Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Real-World Patterns of Utilization and Costs Associated with Second-Generation Oral Antipsychotic Medication for the Treatment of Bipolar Disorder: A Literature Review
Authors Doane MJ, Ogden K , Bessonova L, O'Sullivan AK, Tohen M
Received 21 September 2020
Accepted for publication 6 January 2021
Published 16 February 2021 Volume 2021:17 Pages 515—531
DOI https://doi.org/10.2147/NDT.S280051
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Michael J Doane,1 Kristine Ogden,2 Leona Bessonova,1 Amy K O’Sullivan,1 Mauricio Tohen3
1Health Economics and Outcomes Research, Alkermes, Inc., Waltham, MA, USA; 2Evidence, Worldwide Clinical Trials, Morrisville, NC, USA; 3Department of Psychiatry and Behavioral Sciences, University of New Mexico, Albuquerque, NM, USA
Correspondence: Leona Bessonova
Health Economics and Outcomes Research, Alkermes, Inc., 852 Winter Street, Waltham, MA, 02451-1420, USA
Tel +1 781 609 6439
Email [email protected]
Objective: Treatment with second-generation antipsychotics (SGAs) for bipolar disorder, including bipolar I disorder (BD-I), is common. This review evaluated real-world utilization patterns with oral SGAs in the United States (US) for bipolar disorder (and BD-I specifically when reported) and economic burden associated with these patterns.
Methods: Structured, systematic searches of MEDLINE®, EMBASE®, and National Health Service Economic Evaluation Database identified primary research studies (published 2008– 2018) describing real-world SGA use in adults with bipolar disorder/BD-I.
Results: Among 769 studies screened, 39 met inclusion criteria. Most studies (72%) were analyses of commercial or Medicare/Medicaid claims databases. Patient-related (eg, demographic, comorbidities) and disease-related (eg, mania, psychosis) factors were associated with prescribed SGA. Suboptimal utilization patterns (ie, nonadherence, nonpersistence, treatment gaps, medication switching, and discontinuation) were common for patients treated with SGAs. Also common were SGAs prescribed with another psychotropic medication and SGA combination treatment (use of ≥ 2 SGAs concurrently). Suboptimal adherence and SGA combination treatment were both associated with increased health care resource use (HCRU); suboptimal adherence was associated with higher total direct medical and indirect costs.
Limitations: Different definitions for populations and concepts limited between-study comparisons. Focusing on SGAs limits contextualizing findings within the broader treatment landscape (eg, lithium, anticonvulsants). Given the nature of claims data, prescribing rationale (eg, acute episodes vs maintenance) and factors influencing observed utilization patterns could not be fully derived.
Conclusion: Despite increased use of SGAs to treat bipolar disorder over the last decade, reports of suboptimal utilization patterns of SGAs (eg, nonadherence, nonpersistence) were common as was combination treatment. Patterns of SGA use associated with additional HCRU and/or costs were suboptimal adherence and SGA combination treatment; economic consequences associated with other utilization patterns (eg, nonpersistence) were unclear. Strategies to improve SGA treatment continuity, particularly adherence, may improve clinical and economic outcomes among people living with bipolar disorder.
Keywords: adherence, antipsychotics, economics, mania, mood disorders, prescribing patterns, review
Introduction
Bipolar disorder is a complex and severe mental health disorder that encompasses a variety of subtypes marked by extreme shifts in mood and energy that can lead to cognitive, functional, and social impairment.1,2 The bipolar I disorder (BD-I) subtype, defined as having ≥1 lifetime manic episode,3 accounts for approximately one-quarter of bipolar disorder cases in the United States (US).4 BD-I has a lifetime prevalence of 2.1% and the average age of onset of BD-I is 22 years in the US.2 It is debilitating disorder associated with significant medical and psychiatric comorbidities, as well as high rates of premature mortality resulting from both medical comorbidities and suicide.2,3
Over 90% of those with BD-I who experience a single manic episode transition to having recurrent mood episodes,3 necessitating long-term clinical management involving pharmacologic treatment.1,5,6 A variety of medications are approved to treat or prevent manic episodes, such as “traditional” mood stabilizers (eg, lithium, anticonvulsants including valproate, lamotrigine, and carbamazepine) and antipsychotics. Medication prescribed to resolve an acute episode is generally continued longer-term to prevent new mood episodes and improve patients’ overall functioning.1,7 Guidelines recommend that choice of medication is individualized, informed by response to previous medication(s), patient preferences, the rapidity of response required (combination regimens tend to work more quickly than monotherapy), severity of mania, concerns with adherence, and safety and tolerability profiles.
There are multiple second-generation antipsychotics (SGAs) that are first-line options for initial mood-stabilizing treatment in patients with BD-I, either as monotherapy or in combination with “traditional” mood stabilizers. As of May 2020, seven oral SGAs (aripiprazole, asenapine, cariprazine, olanzapine, quetiapine, risperidone, and ziprasidone) have been approved by the US Food and Drug Administration (FDA) to treat BD-I acute manic/mixed episodes or as BD-I maintenance therapy.8 The first regulatory approvals for SGAs to treat BD-I were granted 20 years ago, and over the period since, the volume of outpatient prescriptions for SGAs has outpaced those for “traditional” mood-stabilizing medications.9–11 From 2013 to 2016, the proportion of outpatient visits that included an SGA prescription was 52.7% compared to 26.4% for those prescribed any mood stabilizer.9
BD-I is associated with considerable disease and economic burden, as well as reduced quality of life, relative to other subtypes of bipolar disorder.2,3,12,13 To better understand real-world use of SGAs to treat patients with mania or predominately manic symptoms in the US, this review was conducted to: 1) characterize real-world utilization patterns with oral SGAs in patients with BD-I [or bipolar disorder in general where BD-I estimates were not available]; and 2) report the relationship between these patterns and HCRU and/or associated medical costs.
