Back to Journals » Lung Cancer: Targets and Therapy » Volume 8
Real-world practice patterns for patients with advanced non-small cell lung cancer: multicenter retrospective cohort study in Japan
Authors Isobe H, Mori K, Minato K, Katsura H , Taniguchi K, Arunachalam A , Kothari S , Cao X, Kato T
Received 26 April 2017
Accepted for publication 8 September 2017
Published 24 October 2017 Volume 2017:8 Pages 191—206
DOI https://doi.org/10.2147/LCTT.S140491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pan-Chyr Yang
Hiroshi Isobe,1 Kiyoshi Mori,2 Koichi Minato,3 Hideki Katsura,4 Kazuko Taniguchi,5 Ashwini Arunachalam,6 Smita Kothari,6 Xiting Cao,6 Terufumi Kato7
1Department of Medical Oncology, KKR Sapporo Medical Center, Hokkaido, 2Department of Thoracic Diseases, Division of Thoracic Oncology, Tsuboi Cancer Center Hospital, Fukushima, 3Department of Respiratory Medicine, Gunma Prefectural Cancer Center, Gunma, 4Division of Respiratory Medicine, Tokyo Women’s Medical University Yachiyo Medical Center, Chiba, 5Market Access, MSD K.K., Tokyo, Japan; 6Center for Observational & Real World Evidence (CORE), Merck & Co., Inc., Kenilworth, NJ, USA; 7Department of Respiratory Medicine, Kanagawa Cardiovascular and Respiratory Center, Kanagawa, Japan
Background: Recommended therapies for advanced/metastatic non-small cell lung cancer (NSCLC) have changed with the advent of targeted therapies. The objectives of this retrospective chart review study were to describe treatment patterns, biomarker testing practices, and health care resource use for advanced NSCLC at 5 sites in Japan.
Patients and methods: We studied anonymized medical record data of patients aged ≥18 years who initiated systemic therapy for newly diagnosed stage IIIB or IV NSCLC from January 2011 through June 2013. Data were analyzed descriptively by histology and mutation status. Overall survival was estimated using the Kaplan–Meier method.
Results: We studied 175 patients, including 43 (25%), 129 (74%), and 3 (2%) with squamous, nonsquamous, and unknown NSCLC histology, respectively; 83% had stage IV NSCLC. Overall, 123 patients (70%) were male; the median age was 70 years (range, 47–86); and 33 (19%) were never-smokers. In the nonsquamous cohort, 105 (81%) and 25 (19%) of patients were tested for epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement, respectively; 44 (42%) had EGFR-positive NSCLC and 2 (8%) had ALK-positive NSCLC, including 26/46 (57%) women and 21/46 (46%) never-smokers. In the squamous cohort, 17 (40%) and 4 (9%), respectively, were tested; 1 EGFR-positive tumor was detected. After first-line therapy, 105 (60%) patients received second-line, and 54/105 (51%; or 31% overall) received third-line therapy. EGFR tyrosine kinase inhibitors were most commonly prescribed for EGFR-positive NSCLC across all lines. In the nonsquamous EGFR/ALK-negative/unknown cohort, most received first-line platinum combinations, particularly younger patients (78% ≥75 years vs 93% <75 years old). The average hospitalization was 21 days/admission. The median (95% CI) overall survival from start of first-line therapy was 9.9 months (7.6–11.7) for all patients and 17.9 months (9.9–24.4) for patients with EGFR/ALK-positive status.
Conclusion: Biomarker testing is common for nonsquamous NSCLC at the 5 Japanese study sites. Treatment is personalized by mutation status and age, per guideline recommendations.
