which may correspond to fewer patients being treated with bDMARDs. Interestingly, there was no change in the number of patients receiving prescriptions for glucocorticoids; however, the number of different glucocorticoids tried was reduced. This study did not analyze daily dose changes of glucocorticoids that could have been impacted by RCI treatment as assessed in previous studies.30,32 Taken together, we observed changes in standard RA treatments that were sustained for up to 1 year after RCI initiation that are consistent with the improvements in clinical measures of disease severity and PROs.
It is notable that approximately half of patients only received one dose of RCI, while the other half of patients received at least 2 doses and more than 10% had 5 or more doses. Additional studies are needed to confirm whether the number of RCI doses correlates to more significant improvement in RA clinical measures and/or further reduction of concomitant medications. Due to the lack of specific dosing data obtained from the EMR for RCI, the estimated treatment duration was based on the date of the first and last prescription. The RCI duration estimate (152±117 days) likely underestimates the total duration, as the time under treatment for the last prescription cannot be determined; however, our findings are consistent with other published reports characterizing the duration of RCI treatment in clinical practice in which the mean duration of treatment was 4.8 months.29
Several recent publications support the use of RCI in patients for whom ACR-recommended RA therapies (glucocorticoids, csDMARDs, tsDMARDs, and bDMARDs) lack efficacy, for acute exacerbation of disease, or for use as an add-on therapy.17,29,30,33 Retrospective analysis of patients with refractory RA demonstrated a significant improvement in physician impression of change after short-term RCI therapy.29 In a similar real-world analysis of RCI use in patients with RA that did not adequately respond to standard-of-care therapies, patients experienced clinically-relevant reduction in disease activity and improved PROs.30 Additionally, a placebo-controlled double-blind withdrawal trial resulted in low disease activity defined by CDAI scores ≤10 after 12 weeks of RCI therapy. Interestingly, low disease activity was sustained in >40% of patients throughout the subsequent 12-week withdrawal period.17
Due to the nature of EMR data, there are limitations in this observational study, namely that they contain information on prescriptions written but not necessarily filled.29,30 For instance, determining dose ranges of both traditional RA medications as well as the dosing pattern of RCI could provide a more complete analysis of the effects of RCI. Patient outcomes were compared regardless of RCI dosing frequency, with more than half receiving one dose of RCI (54%) and the remaining receiving 2 or more doses (46%). When assessing RA treatment patterns, we assume that patients filled prescriptions identified in their chart, but without the addition of pharmacy claims data,34 we cannot validate whether the prescriptions were filled. Comparisons of baseline or pre-index outcomes to post-index outcomes is limited by the measure being analyzed, with only subsets of patients having these values available in their EMR. Additionally, these findings are mostly limited to White, older adult women using commercial insurance or Medicare. A relatively small sample size could also make extrapolation to the larger patient population with RA difficult.
Despite these limitations, data from UR-NICETM, a large rheumatology practice network, captured disease activity and PRO data which are typically not available in large medical claims databases. These findings may inform clinical practice for the use of RCI in treating patients with RA, including expected changes in RA treatment patterns and severity of disease activity. Most notably, RCI reduced the number of concomitant medications and improved both clinical and PRO measures of disease.
Conclusions
Taken together, the findings of this study suggest that at least one dose of RCI can significantly improve clinical outcomes of RA and treatment patterns (by decreasing the need for concomitant medications) for up to 1 year following initiation of therapy. The results of this analysis further characterize patients with RA who initiated RCI therapy and support the real-world effectiveness of RCI for the treatment of RA.
Abbreviations
ACR, American College of Rheumatology; b, biologic; BMI, body mass index; CDAI, Clinical Disease Activity Index; cs, conventional synthetic; DAS28-ESR, Disease Activity Score 28 joint count with ESR; DMARD, disease-modifying antirheumatic drug; EMR, electronic medical record; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; MCR, melanocortin receptor; NSAID, nonsteroidal anti-inflammatory drug; nt, nontraditional; PRO, patient-reported outcome; RA, rheumatoid arthritis; RAPID3, Routine Assessment of Patient Index Data 3; RCI, repository corticotropin injection; ts, targeted synthetic; UR-NICETM, United Rheumatology-Normal Integrated Community EvidenceTM; VAS, visual analogue scale.