Materials and Methods
MEDLINE®, MEDLINE® in-process, EMBASE®, and the National Health Service Economic Evaluation Database (NHS EED) were searched for primary research studies published between 1 January 2008 and 9 July 2018, and abstracts from relevant conferences published between 1 January 2015 and 9 July 2018. Systematic, database-specific search strategies were created using terms related to disease, intervention, and outcomes, and were limited to English-language publications. Structured searches utilized the following Medical Subject Heading terms along with keyword equivalents: bipolar and related disorders, antipsychotic agents, costs and cost analyses, drug prescriptions, drug utilization, and medication adherence. The full search strategy is reported in the Supplementary Material.
The search period for published articles was selected for multiple reasons. First, it captured a decade of published literature on the use of SGAs for the treatment of bipolar disorder at the time the review was conducted. Secondly, since SGAs were first approved in 2001 in the US for the treatment of bipolar disorder, identified papers were likely to describe utilization patterns of SGAs when they were available as an approved treatment for bipolar disorder. Thirdly, most included papers were published after the passage of two federal laws (Mental Health Parity and Addictions Equity Act [MHPAEA] in 2008 and Affordable Care Act [ACA] in 2010, respectively) that changed the insurance landscape and substantially improved mental health pharmacy benefits for patients in the US.14 Finally, a search of conference abstracts from 2015 and 2018 was included to discover any relevant data that may not have been published as a manuscript.
Studies were included if 1) the population of interest was adults with BD-I, mixed subtypes of bipolar disorder including BD-I, or bipolar disorder generally; and 2) the study evaluated choice and dosing of SGAs, patterns of SGA use (eg, adherence, persistence), treatment-specific outcomes (eg, side effects), HCRU, and costs. Papers reporting HCRU or costs were included in this review. Scoping searches informed the broader criterion for study population that included general/mixed bipolar disorders in addition to BD-I, as few studies in pilot searches reported data specific to BD-I patients. Additionally, inclusion in this review was limited to studies conducted in real-world settings (ie, not randomized controlled trials or economic evaluations of specific agents) describing cohorts of at least 100 patients in the US over a period of at least six months. Studies were excluded if they focused on narrow or transient bipolar subtypes (eg, bipolar depression, postpartum bipolar disorder). Review articles were not included, but their reference lists were searched for studies that met inclusion criteria but were not captured via the systematic search.
Article titles and abstracts were screened by a single reviewer. In cases of uncertainty, a second reviewer evaluated the title and abstract to confirm inclusion for full-text review. Full-text review was also performed by a single reviewer, with queries resolved through discussion with a second reviewer. Data from included studies were extracted by one researcher into a structured spreadsheet, which was then validated by a second researcher against source publications. Disagreements on the extracted data were flagged and resolved by discussion between the two researchers. Data specific to BD-I were extracted separately where possible.
Costs were converted to 2018 US dollars (USD), using the Consumer Price Index (CPI) for Medical Care, to facilitate descriptive comparisons across studies reporting similar outcomes.15 If cost-year was not reported, the cost-year was assumed to be the last year of the reported observation period.
Results
A total of 2041 citations were identified across all database searches; all records were combined, and duplicate records and excluded publication types were flagged electronically and removed. The titles and abstracts of the remaining 769 abstracts were screened to determine inclusion. After screening and full-text review, 39 studies met inclusion criteria. Of these, 28 studies (72%) were analyses of commercial or Medicare/Medicaid claims databases and six studies (15%) reported data specific to patients with BD-I. Thirty-seven studies reported the period of data collection (1998–2014); most of these studies (92%) evaluated data collected prior to 2011. Figure 1 presents a PRISMA diagram showing the search and selection process.
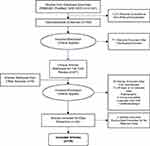 |
Figure 1 Search Results and Study Selection. Abbreviation: NHS EED, National Health Service Economic Evaluation Database. Note: Articles retrieved from other sources refer to papers identified from bibliographic review of relevant published systematic reviews. PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.57 |
Twenty-two studies (56%) reported HCRU or costs of treatment with oral SGAs. Of these, the most frequently studied SGA agent was quetiapine (73%), followed by risperidone (68%), olanzapine (64%), aripiprazole (59%), ziprasidone (50%), and lurasidone (5%). Since most papers were retrospective studies of health care claims, papers did not document whether SGA prescriptions represented treatment for an acute episode or maintenance. Several studies reported aggregated evidence pertaining to SGAs but did not specify the route of administration (ie, oral vs injection), and the route could not be inferred contextually. A list of FDA-approved SGAs for the treatment of BD-I and the chronology of their approvals are included in the Supplementary Material.