Keywords: predictive biomarker, health care resource use, Japan, non-small cell lung cancer, systemic therapy, treatment patterns
Introduction
In Japan, cancer has been the leading cause of death since 1981.1 In 2015, lung cancer was responsible for over 75,000 deaths, representing ~20% of all cancer-related deaths and the number 1 and 2 cause of cancer-related deaths in men and women, respectively.1–4 Over 100,000 new cases of lung cancer are diagnosed annually in Japan, the majority of which (74%) are diagnosed at advanced stages of disease with poor prognosis.1
The Japanese guidelines for treatment of lung cancer recommend that therapy be based on histology, mutation status, and age (<75 vs ≥75 years old).5 For patients with advanced nonsquamous non-small cell lung cancer (NSCLC) harboring a sensitizing mutation in the epidermal growth factor receptor (EGFR) gene or an anaplastic lymphoma kinase (ALK) rearrangement, the guidelines recommend monotherapy with the appropriate EGFR or ALK tyrosine kinase inhibitor (TKI) or a platinum combination with or without bevacizumab. The prevalence of EGFR mutations in NSCLC is higher in Asian than Caucasian patient populations.6–9 The reported prevalence of EGFR mutations in Japanese and East Asian patients with NSCLC is ~40%,6,9 with up to 59% of moderately to well-differentiated adenocarcinomas carrying the mutation.9,10 The EGFR mutation is more common in women and nonsmokers.9,11 The prevalence of ALK-rearranged (ALK-positive) NSCLC is lower, from 4% to 7% by different estimates;12,13 and ALK-positive tumors are more common in younger patients, light or nonsmokers, with adenocarcinoma.12,14 These 2 mutations are usually, but not always, mutually exclusive.7
As the understanding of tumor biology has evolved rapidly in recent years, an understanding of real-world treatment practices is important to benchmark changes in treatment recommendations. Japan was 1 of 9 countries participating in a global study of real-world treatment patterns for advanced NSCLC (also including Australia, Brazil, Canada, Germany, Italy, Korea, Spain, and Taiwan).32 The primary objectives of this multinational, observational study were to describe the treatment patterns, biopsy and biomarker testing practices, and health care resource use (HCRU) for patients who initiated first-line systemic therapy for newly diagnosed stage IIIB or IV NSCLC from January 2011 to July 2013. Here, we report the findings for the Japanese cohort.
Methods
Study design and patients
This retrospective chart review study was conducted at 5 sites in Japan; these sites were selected based on positive responses to a site qualification questionnaire indicating an interest in study participation, experience in managing patients with NSCLC, availability of biomarker testing data, and adequate resources to support participation. Both academic and community oncology centers were considered, with the goal of including from 150 to 200 patients. The 5 study sites included 1 academic center (Yachiyo Medical Center at Tokyo Women’s University), a private hospital (Tsuboi Cancer Center Hospital), a public cancer center (Gunma Prefectural Cancer Center), a general hospital (KKR Sapporo Medical Center), and a specialized hospital (Kanagawa Cardiovascular and Respiratory Center).
The study eligibility period was January 1, 2011, to July 1, 2013. Patients 18 years and older who initiated first-line systemic therapy for newly diagnosed stage IIIB or IV NSCLC during this 2.5-year period were eligible for the study. The date of initiation of first-line therapy for each patient was defined as their index date. Eligible patients were identified by retrospective chart review starting at the end of the eligibility period and working backward in time. Patient follow-up concluded on August 10, 2015, and eligible patients had to have complete medical records until that date (or death, if earlier), thereby ensuring a minimum potential follow-up of over 2 years for each patient.
We required histologic or cytologic confirmation of stage IIIB/IV NSCLC. In the study protocol, investigators were provided with NSCLC staging guidelines according to the TNM classification of the Union for International Cancer Control and the American Joint Committee on Cancer.15,16 Patients who did not initiate systemic therapy for NSCLC were excluded, as were patients enrolled in an interventional clinical trial or other clinical study, those with a concomitant or prior history of other malignancy, and those with an initial diagnosis of an earlier stage of NSCLC (stage I–IIIA) that had progressed to stage IIIB or IV.
The study protocol conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013) and was approved by the head of each study site after review by the institutional ethics committee, reported in the Supplementary materials section. Informed consent requirements were waived by the ethics committees in accordance with Japanese clinical study guidelines for noninterventional research involving pre-existing data.17
Data collection
Electronic case report forms (eCRFs) were used to collect anonymized demographic and clinical data from patient charts by trained chart abstractors at each study site. Each patient was given a unique number as an identifier on the eCRFs. Diagnosis-, biomarker-, and treatment-related data were collected from the index date (i.e., start of first-line therapy) for each patient, including type of biomarker tests, testing dates and results, dose and duration of administered therapies, number of clinic and any emergency visits, and number and duration of hospitalizations.
The Eastern Cooperative Oncology Group (ECOG)18 or Karnofsky scale19 was used to assess performance status on the index date. Maintenance therapy was defined as any therapy given to a patient after first-line induction but before the start of second-line therapy. The duration of each treatment line was calculated (in days as the stop date of treatment line – start date of treatment line +1 day). The primary site investigator confirmed all data entry and provided any assessments that required a medical professional opinion, such as identifying the date of progression after treatment and deciding whether a dose delay, omission of a dose, or utilization of a health care resource occurred secondary to a treatment-related adverse event.
