Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Real-World Experience with Tralokinumab in a Patient with Recalcitrant Atopic Dermatitis: A Case Report
Received 24 August 2022
Accepted for publication 7 December 2022
Published 20 December 2022 Volume 2022:15 Pages 2825—2830
DOI https://doi.org/10.2147/CCID.S382424
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Eva Moennig, Stephan Traidl
Division of Immunodermatology and Allergy Research, Department of Dermatology and Allergy, Hannover Medical School (MHH), Hannover, Germany
Correspondence: Eva Moennig, Division of Immunodermatology and Allergy Research, Department of Dermatology and Allergy, Hannover Medical School (MHH), Carl-Neuberg-Str.1, Hannover, 30625, Germany, Email [email protected]
Abstract: Atopic dermatitis (AD) is a common inflammatory skin disease, which negatively impacts the individual’s quality of life (QoL). In particular, moderate-to-severe AD is frequently difficult to treat. We report a case involving a 40-year-old male who has suffered from AD since early childhood and who also had co-morbid seasonal allergic rhinitis. In June 2021, we initiated treatment with the fully human IgG4 monoclonal antibody tralokinumab that specifically targets IL-13 and the patient has been followed for 38 weeks. During this time period, he received a booster vaccination for COVID-19 (week 18) and developed the disease in April 2022 (both with minimal impact). Tralokinumab treatment reduced AD symptoms, was well tolerated and improved QoL scores, and the patient reported that he was very satisfied with the treatment.
Keywords: atopic dermatitis, COVID-19, systemic therapy, tralokinumab
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by recurrent, intensely pruritic eczematous lesions.1,2 The pathogenesis of AD involves a complex interplay between genetic and environmental factors which result in epidermal barrier impairment, an inflammatory microenvironment, and immune dysregulation.3,4 The immune response is predominantly type-2 immunity-based, and a pivotal role has been suggested for IL-13, among other cytokines.5–8
Tralokinumab is a high-affinity monoclonal antibody that binds to and inhibits IL-13 specifically, blocking its interaction with the IL-13 receptors and neutralizing their biological activity.9 Based on the results of Phase III randomized, placebo-controlled clinical trials (RCTs), tralokinumab was approved in the EU for the treatment of moderate-to-severe AD in adult patients who are candidates for systemic therapy.10 However, at the present time, there do not appear to be long-term real-world effectiveness data pertaining to the use of tralokinumab in community practice where patients with AD are exposed to social/environmental factors associated with day-to-day living. This includes the current coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus.
Herein, we report the case of a male patient who was prescribed tralokinumab for almost 40 weeks for AD, which had proven resistant to conventional first-line therapies. During the course of his AD treatment, he had received a COVID-19 vaccination and, at a later date, he was infected with the SARS-CoV-2 virus. All data presented in this article have been anonymised to ensure patient confidentiality, and written consent for the publication and publication of photographs was obtained from the patient. Institutional approval for publication of this case report was obtained from the Medizinische Hochschule Hannover, although it is not a legal requirement in Germany.
Case Report
A 40-year-old male presented at our dermatological department and was diagnosed with AD as a 1-year-old child. Over the intervening years, the patient has also suffered from seasonal allergic rhinitis with elevated IgE, with symptoms of conjunctivitis/blepharitis and swollen eyes in the spring/summer time. The patient had never experienced any signs or symptoms of asthma.
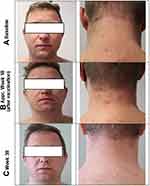
Conventional treatments such as moisturizers, higher potency (group 3 or 4, Niedner classification) corticosteroids, topical calcineurin inhibitors and UV-A1 phototherapy had been tried with limited success and usually with flare-up of AD symptoms. In June 2021, the patient presented to the dermatology department with pruritic eczematous lesions, including the face and neck (Figure 1A, baseline) as well as symptoms of an allergic rhinoconjunctivitis. A full diagnostic examination was performed, including measures of QoL (Table 1). A relapse of severe AD was diagnosed with significant eye involvement leading to the initiation of tralokinumab treatment. Because co-morbid eye symptoms were particularly troublesome for this patient during the spring allergy season, we chose to treat his AD with tralokinumab rather than dupilumab since it may be associated with a lower incidence of conjunctivitis compared with dupilumab.11–13 Tralokinumab (Adtralza®) was administered as a subcutaneous injection at an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week. Concomitant topical class II corticosteroids and pimecrolimus cream were also applied as required.