Trends in SGA Use and Dosing
Trends in SGA Use
Results of studies suggest that SGA use increased rapidly from 1998 to 2011. In an analysis of Department of Veterans Affairs (VA) health care claims data between 2003 and 2010, SGAs replaced lithium, valproate, and carbamazepine/oxcarbazepine as the most commonly initiated mood-stabilizing treatments for bipolar disorder by 2007.11 A study of commercial health care claims for outpatient visits for bipolar disorder reported SGA prescribing grew from 18% to 49% between 1998 and 2009. This trend began with off-label use, most commonly with olanzapine and risperidone, prior to the first FDA approvals of SGAs in BD-I.10
Another study examined trends in the mix of SGAs prescribed at a bipolar disorder specialty clinic over a 12-year period (2000–2011). During this interval, use of quetiapine and aripiprazole more than doubled, while use of olanzapine and risperidone decreased by more than half. It was suggested that these trends may have been driven by differences in tolerability (eg, fewer side effects associated with aripiprazole, weight gain observed with olanzapine, and extrapyramidal symptoms associated with risperidone), and by improved efficacy observed with quetiapine in bipolar depression.16
SGA Dosing
Eight studies described dosing patterns for aripiprazole, olanzapine, quetiapine, risperidone, or ziprasidone (Table 1). Seven papers reported daily doses for quetiapine that fell below the recommended dose range specified in the product label.17–23 Due to its sedating properties, three studies suggested low dose quetiapine may have been prescribed to treat insomnia and/or anxiety; however, the prescribing rationale for subtherapeutic dose ranges reported could not be confirmed.18,20,24 Daily doses reported for the other four SGAs generally corresponded to the lower end of the recommended dose range in product labels,17–23 with two separate analyses suggesting that dosing at the lower end of therapeutic ranges may be associated with attempts to manage side effects and improve tolerability.20,22 Another study of Medicaid patients with bipolar disorder for whom oral SGAs were prescribed (study period 2000 to 2008) found that fewer than half (45%) received clinically recommended doses after two months of treatment. The proportion of patients receiving doses lower than recommended varied by SGA; the majority of patients receiving quetiapine (72%) received doses below the clinically recommended range, followed by risperidone (45%), olanzapine and ziprasidone (both 35%), and aripiprazole (20%).24 A prospective naturalistic study (reporting period 1998–2005) reporting on the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) found that average daily doses of some SGAs were higher in patients treated with more than one SGA compared to those on SGA monotherapy.18 In a separate analysis from STEP-BD, SGA dosing varied by patient age: younger (vs older) patients were prescribed higher doses for four out of five SGA agents studied.17
 |
Table 1 SGA Dosing |
Choice of SGA
Prescribing patterns described in the literature suggest patient-related (age, gender, race, comorbidities, and treatment history) and disease-related factors (clinical symptoms, eg, presence of mania or psychosis) may influence prescribers’ choice of SGA. Younger patients (age 25–34 years) were less likely to receive risperidone (vs olanzapine), while older patients (age 45–64 years) were less likely to receive quetiapine (vs olanzapine). Compared with olanzapine, African-American patients were more likely to receive risperidone and women were more likely to receive quetiapine.25 Other patient-related factors associated with prescribers’ choice of SGA were the presence of comorbid metabolic conditions (eg, diabetes, obesity); for example, selecting a regimen less likely to induce additional weight gain.11,25,26 Another study found patients who received treatment with lithium were less likely to be prescribed SGA medications.27
Disease-related factors associated with initiating SGA treatment included having a complex clinical profile,26 presence of psychosis,11,27 mania, or receiving treatment in an inpatient (vs outpatient) setting.27 Additionally, patients’ pre-existing risks for adverse events (AEs) can limit the choice of SGA prescribed. A retrospective analysis estimated the prevalence of pre-existing risk factors for AEs and potential drug–drug interactions for a cohort of bipolar patients newly initiating SGA treatment. After comparing patients’ comorbid conditions and concomitant medications against the warnings and precautions from product package inserts, the prevalence of pre-existing AE risk factors was estimated to range from 25% to 88% by individual SGA (aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone).28
Utilization Patterns with Oral SGA Medication
Twenty-three studies described real-world utilization patterns observed with oral SGAs including adherence, persistence, treatment gaps, medication switching, discontinuation, and combination treatment (use of ≥2 concurrent psychotropic medications). Suboptimal treatment outcomes reported in association with these patterns are described in the sections that follow, noting authors’ definitions for these measures.
Adherence
Studies reporting on adherence are shown in Table 2. Some studies reported on adherence to bipolar disorder medication aggregated across several drug classes where SGA-specific adherence data were not available.
 |
Table 2 Adherence with SGAs and BD Medications (Multiple Classes Including SGAs) |
Medication possession ratio (MPR) was the most common measure of adherence, and many analyses of health care claims defined adherence as an MPR of ≥0.8.24,29–31 Utilizing this definition, fewer than 39% of patients with BD-I were adherent with their oral SGA medication at one year in two separate studies.30 A study of bipolar disorder patients taking SGAs, first-generation antipsychotics (FGAs), or mood stabilizer medications found about one-third (35.3%) were adherent with their regimen over a year of follow-up.29 There were three analyses that reported relatively high adherence rates for patients prescribed antipsychotics (MPRs ranged from 0.80 to 1.02);19,32,33 however, these adherence rates may have been due to assessing MPR over treatment episodes (defined as period of ≥2 sequential antipsychotic prescriptions), rather than over a discrete evaluation period (eg, 1 year).