Statistical analyses
We performed descriptive analyses of demographic, diagnostic, treatment-related, and HCRU data. Results were reported using summary statistics, including frequency count and percentage for categorical variables, and patient number, mean, SD, median, and range for continuous and count variables. The proportion of missing data was reported for key variables; missing data were not imputed.
Treatment patterns were evaluated by line of therapy and histology, EGFR-mutation and ALK-rearrangement status, and age group (<75 years vs ≥75 years old). We categorized treatment regimens into 4 major categories: platinum-based combination, nonplatinum combination, single agent, and targeted therapy (i.e., EGFR/ALK TKI). Overall survival (OS) was estimated using the Kaplan–Meier product-limit method.
This was an observational study with no a priori hypothesis testing; therefore, we did not undertake a formal calculation of sample size and statistical power.
Analyses were carried out using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
Five sites in Japan enrolled a total of 175 patients, including 43 (25%), 129 (74%), and 3 (2%) with squamous, nonsquamous, and unknown NSCLC histology, respectively. Index dates ranged from January 24, 2011 to June 27, 2013.
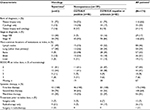
Overall, 123 (70%) patients were male; the median age was 70 years and age range, from 47 to 86 years. Approximately one-third of patients were 75 years or older (Table 1). In the squamous cohort, the proportion of male patients was higher than in the nonsquamous cohort (86% vs 65% male), as was the proportion of current and former smokers (98% vs 76% in the nonsquamous cohort). NSCLC was at stage IV upon diagnosis for most patients overall (83%), and for 70% of those in the squamous cohort (Table 2). Lymph nodes, lung (other than the primary site), and bone were the most common sites of metastases. The majority of patients (84%) had an ECOG performance status of 0 or 1 (Table 2).
Patient demographic and clinical characteristics are reported in Tables 1 and 2 according to EGFR mutation and ALK rearrangement status. In the nonsquamous cohort, patients with positive EGFR or ALK mutation status, compared with patients with negative or unknown results, were more likely to be female (57% vs 23%) and more likely to be never-smokers (46% vs 11%).
Biopsy and biomarker testing patterns
The diagnosis of NSCLC was made by tissue biopsy for most patients, including 66% solely with biopsy and 14% with biopsy and cytology (Table 2). Overall, the majority of patients (91%) had at least 1 biopsy during the study (Table 3).
Three-quarters of patients overall (n=130; 74%) had at least 1 biomarker test performed, including 17 (40%), and 110 (85%) of those in squamous and nonsquamous cohorts, respectively, and all 3 patients in the unknown histology cohort (Table 3). Overall, 125 (71%) and 30 (17%) patients were tested for EGFR mutation and ALK rearrangement, respectively. The biomarker test results are reported in Table 3.
Treatment patterns
As per eligibility criteria, all 175 patients received first-line therapy. A total of 105 (60%) patients continued to second-line therapy, including 30/43 (70%) with squamous NSCLC, 30/46 (65%) with EGFR/ALK-positive nonsquamous NSCLC, and 43/83 (52%) with EGFR/ALK-negative or -unknown nonsquamous NSCLC (Table 4). Of the patients who received second-line therapy, 54/105 (51%; or 31% overall) received third-line therapy. Two of the 3 patients with unknown histology received second- and third-line therapy.
The most common first-, second-, and third-line therapies administered to patients in the study are summarized in Table 4 by regimen category for all patients and according to histology, EGFR/ALK mutation status for the nonsquamous cohort, and age group. (More detail on specific regimens is provided in Tables S1–S4).
For first-line therapy, two-thirds of patients (66%), including 84% and 60% of patients in squamous and nonsquamous histology cohorts, respectively, received a platinum-based combination, most commonly carboplatin–paclitaxel. One-quarter (25%) of patients, including all but 1 with positive EGFR or ALK mutation status, received an EGFR/ALK TKI as first-line therapy (Table 4; Table S3). For second-line therapy, the majority of the 105 patients (47%) received a single agent, most commonly docetaxel in the squamous cohort (47%) and pemetrexed (22%) or docetaxel (21%) in the nonsquamous cohort. There was no consistent pattern of therapy for the 54 patients who continued to third-line therapy. Single agents were commonly administered (44% overall), including 6 different agents in the squamous cohort and docetaxel (16%) and pemetrexed (11%) in the nonsquamous cohort, while 24% of patients received erlotinib, and 22% received a platinum combination, most of them <75 years old in the nonsquamous cohort (Table 4; Tables S1–S4).