Compliance with Ethics Guidelines
The management of study data conformed to all applicable Health Insurance Portability and Accountability Act rules. All data were de-identified throughout the study to preserve patient anonymity and confidentiality. This observational study was conducted under the research exception provisions of the Privacy Rule, 45 CFR 164.514(e), and was exempt from Institutional Review Board informed consent requirements.
Acknowledgments
Professional writing and editorial support were provided by MedLogix Communications, LLC, Itasca, Illinois, under the direction of the authors and were funded by Mallinckrodt Pharmaceuticals. We thank Sung-Woo Ahn for assistance with data analysis.
Disclosure
KH, GJW, and JN are employees of Mallinckrodt Pharmaceuticals. PH and MP are paid independent consultants for Mallinckrodt Pharmaceuticals. MF is an employee at KMK consulting and a paid analyst for Mallinckrodt Pharmaceuticals. BD is an employee of United Rheumatology. The authors report no other conflicts of interest in this work.
References
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi:10.1016/S0140-6736(16)30173-8
2. Strand V, Tundia N, Song Y, Macaulay D, Fuldeore M. Economic burden of patients with inadequate response to targeted immunomodulators for rheumatoid arthritis. J Manag Care Spec Pharm. 2018;24(4):344–352. doi:10.18553/jmcp.2018.24.4.344
3. Markusse IM, Dirven L, Gerards AH, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther. 2015;17(1):232. doi:10.1186/s13075-015-0730-2
4. Singh JA, Saag KG, Bridges SL
5. Kuijper TM, Lamers-Karnebeek FB, Jacobs JW, Hazes JM, Luime JJ. Flare rate in patients with rheumatoid arthritis in low disease activity or remission when tapering or stopping synthetic or biologic DMARD: a systematic review. J Rheumatol. 2015;42(11):2012–2022. doi:10.3899/jrheum.141520
6. Mallinckrodt AR. HP Acthar Gel (Repository Corticotropin Injection). Mallinckrodt ARD; 2021.
7. Huang YJ, Galen K, Zweifel B, Brooks LR, Wright AD. Distinct binding and signaling activity of Acthar Gel compared to other melanocortin receptor agonists. J Recept Signal Transduct Res. 2020;1–9. doi:10.1080/10799893.2020.1818094
8. Wang X, Pham L, Poola N, Brooks LR, Due B. Comparison of steroidogenic exposure following the administration of repository corticotropin injection with a synthetic ACTH1-24 depot and methylprednisolone in healthy subjects. Clin Pharmacol Drug Dev. 2020. doi:10.1002/cpdd.894
9. Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. ScientificWorldJournal. 2010;10:1840–1853. doi:10.1100/tsw.2010.173
10. Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56(1):1–29. doi:10.1124/pr.56.1.1
11. Ahmed TJ, Montero-Melendez T, Perretti M, Pitzalis C. Curbing inflammation through endogenous pathways: focus on melanocortin peptides. Int J Inflam. 2013;2013:985815. doi:10.1155/2013/985815
12. Olsen NJ, Decker DA, Higgins P, et al. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17(1):300. doi:10.1186/s13075-015-0823-y
13. Healy LMJJ, Lin YH, Rao V, Antel JP, Wright D. ePosters. Mult Scler. 2017;23(3_suppl):680–975. doi:10.1177/1352458517731285
14. Wright D, Sharma P, Galen K, Fitch R. AB0082 reduced steroidogenic activity of repository corticotropin injection (RCI) induces a distinct cytokine response following T cell activation in vivo. Ann Rheum Dis. 2019;78:1504.