 |
Table 1 Evolution in Measures of Disease Severity in a Patient with Atopic Dermatitis During Treatment with Tralokinumab |
During tralokinumab treatment, the patient achieved stable disease and a reduction of the eye symptoms during follow-up. Improvement was observed in most measures of disease severity after 12 weeks of treatment (Table 1). Follow-up visits at weeks 32 and 34 showed that disease measure scores were either maintained or further improved during continued treatment. The last follow-up visit took place at week 38, and tralokinumab continued to sustain clinical benefit during long-term treatment. The evolution of AD symptom and QoL scores from baseline to week 38 are shown in Figure 2. Major improvements associated with tralokinumab therapy were reported for skin lesions (EASI, IGA), pruritus (itch) and other patient-related outcomes (Table 1). EASI was reduced by 75.5% by week 38 vs baseline and pruritus was reduced from an NRS of 6 at baseline to 2 at week 38. Additional benefits with tralokinumab included less fatigue, better sleep patterns and improved QoL. In particular, DLQI improved by 21 points and sleeplessness was completely alleviated by week 38 (Table 1; Figure 2). Based on these important clinical benefits, the patient reported that he was very satisfied with treatment.
In the real-world, more and more patients are impacted by the COVID-19 pandemic, either as a result of being vaccinated or from catching the disease. In the current case, the patient received a COVID-19 booster vaccination at about week 18 of treatment with tralokinumab. This was associated with an initial exacerbation of AD (Figure 1B) but good improvement of symptoms by week 32 and up to week 38 (Figure 1C, Week 38). Furthermore, in April 2022, the patient developed a COVID-19 infection, but this had no observable impact on AD status and the skin remained stable. Tralokinumab treatment was continued unchanged with no adverse consequences.
Discussion
At the present time, there are no real-world effectiveness findings available for tralokinumab, and we report this case of a patient with a long-term history of recalcitrant AD and comorbid rhinoconjunctivitis who was initiated on systemic therapy for moderate-to-severe disease at the age of 40 years. Because co-morbid eye symptoms were particularly troublesome for this patient during the spring allergy season, we chose to treat his AD with tralokinumab. The beneficial results of treatment with tralokinumab in this case include relief of the most bothersome AD symptoms for this individual (skin lesions and itch) as well as completely alleviating the swollen eyes/conjunctival irritation. This latter finding is consistent with results from the ECZTRA clinical trial programme, which reported a low rate of conjunctival adverse effects (5.4%).14
In addition, lifestyle factors such as sleeplessness and fatigue were improved, and all these factors contributed to higher QoL ratings by the patient as well as IGA. These results mirror findings from three phase III RCTs conducted in adults with moderate-to-severe AD not adequately controlled by topical corticosteroids. These RCTs demonstrated that tralokinumab (300 mg subcutaneously every 2 weeks) was significantly superior to placebo regarding the primary endpoints (IGA score of 0 or 1 and improvement of eczema area severity index of at least 75%) and DLQI at week 16 and was well tolerated up to 52 weeks of treatment.15,16
The current case is notable because of the long-term follow-up (38 weeks) during the COVID-19 pandemic, and the patient received a COVID-19 vaccination during treatment and this caused a slight deterioration of his AD which quickly resolved with continued administration. The patient also developed COVID-19, and this had no material effect on the control of his AD symptoms. In addition to the potential effects of COVID-19 on the effectiveness of tralokinumab therapy, there is also the possibility that treatment may affect the individual’s ability to respond to the disease. Among 51 patients with moderate-to-severe AD who developed COVID-19 while participating in the open-label long-term extension trial of tralokinumab (ECZTEND, NCT 03587805), 96% of cases were mild or moderate and all patients continued tralokinumab treatment following initial diagnosis.17,18 These results suggest that tralokinumab has no significant impact on patients’ ability to react to the SARS-CoV-2 virus responsible for COVID-19.18
This is the first case to be reported in the literature investigating the effectiveness of tralokinumab in a patient with moderate-to-severe AD treated long term in the real-world setting of community practice. Despite providing information about the use of tralokinumab during the COVID-19 pandemic, the findings should be viewed cautiously and are not generalizable to other patients with AD.