In the nonsquamous cohort, 39 of 44 (89%) patients with EGFR-positive status received an EGFR TKI in first-line therapy; all but 1 received gefitinib (38/39; 97%). The 2 patients with ALK-positive status received crizotinib as first-line therapy; neither one continued therapy beyond first-line. Half or more of patients with EGFR-positive status were prescribed an EGFR TKI for second-line therapy (15/30; 50%), most commonly erlotinib (11/15; 73%), and for third-line therapy (9/15; 60%), all erlotinib (Table 4; Table S3). By contrast, the patients with negative, unknown, or untested mutation status most commonly received a platinum combination (89%) as first-line and single agents as second-line (67%) and third-line (50%), while patients ≥75 years old received fewer platinum combinations and more single agents than younger patients (Table 4; Table S2). Six percent of patients, overall, received maintenance therapy after first-line (data not shown).
The median (range) durations of treatment for patients with EGFR/ALK-positive mutation status were 181 (4–1166), 34 (1–606), and 19 (1–211) days for first-, second-, and third-line therapy, respectively; and the corresponding durations for those with EGFR-negative or unknown mutation status were 68 (1–451), 28 (1–985), 43 (1–394) days, respectively.
Overall survival
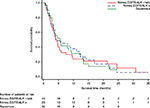
The median OS for the full population was 9.9 months (95% CI, 7.6–11.7) from the start of first-line therapy and 4.7 months (95% CI, 3.8–5.8) from the start of second-line therapy.
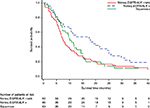
For the squamous cohort, median OS was 10.1 months (95% CI, 7.3–14.4) and 5.6 months (95% CI, 3.1–9.4) from the start of first- and second-line therapy, respectively. As depicted in the Kaplan–Meier plots (Figures 1 and 2), the survival curves for the nonsquamous EGFR/ALK-positive and EGFR/ALK–negative cohorts separated distinctly between 5 and 30 months after initiation of first-line therapy, while the curves after second-line therapy were similar. The median OS for patients with EGFR/ALK-negative or unknown status was 6.9 months (95% CI, 5.6–10.0) and 4.0 months (95% CI, 2.8–4.8) from the start of first- and second-line therapy, respectively, while that for patients with EGFR/ALK-positive status was 17.9 months (95% CI, 9.9–24.4) and 4.0 months (3.3–12.0), respectively.
Health care resource use
The numbers of hospital inpatient admissions and inpatient visits without overnight stay per 100 patient-weeks were similar during first- and second-line therapy, as summarized in Table 5: namely, 5.5 vs 5.1 inpatient admissions and 37.7 vs 38.2 inpatient visits without overnight stay, respectively. The number of emergency department visits was relatively low but doubled with increasing line of therapy from 0.2 to 0.4 emergency department visits per 100 patient-weeks. Similarly, the overall average length of hospitalization increased from 19.1 days in first-line to 21.7 days in second-line therapy. The average length of hospitalization was longest for the EGFR/ALK-positive nonsquamous cohort in first-line (23.7 days) and for the squamous cohort in second-line (26.0 days), although both of these cohorts had the fewest hospital admissions per 100 patient-weeks (Table 5). The average hospital stay overall was 21 days/admission. The total number of image tests was greatest in first-line, although the number per 100 patient-weeks was similar in the 2 lines of therapy.
Discussion
This retrospective observational study has given us the opportunity to evaluate real-world clinical management of patients who initiated systemic therapy for advanced NSCLC at 5 sites in Japan from 2011 to 2013. We found that the proportions of patients with nonsquamous NSCLC tested for EGFR mutation and ALK rearrangement at these sites were 81% and 19%, respectively, with 42% and 8% EGFR- and ALK-positive, respectively. In the squamous cohort, the proportions tested were 40% and 9%, respectively, with only 1 EGFR-positive tumor detected. The treatment patterns for patients with EGFR/ALK-positive nonsquamous NSCLC differed substantially from those with mutation-negative (or unknown) nonsquamous NSCLC. The majority of patients with EGFR- or ALK-positive tumors received an appropriate TKI, suggesting that treatment was personalized according to mutation status.