15. Hayes K, Panaccio MP, Goel N, Fahim M. Patient characteristics and indicators of treatment initiation with repository corticotropin injection in patients with rheumatoid arthritis: a claims database analysis. Rheumatol Ther. 2021;8(1):327–346. doi:10.1007/s40744-020-00272-x
16. Fischer PA, Rapoport RJ. Repository corticotropin injection in patients with rheumatoid arthritis resistant to biologic therapies. Open Access Rheumatol. 2018;10:13–19. doi:10.2147/OARRR.S153307
17. Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327–344. doi:10.1007/s40744-020-00199-3
18. Fleischmann R, Furst DE. Safety of repository corticotropin injection as an adjunctive therapy for the treatment of rheumatoid arthritis. Expert Opin Drug Saf. 2020;19(8):935–944. doi:10.1080/14740338.2020.1779219
19. Philbin M, Niewoehner J, Wan GJ. Clinical and economic evaluation of repository corticotropin injection: a narrative literature review of treatment efficacy and healthcare resource utilization for seven key indications. Adv Ther. 2017;34(8):1775–1790. doi:10.1007/s12325-017-0569-9
20. Gillis T, Crane M, Hinkle C, Wei N. Repository corticotropin injection as adjunctive therapy in patients with rheumatoid arthritis who have failed previous therapies with at least three different modes of action. Open Access Rheumatol. 2017;9:131–138. doi:10.2147/OARRR.S131046
21. Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8.
22. Rheumatology. Routine Assessment of Patient Index Data. [survey] Available from: https://www.rheumatology.org/Portals/0/Files/RAPID3%20Form.pdf. Accessed May 20, 2021.
23. Kumar BS, Suneetha P, Mohan A, Kumar DP, Sarma KVS. Comparison of disease activity score in 28 joints with ESR (DAS28), Clinical Disease Activity Index (CDAI), Health Assessment Questionnaire Disability Index (HAQ-DI) & Routine Assessment of Patient Index Data with 3 measures (RAPID3) for assessing disease activity in patients with rheumatoid arthritis at initial presentation. Indian J Med Res. 2017;146(Supplement):S57–S62. doi:10.4103/ijmr.IJMR_701_15
24. Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol. 2008;35(11):2136–2147. doi:10.3899/jrheum.080182
25. Ward MM, Castrejon I, Bergman MJ, Alba MI, Guthrie LC, Pincus T. Minimal clinically important improvement of routine assessment of patient index data 3 in rheumatoid arthritis. J Rheumatol. 2019;46(1):27–30. doi:10.3899/jrheum.180153
26. Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol. 2007;34(8):1674–1683.
27. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi:10.1016/S0140-6736(01)06075-5
28. Feng X, Xu X, Shi Y, et al. Body mass index and the risk of rheumatoid arthritis: an updated dose-response meta-analysis. Biomed Res Int. 2019;2019:3579081. doi:10.1155/2019/3579081
29. Ho-Mahler N, Turner B, Eaddy M, Hanke ML, Nelson WW. Treatment with repository corticotropin injection in patients with rheumatoid arthritis, systemic lupus erythematosus, and dermatomyositis/polymyositis. Open Access Rheumatol. 2020;12:21–28. doi:10.2147/OARRR.S231667
30. Busch H, Wan GJ, Niewoehner J, Houston P, Su Y, Panccio MP. Real-world treatment patterns for repository corticotropin injection in patients with rheumatoid arthritis. Rheumatol Ther. 2021;submitted.
31. Hammer HB, Michelsen B, Provan SA, et al. Tender joint count and inflammatory activity in patients with established rheumatoid arthritis: results from a longitudinal study. Arthritis Care Res. 2020;72(1):27–35. doi:10.1002/acr.23815
32. Chopra I, Qin Y, Kranyak J, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Ther Adv Respir Dis. 2019;13:1753466619888127. doi:10.1177/1753466619888127
33. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1108–1123. doi:10.1002/art.41752
34. Gliklich RE, Leavy MB, Dreyer NA, eds. Registries for Evaluating Patient Outcomes: A User’s Guide.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.