Conclusions
In an adult with AD that had proven recalcitrant to conventional therapies, tralokinumab produced long-term relief of symptoms such as skin lesions, pruritus and sleeplessness, as well as improvements in measures of QoL and overall well-being and this was unaffected by COVID-19 infection. Larger observational studies are warranted with tralokinumab to fully understand the benefits that it might provide in real-world clinical practice.
Acknowledgments
Writing assistance for this article was provided by Kerry Dechant, ISMPP CMPP™ and Peter Weber PhD, ISMPP CMPP™, on behalf of Content Ed Net (Munich, Germany) with funding from LEO Pharma GmbH (Neu-Isenburg, Germany).
Funding
The work was supported by Leo Pharma.
Disclosure
Dr Eva Moennig reports personal fees from Leo Pharma, during the conduct of the study. Dr Stephan Traidl reports personal fees from Leo Pharma, during the conduct of the study; personal fees from Janssen, Sanofi, LaRoche-Posay, Novartis, and Lilly Pharma, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Traidl S, Werfel T, Traidl-Hoffmann C. Atopic Eczema: pathophysiological Findings as the Beginning of a New Era of Therapeutic Options. Handb Exp Pharmacol. 2022;268:101–115. doi:10.1007/164_2021_492
2. Wollenberg A, Christen-Zäch S, Taieb A, et al. European Task Force on Atopic Dermatitis/EADV Eczema Task Force. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744. doi:10.1111/jdv.16892
3. Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40(2):84–92. doi:10.2500/aap.2019.40.4202
4. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi:10.1016/S0140-6736(20)31286-1
5. Szegedi K, Lutter R, Res PC, et al. Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. J Eur Acad Dermatol Venereol. 2015;29:2136–2144. doi:10.1111/jdv.13160
6. Tsoi LC, Rodriguez E, Degenhardt F, et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139:1480–1489. doi:10.1016/j.jid.2018.12.018
7. Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2019;75:54. doi:10.1111/all.13954
8. Tubau C, Puig L. Therapeutic targeting of the IL-13 pathway in skin inflammation. Expert Rev Clin Immunol. 2021;17:15–25. doi:10.1080/1744666X.2020.1858802
9. Duggan S. Tralokinumab: first Approval. Drugs. 2021;81(14):1657–1663. doi:10.1007/s40265-021-01583-1
10. Tralokinumab (Adtralza) 150 mg solution for injection. Summary of Product Characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/adtralza-epar-product-information_en.pdf.
11. Wollenberg A, Weidinger S, Worm M, Bieber T. Tralokinumab in atopic dermatitis. J Dtsch Dermatol Ges. 2021;19(10):1435–1442. doi:10.1111/ddg.14545
12. Wollenberg A, Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–141. doi:10.1016/j.jaci.2018.05.029
13. Neagu N, Dianzani C, Avallone G, et al. Dupilumab ocular side effects in patients with atopic dermatitis: a systematic review. J Eur Acad Dermatol Venereol. 2022;36(6):820–835. doi:10.1111/jdv.17981
14. European Medicines Agency. Summary of product characteristics: adtralza 150 mg solution for injection in pre-filled syringe; 2021. Available from: https://ec.europa.eu/health/documents/community-register/2021/20210617151835/anx_151835_en.pdf.
15. Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi:10.1111/bjd.19574
16. Silverberg JI, Toth D, Bieber T, et al. ECZTRA 3 study investigators. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450–463. doi:10.1111/bjd.19573
17. ClinicalTrials.gov. An Open-label, Single-arm, Multi-centre, Long-term Extension Trial to Evaluate the Safety and Efficacy of Tralokinumab in Subjects With Atopic Dermatitis Who Participated in Previous Tralokinumab Clinical Trials. Available from: https://clinicaltrials.gov/ct2/show/NCT03587805.
18. Langley R, Guttman‐Yassky E, Corriveau J, et al. COVID-19 in tralokinumab-treated patients with moderate-to-severe atopic dermatitis: case series from the ECZTEND long-term extension trial. Br J Dermatol. 2021;185(3):1264–1265. doi:10.1111/bjd.20657
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.