The Japanese lung cancer guidelines recommend that older patients (≥75 years) with nonsquamous EGFR-negative advanced NSCLC receive nonplatinum monotherapy or a carboplatin combination.5 We did indeed find that older patients were less likely to receive a platinum combination and more likely to receive a single agent than younger patients, as recommended.
The EGFR-mutation testing rates in our study were higher than those in a prior retrospective study in the Asia-Pacific region, which found that, in 2011, the testing rates for EGFR mutation were 65% in Japan, the highest of 11 countries surveyed, and included 69% of adenocarcinomas and 50% of squamous NSCLC.6 While EGFR-mutation testing of squamous NSCLC is not considered necessary by the guidelines because of low levels of positivity, the relatively high testing rates for squamous NSCLC in that study and in ours (40%) could perhaps be explained by concurrent histopathological and biomarker testing of the same sample. Indeed, only 1 squamous tumor in our study and 3% of squamous tumors in the prior study were EGFR-positive.6
The Japanese guidelines now recommend that all EGFR-negative tumors be tested for ALK rearrangements to help clinicians choose the most appropriate therapy for their patients.5 The low rates of ALK testing in our study are likely because testing was relatively new, not available at all hospitals, and not considered reliable during much of the eligibility period (January 2011 through June 2013); moreover, the ALK inhibitors crizotinib and alectinib were not launched in Japan until May 2012 and September 2014, respectively.
We found that patients with advanced NSCLC experienced substantial HCRU and lengthy hospitalizations. The long duration of hospitalizations was not unexpected because of the common practice until recently of observing patients in hospital for 4 weeks to manage potential adverse events, because of precautions on the gefitinib label20 and a publicized death from interstitial pneumonia of a gefitinib-treated patient in the early 2000s. Indeed, patients in the EGFR/ALK-positive cohort had the lengthiest hospital stays in first-line (but not in second-line).
The median OS outcomes for patients in this study were inferior to those found in Phase III clinical trials.21,22 Older age and the presence of bone metastases have been identified as negative prognostic factors for lung cancer survival in large population-based studies.23–25 Our patient population included many older patients (30% were ≥75 years old) as well as patients with bone metastases at the index date (33% of the study population). In addition, 16% of patients had ECOG performance status of 2–3, including 5 of 26 patients, or almost one-fifth of those in the EGFR/ALK-positive nonsquamous cohort with ECOG data; therefore, it is possible that these real-world patients were sicker than patients eligible to enroll in clinical trials, which usually require an ECOG performance status of 0 or 1. Nonetheless, as expected, patients with EGFR/ALK-positive nonsquamous NSCLC, most of whom received an EGFR/ALK TKI as first-line therapy, had substantially better OS than those with negative mutation status (median, 18 months vs 7 months from first-line initiation). The median OS from the initiation of second-line therapy for the EGFR/ALK-positive cohort was only 4 months. This may be because some patients with disease progression on first-line gefinitib received erlotinib in second-line.
This noninterventional study provides information on real-world patterns of treatment, biopsy, biomarker testing, and HCRU among patients with stage IIIB/IV NSCLC at several sites in Japan, including the continuum of therapy from first- through third-line regimens. In Japan, most drugs are reimbursed; therefore, doctors can choose therapeutic regimens without limitations imposed by insurance or expense considerations. Overall, 70% of patients with squamous NSCLC continued to second-line therapy, and 35% received third-line therapy; similar proportions to those reported by Minami et al26 (66% and 33%, respectively) in their single-center study of Japanese patients with stage IIIB/IV squamous NSCLC in 2007–2015. Instead, 52% and 27% of patients in our study with EGFR/ALK-negative/unknown nonsquamous NSCLC received second-line and third-line therapy, respectively, somewhat lower proportions than reported by Minami et al27 (61% and 34%, respectively) in their parallel study of EGFR-negative advanced lung adenocarcinoma. Our study differs from other prior observational studies in Japan, most of which were focused on evaluating outcomes with prespecified NSCLC therapies.28–31
The limitations of our study include those common to all retrospective chart review studies, such as potential transcription errors and missing data. In Japan, patient charts are not transferred when patients move to a new hospital; therefore, it is possible that some treatment regimens were incomplete, and thus not reflective of actual prescribing, if patients had changed hospital. While we provided training to abstractors and employed a standardized eCRF with prespecified variable definitions, we did not conduct pilot testing or an intra-rater reliability assessment. Other study limitations are the small sample size in some cohorts, giving wide OS and treatment duration ranges, and the lack of outcome data associated with specific therapies. Moreover, it would have been of interest to examine the prevalence of different EGFR mutation types; however, these data were not collected.