Other studies collected data on medication adherence via self-report from patients, and reported adherence estimates that are similar to those calculated using MPR. During 48,287 follow-up visits among 3640 patients, fewer than half (46.4%) self-reported being adherent with their bipolar disorder medications, which was defined as having missed fewer than 25% of total doses of any one medication.34 In another patient survey, 33.8% self-reported missing at least one dose of bipolar disorder medications in the prior 10 days.35
Less frequently utilized metrics of adherence included cumulative medication acquisition (CMA), cumulative medication gap (CMG), and proportion of days covered (PDC). A cost analysis of Medicaid claims for bipolar disorder patients newly started on SGA monotherapy with olanzapine, risperidone, or quetiapine reported adherence over a 1-year period as measured by CMA and CMG.25 Across the three treatment cohorts, similar rates were reported for CMA (70% to 76%) and CMG (35% to 38%), respectively. An analysis of multi-state Medicaid claims for bipolar disorder patients newly prescribed SGA therapy reported a PDC of 72% over 1 year of follow-up.36
Four analyses evaluated sociodemographic, clinical, and/or treatment-related characteristics associated with suboptimal adherence with oral SGA therapy, which included comorbid substance use disorder,19,31 a history of suicide attempts,24 and older age (Table 3).19,24,33 Two studies examined adherence by prescribed SGA dose. An analysis of commercial health care claims reported those with predominately manic/mixed symptoms who received risperidone, olanzapine, and FGAs had reduced adherence (MPR) with higher doses in every 3-month treatment segment over a 15-month period, an effect that was not observed among those receiving aripiprazole, quetiapine, or ziprasidone.19 In a cohort of Medicaid bipolar patients for whom SGAs were prescribed, only 45% had clinically recommended doses after two months of treatment, and of these, only 58% were adherent (MPR ≥0.8). Level of adherence was not reported for the 55% of patients who received either subtherapeutic or high doses of the index SGA treatment, nor were reasons for prescriptions that fell outside of the clinically recommended dose range described.24
 |
Table 3 Factors Associated with Suboptimal Adherence with SGA Regimen in Analyses of Health Care Claims |
One study surveyed patients about factors that contributed to suboptimal adherence with their bipolar disorder medications, such as missing doses or stopping treatment. Patients cited two frustrations that negatively impacted medication adherence: having to take medicine daily (51.1%) and side effects associated with mood-stabilizing psychotropic medications (40.2%). Patients who self-reported suboptimal adherence pointed to adverse effects of bipolar disorder medications such as weight gain (58.5%), excessive sedation (54.2%), and physical awkwardness or tremor (33.1%) to explain their incomplete adherence with prescribed therapies.35
Persistence, Treatment Gaps, Switching, and Discontinuation
Studies reporting on treatment persistence and duration of treatment/treatment gaps are summarized in Tables 4 and 5. Although definitions of persistence varied across studies, regardless of the assessment criteria used, persistence with treatment was generally low. An analysis of Medicaid health care claims found that only 18% of patients who were prescribed SGAs at clinically recommended doses were persistent with therapy defined as no gap (>30 days between refills) over a 1-year period. Median time to SGA-nonpersistence (30-day gap in treatment) was approximately three months (96 days), with modest variation depending on the prescribed SGA (low of 72 days with olanzapine; high of 117 with ziprasidone).24 An analysis of commercial health care claims for employees with bipolar disorder found greater persistence among adherent (MPR ≥0.8) patients; although, over 1 year of follow-up these patients had more treatment gaps (3.62 vs 2.60) for shorter periods (15.57 vs 58.89 days) than those with a MPR <0.8.29 In evaluating dosing regularity, drug holidays (missing ≥3 consecutive days of medication, found in 35.8% of analysis periods), single-day omissions (present in 64.7% of periods) and self-directed changes in daily dosing (86.7% of periods) were common.37
 |
Table 4 Persistence with Oral SGAs or BD Medication |
 |
Table 5 Duration of Therapy and Treatment Gaps with SGAs |
Of the studies describing utilization patterns for patients newly initiated on SGAs, only one study reported rates of switching treatment and two studies described rates of discontinuation with the index SGA medication. A study of utilization patterns for BD-I patients newly prescribed SGA treatment over a 1-year period reported 8.4% of patients switched treatment (changing from index SGA to another) and most (63.4%) discontinued their index SGA medication. The average time to discontinuation was approximately 2 months, with only one-third of patients who discontinued their index SGA resuming any type of antipsychotic medication within the remaining 1-year evaluation period.30 A second retrospective study reported that discontinuation rates varied by individual SGA (67% for ziprasidone to 83% for aripiprazole) and fewer than 5% of patients completed a full year of taking their index SGA medication.22
Combination Treatment
Fourteen studies reported use of concomitant psychotropic medications in patients prescribed SGAs (Table 6), including SGA combination treatment (use of ≥2 SGAs concurrently) and concomitant use of SGAs with other psychotropic medications (eg, antidepressants, traditional mood stabilizers). The prevalence of SGA combination treatment ranged from 1% in a sample of patients newly initiated on SGAs to 23% of SGA-treated patients insured by a large commercial health plan.26,30 In the STEP-BD study, SGA combination treatment (vs monotherapy) was associated with no improvement in clinical status and slightly poorer global functioning, although the effect size was small. In addition, there was no association between use of combination treatment and illness severity based on number of comorbid diagnoses, duration of illness, number of manic or depressive episodes, or clinical measures collected at baseline.18
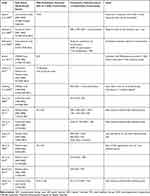 |
Table 6 Prevalence of Combination Treatment |
Combination treatment with SGAs and another class of psychotropic medication was reported more frequently than SGA combination treatment, with rates that ranged from 5% to 78%. Different sample characteristics and inclusion criteria associated with concomitant treatments at baseline contributed to the wide range reported.22,26 Complex combination treatment (ie, four or more bipolar disorder-related psychotropic medications concomitantly) was reported among 38% of patients receiving a SGA regimen in the STEP-BD study.38
HCRU and Costs Associated with Utilization Patterns