We included data from a convenience sample of patients at specialized study centers who presented with newly diagnosed stage IIIB/IV NSCLC and initiated first-line therapy, excluding those participating in clinical trials, thereby limiting the generalizability of our findings. Although our population does not represent the full Japanese population with NSCLC, our findings complement clinical trial data. We have described NSCLC treatment patterns by mutation status and age group, in line with the treatment guidelines, thus providing a benchmark for care provided in recent years.
These results from 2011 onward may not be reflective of the current clinical landscape. The treatment options for NSCLC are rapidly increasing with the emergence of checkpoint-based immunotherapies, such as pembrolizumab and nivolumab, and the newer targeted therapies, such as the third-generation EGFR TKIs (e.g., osimertinib) and the second- and third-generation ALK inhibitors. The importance of predictive biomarker testing and the role of personalized treatment are increasingly relevant in light of these expanding treatment options. With the use of immunotherapies and ever-improving knowledge about targetable mutations, we believe there is cause for optimism regarding future therapy for advanced NSCLC based on our finding that patients in the nonsquamous cohort with EGFR/ALK-positive status, most of whom were treated with an EGFR/ALK TKI, had a median OS of 18 months, almost double that for the full study population (10 months).
In conclusion, we found that the majority of patients with nonsquamous NSCLC were tested for predictive biomarkers, most commonly for EGFR mutation. Treatment for both older patients and those with EGFR/ALK-positive nonsquamous NSCLC at these 5 sites in Japan is personalized according to mutation status and is in concordance with guideline recommendations.
Acknowledgments
This study was sponsored by Merck & Co., Inc., Kenilworth, NJ, USA. We thank QuintilesIMS (Cambridge, MA, USA) for data acquisition and statistical support, Yu Feng and Mary Anne Rutkowski (Merck & Co., Inc., Kenilworth, NJ, USA) for statistical programming support, and Reshma Shinde (Merck & Co., Inc., Kenilworth, NJ, USA) for editorial and administrative support. Medical writing and editorial assistance was provided by Elizabeth V. Hillyer, DVM. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure
Hiroshi Isobe has received payment for speaking engagements from AstraZeneca, Bristol-Myers Squibb, Kyowa Hakko Kirin, Chugai, Boehringer Ingelheim, Ono Pharmaceutical Co. Ltd., and Eli Lilly, Japan KK. Koichi Minato has received research funding for his institution from Ono Pharmaceutical Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Kazuko Taniguchi is an employee of MSD KK, and Ashwini Arunachalam, Smita Kothari, and Xiting Cao are employees of Merck & Co., Inc., Kenilworth, NJ, USA, the sponsor of this study. Terufumi Kato has received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Kyowa Hakko Kirin, Lilly, Ono, Pfizer, Roche, and Taiho, and has received research funding from Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly, Merck Sharp & Dohme, Ono, Parexel, Pfizer, Quintiles, Taiho, Takeda, Yakult Honsha. The authors report no other conflicts of interest in this work.