Studies reporting the effects of the utilization patterns observed with SGAs on HCRU and costs are summarized in Table 7. Generally, patients with lower antipsychotic adherence were at greater risk of hospitalizations and emergency room visits;31,39 conversely, those who had higher rates of adherence had lower risks of hospitalization39,40 and lower outpatient psychiatric care expenditures.32 Having an MPR ≥0.75 after a year of treatment was associated with lower risk of all-cause (OR=0.730) and psychiatric-related rehospitalizations (OR= 0.759). Additionally, improvements in MPR above the 75% threshold further decreased odds of both types of rehospitalization.40 Similarly, bipolar disorder patients with greater adherence to antipsychotic medication also had lower subsequent total and outpatient psychiatric care expenditures driven by decreased risk of requiring acute mental health care (ie, hospitalization, ER visit). Among patients with predominately manic/mixed symptoms followed over 15 months, a single-point increase in MPR was significantly associated with a $192-$686 quarterly reduction in total expenditures and a $112-$583 quarterly reduction in outpatient psychiatric care over every 3-month period of treatment (2018 USD). This inverse relationship between MPR and mental health care expenditures (total and outpatient) was also observed for patients with predominantly depressive symptoms; however, this association was significant in only one of the 3-month treatment periods (months 10 to 12).32 In addition, in a study of Medicaid patients receiving oral or injectable antipsychotic medications over a 1-year period, those with suboptimal adherence (MPR<0.8) were more likely than adherent patients to have an all-cause ER visit (73% vs 57%) or hospitalization (42% vs 37%), respectively.31
 |
Table 7 Utilization Patterns with SGAs Associated with Increased HCRU or Costs |
Increased use of both general medical and psychiatric services was reported for patients receiving SGA combination treatment (use of ≥2 SGAs concurrently) compared to SGA monotherapy in a longitudinal cohort study over a mean follow-up duration of 21 months. BD-I patients with SGA combination treatment had 80% more general medical service visits (3.6 vs 2.0 visits) and more than twice the psychiatric treatment visits (6.4 vs 2.1 visits) than patients receiving SGA monotherapy. Regression analyses that included factors considered proxies for illness severity (eg, age, illness duration, and use of other psychotropic medications) confirmed there was an independent association between SGA combination treatment and medical and psychiatric HCRU.18
Discussion
Since 2000, expanded approval of SGAs to treat bipolar disorder has led to their increased use in the clinical management of manic and mixed acute episodes, and as a maintenance treatment, relative to traditional mood stabilizers. Prescribing trends reported in this review10,11,16 align with a recent US analysis of National Ambulatory Medical Care Survey (NAMCS) data that found SGA prescriptions in the outpatient setting grew from 12.4% to 51.4% over the periods from 1997 to 2000 and 2013 to 2016, respectively. Meanwhile, during these same 4-year periods, use of traditional mood stabilizers, such as lithium, valproate, and carbamazepine/oxcarbazepine, declined substantially from 62.3% to 26.4%.9
A greater proportion of outpatient visits may include prescriptions for SGA treatment than traditional mood stabilizers; yet, treatment prevalence rates for bipolar disorder in the US remain low.41 In a recent epidemiological study for example, the 12-month treatment rate for BD-I was 46%.2 Among those who do receive any type of oral medication, adherence (MPR ≥80%) is achieved by roughly 60% of patients.41 This review of real-world studies reported rates of adherence to SGA treatment that were lower still, ranging from 38.9% to as low as 8.3%.30,31 Other notable suboptimal patterns described included use of SGAs outside of, or at the lower end of, therapeutic recommended ranges;24 low rates of persistence,24,30 and long treatment gaps (eg, >30 days).24,31 The majority of these findings were drawn from retrospective analyses of health care claims (72% of studies in this review), which shed little light on reasons for these trends, or the degree to which factors associated with SGA medication (eg, efficacy or tolerability) or other factors (eg, patient or clinical status) may have influenced these findings. This review also highlights a large gap in the knowledge base, underscoring the need for more prospective, real-world research that incorporates input from patients and clinicians to help illuminate what factors may be influencing utilization patterns observed with SGA treatment, as well as their economic impact on patients and health systems.
A common reason for nonadherence to bipolar medication cited in the extant literature is patients’ experience of side effects. Studies exploring factors associated with nonadherence to mood-stabilizing treatment (including antipsychotics) have found that patients’ negative attitudes toward medications,42–44 worry about medication,45 and adverse effects of medication (eg, weight gain, cognitive effects, sedation) contribute to nonadherence.44,46 In a recent study of BD-I patients taking oral antipsychotics, experience of medication side effects was cited as a reason for stopping medication nearly half of the time. When participants were asked to describe the adverse effects of antipsychotics they wanted to avoid most in a new medication, the most common answers were medication-induced anxiety (50%), weight gain (48%), and “feeling like a zombie” (47%).47 Similar findings were reported in this review, with 40% of patients (in a survey) attributing their nonadherence with mood-stabilizing psychotropic medications to side effects, suggesting a need for treatments with better benefit/risk profiles to improve patients’ adherence with medication.
The association between treatment nonadherence and poor clinical/economic outcomes, coupled with evidence that modest improvements in adherence can significantly reduce HCRU and/or costs associated with inadequate symptom control,32,39, call for increased efforts to support patients in maintaining continuous pharmacotherapy. In a 2018 retrospective study, published outside the date range for this review, analyses showed patients newly initiated on antipsychotic medication who were fully adherent (PDC ≥80%) for ≥6 months had significantly lower adjusted rates of psychiatric hospitalization (6.0%) compared to those who were partially adherent (8.3%, PDC ≥40% and <80%) or nonadherent (8.8%, PDC <40%).48 To this end, building upon interventions targeted to other factors associated with nonadherence, such as simplifying medication regimens,38,49,50 programming medication reminders,44,51 and programs to strengthen the patient-clinician therapeutic alliance5,6,44 need to be a research priority as well as part of ongoing clinical management of patients.