References
Center for Cancer Control and Information Services, National Cancer Center (ganjoho.jp). Cancer statistics in Japan - 2015; 2016. Available from: http://ganjoho.jp/en/professional/statistics/brochure/2015_en.html. Accessed April 24, 2017. | ||
Japan Center for Cancer Control and Information Services. Projected Cancer Statistics; 2016. Available from: http://ganjoho.jp/en/public/statistics/short_pred.html. Accessed April 24, 2017. | ||
Katanoda K, Kamo K, Saika K, et al. Short-term projection of cancer incidence in Japan using an age-period interaction model with spline smoothing. Jpn J Clin Oncol. 2014;44(1):36–41. | ||
Japan Census Data; 2015. Available from: http://www.e-stat.go.jp/SG1/estat/List.do?lid=000001158057. Accessed April 24, 2017. | ||
Japan Lung Cancer Society. Treatment guidelines for lung cancer [In Japanese]; 2015. Available from: http://www.haigan.gr.jp/guideline/2015/2/150002050100.html. Accessed April 24, 2017. | ||
Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol. 2015;10(3):438–445. | ||
Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–4281. | ||
Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11(3):190–198. | ||
Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64(24):8919–8923. | ||
Li S, Choi YL, Gong Z, et al. Comprehensive characterization of oncogenic drivers in Asian lung adenocarcinoma. J Thorac Oncol. 2016;11(12):2129–2140. | ||
Matsuo K, Ito H, Yatabe Y, et al. Risk factors differ for non-small-cell lung cancers with and without EGFR mutation: assessment of smoking and sex by a case-control study in Japanese. Cancer Sci. 2007;98(1):96–101. | ||
Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014;6:423–432. | ||
Hida T, Nakagawa K, Seto T, et al. Pharmacologic study (JP28927) of alectinib in Japanese patients with ALK+ non-small-cell lung cancer with or without prior crizotinib therapy. Cancer Sci. 2016;107(11):1642–1646. | ||
Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. | ||
Union for International Cancer Control (UICC). TNM Classification of Malignant Tumours. 7th ed. Geneva: Wiley-Blackwell; 2009. | ||
American Joint Committee on Cancer. AJCC Lung Cancer Staging, 7th ed.; 2010. Available from: https://cancerstaging.org/references-tools/deskreferences/Pages/AJCC-7th-Ed-Cancer-Staging-Manual.aspx. Accessed April 24, 2017. | ||
Japan Ministry of Health Labour and Welfare. Ethical guidelines for medical and health research involving human subjects; 2015. Available from: http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf. Accessed April 24, 2017. | ||
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. | ||
Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A(7):1135–1141. | ||
AstraZeneca. Iressa (gefitinib) Presribing Information [in Japanese]; 2002. Available from: http://www.info.pmda.go.jp/downfiles/ph/PDF/670227_4291013F1027_1_30.pdf. Accessed April 24, 2017. | ||
Morikawa N, Minegishi Y, Inoue A, et al. First-line gefitinib for elderly patients with advanced NSCLC harboring EGFR mutations. A combined analysis of North-East Japan Study Group studies. Expert Opin Pharmacother. 2015;16(4):465–472. | ||
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. | ||
Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in Taiwan: a population-based cancer registry. J Thorac Oncol. 2013;8(9):1128–1135. | ||
Eberle A, Jansen L, Castro F, et al. Lung cancer survival in Germany: a population-based analysis of 132,612 lung cancer patients. Lung Cancer. 2015;90(3):528–533. | ||
Cetin K, Christiansen CF, Jacobsen JB, Norgaard M, Sorensen HT. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86(2):247–254. | ||
Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Outcomes and prognostic factors of chemotherapy for patients with locally advanced or metastatic pulmonary squamous cell carcinoma. Lung Cancer (Auckl). 2016;7:99–110. | ||
Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Trajectory of chemotherapy for patients with EGFR wild-type advanced pulmonary adenocarcinoma: a single-institution retrospective study. Lung Cancer (Auckl). 2017;8:21–30. | ||
Nakamura H, Satoh H, Kaburagi T, et al. Bevacizumab-containing chemotherapy for non-small cell lung cancer patients: a population-based observational study by the Ibaraki thoracic integrative (POSITIVE) research group. Med Oncol. 2012;29(5):3202–3206. | ||
Kurishima K, Satoh H, Kaburagi T, et al. Erlotinib for elderly patients with non-small-cell lung cancer: Subset analysis from a population-based observational study by the Ibaraki Thoracic Integrative (POSITIVE) Research Group. Mol Clin Oncol. 2013;1(5):828–832. | ||
Kaburagi T, Satoh H, Hayashihara K, et al. Observational study on the efficacy and safety of erlotinib in patients with non-small cell lung cancer. Oncol Lett. 2013;5(2):435–439. | ||
Nishiyama A, Katakami N, Yoshioka H, et al. Retrospective efficacy and safety analyses of erlotinib, pemetrexed, and docetaxel in EGFR-mutation-negative patients with previously treated advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2015;89(3):301–305. | ||
de Castro J, Tagliaferri P, de Lima VCC, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care (Engl). In press 2017. |
Supplementary materials
Approving ethics committees
The study protocol conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013) and was approved by the head of each study site after review by the institutional ethics committee (EC).
  |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.