This review has several limitations. It considered only studies published between 2008 and 2018, in English, describing US data. Most studies (92%) evaluated data collected prior to 2011; these older data may not reflect contemporary practice patterns. Most studies used pre-DSM-5 definitions of bipolar disorder subtypes. Because DSM-5 criteria broaden the definition of bipolar disorder,52 outcomes reported from studies using earlier DSM criteria may not be representative of current clinical experience. In addition, publications were excluded if they considered fewer than 6 months of treatment, which may have excluded relevant publications. Wherever possible, the primary focus of investigation was BD-I. However, in the absence of relevant research for BD-I populations, evidence related to general bipolar disorder populations was described. Including findings based on individuals across bipolar disorder subtypes could conceivably mischaracterize results as applied to BD-I patients.
There are many treatment options for bipolar disorder; focusing on SGA therapy may limit the degree to which these findings may be applied within the broader treatment landscape. Some of the real-world studies did not break results out by drug class or by SGA agent. This led to some additional limitations. For example, it was not always possible to separate out the effects of individual drug classes on adherence or persistence. Most real-world papers were retrospective analyses of health care claims data; it is well established that data collected for reimbursement are subject to coding errors and sampling issues that can limit the generalizability of the patterns observed. Further, claims provide no direct information from clinicians or patients describing the reasons for initiating/stopping treatment with SGAs (eg, treatment for acute episodes vs maintenance), the planned duration of treatment, choice of dose, or choice to prescribe combination regimens in the real-world setting. Few analyses considered disease severity or complexity; thus, the degree to which severity of bipolar symptoms or medical comorbidities may have influenced the associated data reported are unknown. Finally, although the terminology used to describe adherence and persistence was similar across studies, operational definitions varied, potentially influencing the comparability of findings, as well as the interpretability of reported results. However, the overall focus on real-world evidence was a strength of this review in terms of understanding current clinical practice, its effects on outcomes, and barriers to improved SGA treatment.
Conclusion
SGA treatment is routinely prescribed to treat bipolar disorder, yet reports of suboptimal utilization patterns (ie, nonadherence, nonpersistence, treatment gaps, medication switching, and discontinuation) with SGAs are common. Also common were SGAs prescribed with another psychotropic medication and SGA combination treatment. Of the utilization patterns described in this review, two (suboptimal adherence, SGA combination treatment) were found to have a likely economic impact for patients and the health care system. Both suboptimal adherence and SGA combination treatment were associated with increased HCRU. Additionally, increased direct and indirect medical costs were observed in SGA-treated cohorts with suboptimal adherence. Other utilization patterns with the potential to affect HCRU or costs included nonpersistence, treatment gaps (ie, treatment episodes with one or more periods of no SGA treatment), switching, and early discontinuation of SGA medication; however, cost estimates associated with these patterns were not reported in the identified literature. Strategies to improve treatment continuity, particularly adherence with SGA medications, as well as to reduce the need for combination treatments may improve clinical and economic outcomes among people living with bipolar disorder.
Acknowledgments
The authors thank Nancy Neil, PhD (Worldwide Clinical Trials, Inc., USA), who assisted in the planning and conduct of the literature searches and analyses. Medical writing and editorial support for the preparation of the manuscript, under the direction of the authors, was provided by Jo Whelan, MSc (Textpharm Limited, UK) and was funded by Alkermes, Inc. In October of 2019, data included in this manuscript were presented as a poster at the Academy of Managed Care Pharmacy (AMCP) NEXUS 2019 annual meeting in National Harbor, Maryland. The poster’s abstract was published in “Meeting Abstracts” in Journal of Managed Care & Specialty Pharmacy, Supplement Vol 25(10-a): https://www.jmcp.org/doi/pdf/10.18553/jmcp.2019.25.10-a.s1.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
Funding for the planning and conduct of the systematic literature review and the preparation of this manuscript was provided by Alkermes, Inc.
Disclosure
Leona Bessonova, Michael J. Doane, and Amy K. O’Sullivan are employees of Alkermes, Inc.; Leona Bessonova and Amy K. O’Sullivan may own stock/options in the company and Michael J. Doane owns Alkermes, Inc stock. Kristine Ogden is an employee of Worldwide Clinical Trials, Inc., which has received consulting fees from Alkermes, Inc. for conducting this study. Leona Bessonova reports personal fees from Alkermes Inc., outside the submitted work. Mauricio Tohen was an employee of Lilly (1997 to 2008) and has received honoraria from or consulted for Abbott, AstraZeneca, Alkermes, Allergan, Bristol-Myers Squibb, GlaxoSmithKline, Intracellular Therapies, Lilly, Johnson & Johnson, Otsuka, Merck, Gedeon Richter Plc, Sunovion, Forest, Roche, Elan, Lundbeck, Teva, Pamlab, Minerva, Neurocrine, Pfizer, Wyeth and Wiley Publishing; his spouse was a full time employee at Lilly (1998–2013).
References
1. Vieta E, Berk M, Schulze TG, et al. Bipolar disorders. Nat Rev Dis Primers. 2018;4(1):18008. doi:10.1038/nrdp.2018.8
2. Blanco C, Compton WM, Saha TD, et al. Epidemiology of DSM-5 bipolar I disorder: results from the national epidemiologic survey on alcohol and related conditions - III. J Psychiatr Res. 2017;84:310–317. doi:10.1016/j.jpsychires.2016.10.003
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
4. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. doi:10.1001/archgenpsychiatry.2011.12
5. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180–195.
6. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170.
7. Bobo WV. The diagnosis and management of bipolar I and II disorders: clinical practice update. Mayo Clin Proc. 2017;92(10):1532–1551. doi:10.1016/j.mayocp.2017.06.022
8. U.S. Food and Drug Administration. FDALabel databases. 2020. Available from: https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/57988.
9. Rhee TG, Olfson M, Nierenberg AA, Wilkinson ST. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;appiajp202019091000.
10. Pillarella J, Higashi A, Alexander GC, Conti R. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998–2009. Psychiatr Serv. 2012;63(1):83–86. doi:10.1176/appi.ps.201100092
11. Miller CJ, Li M, Penfold RB, et al. The ascendancy of second-generation antipsychotics as frontline antimanic agents. J Clin Psychopharmacol. 2015;35(6):645–653. doi:10.1097/JCP.0000000000000405
12. Bessonova L, Doane MD, Ogden K, O’Sullivan AK, Tohen M. The economic burden of bipolar disorder in the United States: a systematic literature review. Clinicoecon Outcomes Res. 2020;Volume 12:481–497. doi:10.2147/CEOR.S259338
13. Jin H, McCrone P. Cost-of-illness studies for bipolar disorder: systematic review of international studies. Pharmacoeconomics. 2015;33(4):341–353. doi:10.1007/s40273-014-0250-y
14. Frank RG, Beronio K, Glied SA. Behavioral health parity and the affordable care act. J Soc Work Disabil Rehabil. 2014;13(1–2):31–43. doi:10.1080/1536710X.2013.870512
15. United States Bureau of Labor Statistics. Consumer price index. 2018. Available from: http://www.bls.gov/cpi/.
16. Hooshmand F, Miller S, Dore J, et al. Trends in pharmacotherapy in patients referred to a bipolar specialty clinic, 2000–2011. J Affect Disord. 2014;155:283–287. doi:10.1016/j.jad.2013.10.054
17. Al Jurdi RK, Marangell LB, Petersen NJ, Martinez M, Gyulai L, Sajatovic M. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922–933. doi:10.1097/JGP.0b013e318187135f
18. Brooks JO, Goldberg JF, Ketter TA, et al. Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the systematic treatment enhancement program for bipolar disorder. J Clin Psychiatry. 2011;72(2):240–247. doi:10.4088/JCP.09m05214yel
19. Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH. The association between treatment adherence and antipsychotic dose among individuals with bipolar disorder. Int Clin Psychopharmacol. 2008;23(6):305–316. doi:10.1097/YIC.0b013e32830b0f88
20. Jing Y, Johnston SS, Fowler R, Bates JA, Forbes RA, Hebden T. Comparison of second-generation antipsychotic treatment on psychiatric hospitalization in medicaid beneficiaries with bipolar disorder. J Med Econ. 2011;14(6):777–786. doi:10.3111/13696998.2011.625066
21. Jing Y, Kim E, You M, Pikalov A, Tran QV. Healthcare costs associated with treatment of bipolar disorder using a mood stabilizer plus adjunctive aripiprazole, quetiapine, risperidone, olanzapine or ziprasidone. J Med Econ. 2009;12(2):104–113. doi:10.3111/13696990903044092
22. Kim E, You M, Pikalov A, Van-Tran Q, Jing Y. One-year risk of psychiatric hospitalization and associated treatment costs in bipolar disorder treated with atypical antipsychotics: a retrospective claims database analysis. BMC Psychiatry. 2011;11:6. doi:10.1186/1471-244X-11-6
23. Kim E, Maclean R, Ammerman D, et al. Time to psychiatric hospitalization in patients with bipolar disorder treated with a mood stabilizer and adjunctive atypical antipsychotics: a retrospective claims database analysis. Clin Ther. 2009;31(4):836–848. doi:10.1016/j.clinthera.2009.04.022
24. Rascati KL, Richards KM, Ott CA, et al. Adherence, persistence of use, and costs associated with second-generation antipsychotics for bipolar disorder. Psychiatr Serv. 2011;62(9):1032–1040. doi:10.1176/ps.62.9.pss6209_1032
25. Qiu Y, Christensen DB, Fu AZ, Liu GG. Cost analysis in a medicaid program for patients with bipolar disorder who initiated atypical antipsychotic monotherapy. Curr Med Res Opin. 2009;25(2):351–361. doi:10.1185/03007990802634077
26. Tohen M, Ng-Mak D, Rajagopalan K, Halpern R, Chuang CC, Loebel A. Patient characteristics associated with use of lurasidone versus other atypical antipsychotics in patients with bipolar disorder: analysis from a claims database in the United States. Prim Care Companion CNS Disord. 2017;19(3). doi:10.4088/PCC.16m02066
27. Prabhakar M, Haynes WG, Coryell WH, et al. Factors associated with the prescribing of olanzapine, quetiapine, and risperidone in patients with bipolar and related affective disorders. Pharmacotherapy. 2011;31(8):806–812. doi:10.1592/phco.31.8.806
28. Citrome L, Johnston S, Nadkarni A, Sheehan JJ, Kamat SA, Kalsekar I. Prevalence of pre-existing risk factors for adverse events associated with atypical antipsychotics among commercially insured and medicaid insured patients newly initiating atypical antipsychotics. Curr Drug Saf. 2014;9(3):227–235. doi:10.2174/1574886309666140601211551
29. Bagalman E, Yu-Isenberg KS, Durden E, Crivera C, Dirani R, Bunn WB
30. Chen W, Deveaugh-Geiss AM, Palmer L, Princic N, Chen YT. Patterns of atypical antipsychotic therapy use in adults with bipolar I disorder in the USA. Hum Psychopharmacol. 2013;28(5):428–437. doi:10.1002/hup.2326
31. Lang K, Korn J, Muser E, Choi JC, Abouzaid S, Menzin J. Predictors of medication nonadherence and hospitalization in medicaid patients with bipolar I disorder given long-acting or oral antipsychotics. J Med Econ. 2011;14(2):217–226. doi:10.3111/13696998.2011.562265
32. Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH. Antipsychotic treatment adherence and associated mental health care use among individuals with bipolar disorder. Clin Ther. 2008;30(7):1358–1374. doi:10.1016/S0149-2918(08)80062-8
33. Gianfrancesco FD, Sajatovic M, Tafesse E, Wang RH. Association between antipsychotic combination therapy and treatment adherence among individuals with bipolar disorder. Ann Clin Psychiatry. 2009;21(1):3–16.
34. Perlis RH, Ostacher MJ, Miklowitz DJ, et al. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry. 2010;71(3):296–303. doi:10.4088/JCP.09m05514yel
35. Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol. 2008;23(2):95–105. doi:10.1002/hup.908
36. Seabury SA, Goldman DP, Kalsekar I, Sheehan JJ, Laubmeier K, Lakdawalla DN. Formulary restrictions on atypical antipsychotics: impact on costs for patients with schizophrenia and bipolar disorder in medicaid. Am J Manag Care. 2014;20(2):e52–e60.
37. Pilhatsch M, Glenn T, Rasgon N, et al. Regularity of self-reported daily dosage of mood stabilizers and antipsychotics in patients with bipolar disorder. Int J Bipolar Disord. 2018;6(1):10. doi:10.1186/s40345-018-0118-8
38. Goldberg JF, Brooks JO
39. Lage MJ, Hassan MK. The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry. 2009;8:7. doi:10.1186/1744-859X-8-7
40. Hassan M, Lage MJ. Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge. Am J Health Syst Pharm. 2009;66(4):358–365. doi:10.2146/ajhp080374
41. Greene M, Paladini L, Lemmer T, Piedade A, Touya M, Clark O. Systematic literature review on patterns of pharmacological treatment and adherence among patients with bipolar disorder type I in the USA. Neuropsychiatr Dis Treat. 2018;14:1545–1559. doi:10.2147/NDT.S166730
42. Barraco A, Rossi A, Nicolo G. Description of study population and analysis of factors influencing adherence in the observational Italian study “Evaluation of Pharmacotherapy Adherence in Bipolar Disorder” (EPHAR). CNS Neurosci Ther. 2012;21(1):110–118. doi:10.1111/j.1755-5949.2010.00225.x
43. Sajatovic M, Ignacio RV, West JA, et al. Predictors of nonadherence among individuals with bipolar disorder receiving treatment in a community mental health clinic. Compr Psychiatry. 2009;50(2):100–107. doi:10.1016/j.comppsych.2008.06.008
44. Jawad I, Watson S, Haddad PM, Talbot PS, McAllister-Williams RH. Medication nonadherence in bipolar disorder: a narrative review. Ther Adv Psychopharmacol. 2018;8(12):349–363. doi:10.1177/2045125318804364
45. Scott J, Pope M. Nonadherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63(5):384–390. doi:10.4088/jcp.v63n0502
46. Mago R, Borra D, Mahajan R. Role of adverse effects in medication nonadherence in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):363–366. doi:10.1097/HRP.0000000000000017
47. Bessonova L, Velligan DI, Weiden PJ, et al. Antipsychotic treatment experiences of people with bipolar I disorder: patient perspectives from an online survey. BMC Psychiatry. 2020;20(1):354. doi:10.1186/s12888-020-02767-x
48. Broder MS, Greene M, Chang E, Hartry A, Yan T, Yermilov I. Atypical antipsychotic adherence is associated with lower inpatient utilization and cost in bipolar I disorder. J Med Econ. 2019;22(1):63–70. doi:10.1080/13696998.2018.1543188
49. Fung VC, Overhage LN, Sylvia LG, et al. Complex polypharmacy in bipolar disorder: side effect burden, adherence, and response predictors. J Affect Disord. 2019;257:17–22. doi:10.1016/j.jad.2019.06.050
50. Goldberg JF. Complex combination pharmacotherapy for bipolar disorder: knowing when less is more or more is better. FOCUS. 2019;17(3):218–231. doi:10.1176/appi.focus.20190008
51. Levin JB, Sajatovic M, Rahman M, et al. Outcomes of psychoeducation and a text messaging adherence intervention among individuals with hypertension and bipolar disorder. Psychiatr Serv. 2019;70(7):608–612. doi:10.1176/appi.ps.201800482
52. Angst J. Bipolar disorders in DSM-5: strengths, problems and perspectives. Int J Bipolar Disord. 2013;1:12. doi:10.1186/2194-7511-1-12
53. Aparasu RR, Jano E, Bhatara V. Concomitant antipsychotic prescribing in US outpatient settings. Res Social Adm Pharm. 2009;5(3):234–241. doi:10.1016/j.sapharm.2008.08.005
54. Baldessarini R, Henk H, Sklar A, Chang J, Leahy L. Psychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherence. Psychiatr Serv. 2008;59(10):1175–1183. doi:10.1176/ps.2008.59.10.1175
55. Guo JJ, Keck PE
56. Qiu Y, Fu AZ, Liu GG, Christensen DB. Healthcare costs of atypical antipsychotic use for patients with bipolar disorder in a medicaid programme. Appl Health Econ Health Policy. 2010;8(3):167–177. doi:10.2165/11318830-000000000-00000
57. Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10).
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
